Abstract
Background
A minority of patients who experience awareness and/or pain during surgery subsequently develop posttraumatic stress disorder. In a rodent model of posttraumatic stress disorder, stress-enhanced fear learning (SEFL), rats are pre-exposed to a stressor of 15 footshocks. Subsequent exposure to a single footshock produces an enhanced fear response. This effect is akin to sensitized reactions shown by some posttraumatic stress disorder patients to cues previously associated with the traumatic event.
Methods
We studied the effect of isoflurane and nitrous oxide on SEFL. Rats were exposed to the inhaled anesthetic during or after a 15-footshock stressor. Then rats were given a single footshock in a different environment. Their fear response was quantified in response to the 15-footshock and single-footshock environments. SEFL longevity was tested by placing a 90-day period between the 15 footshocks and the single footshock. In addition, the intensity of the footshock was increased to evaluate treatment effectiveness.
Results
Increasing isoflurane concentrations decreased SEFL when given during, but not after, the stressor. At 0.40 minimum alveolar concentration, isoflurane given during the stressor blocked SEFL 90 days later. A three-fold increase in the stressor intensity increased the isoflurane concentration required to block SEFL to no more than 0.67 minimum alveolar concentration. As with isoflurane, nitrous oxide suppressed SEFL at a similar minimum alveolar concentration fraction.
Conclusions
These results suggest that sufficient concentrations (perhaps 0.67 minimum alveolar concentration or less) of an inhaled anesthetic may prevent SEFL.
Introduction
Surgery can impose both physical and mental stress. Patients given insufficient anesthesia during surgery may experience awareness and/or pain during their procedure. These patients are at increased risk of developing Posttraumatic Stress Disorder (PTSD) subsequently.1 Such patients display hallmark signs of PTSD including nightmares, flashbacks, general anxiety, chronic fear, and avoidance of trauma reminders, such as the hospital.2,3
It is ethically difficult to prospectively study PTSD in humans. Animal models present an alternative approach to study the disorder. While animal models cannot completely reflect a human psychological state, they may be used to study aspects of these states. In one such model, Stress-Enhanced Fear Learning (SEFL),4,5 rats are placed in an environment and given 15 footshocks, which simulates a stressful or “traumatic” event. The following day, they are placed in a second environment and given a single footshock, a reminder of the original stressful event. Over the following two days, the animals are tested for their fear to each environment in the absence of shock. The fear response measured is freezing, or immobility, which is an adaptive reaction of rodents to fearful stimuli.6 Typically, freezing increases asymptotically to a maximum as the number of shocks given increases.7 When animals are placed back in the 15-shock environment, they show a fear response proportional to the number of shocks received there.4 Pre-exposure to the 15-shock stressor enhances the fear response in the 1-shock environment. Animals not pre-exposed to the stressor show freezing proportional to one shock while animals pre-exposed to the 15-shock stressor show enhanced freezing.4
This model produces two dissociable effects. One effect is associative fear conditioning to the different cues present in each environment. Associative fear implies that there is a learned connection between two specific events such that the occurrence of one activates the representation of the other. In this case, placement into the context elicits the memory of the shock, which results in a fear response. The second effect is a permanent change in the neurobiological mechanisms that mediate fear conditioning such that a new stressful event produces greatly exaggerated learning of a new fear response, SEFL. Associative fear to the reminder should be low (in the absence of shock pre-exposure), but it is enhanced in pre-shocked animals due to a non-associative mechanism. Evidence from previous studies suggests that distinct mechanisms mediate associative fear conditioning versus SEFL.4 For example, despite elimination of associative fear to the 15-shock environment by extinction (repeated presentations of the context until there is no conditional fear) or pharmacological means, SEFL still persists.4
Previously, we have examined isoflurane's ability to block associative fear conditioning. Isoflurane given during training dose dependently reduces conditional fear during test.8,9 The present study assessed the capacity of inhaled anesthetics to suppress PTSD-like symptomotology, as measured in the SEFL animal model. Our goal was to determine what concentration of a prototypic inhaled anesthetic, isoflurane, suppressed SEFL (Experiment 1); whether post-stressor administration of isoflurane suppressed SEFL (Experiment 2); whether the suppression persisted (Experiment 3); whether the intensity of the stressor affected the isoflurane concentration required to suppress SEFL (Experiments 4 and 5); and whether nitrous oxide suppressed SEFL (Experiment 6).
Materials and Methods
The protocols for all studies were approved by the University of California San Francisco Committee on Animal Care (San Francisco, CA, USA). We used 70-90 days old naïve adult male Long Evans rats (Harlan; Indianapolis, IN). The rats were housed 2-3 per cage (42 cm long × 25 cm wide × 20 cm high), and food and water were available ad libitum. The animals were kept on a 12:12-hr light-dark cycle, with lights coming on at 6:00 am, and all experimental procedures took place during the light cycle. All rats were handled daily for approximately 20 s each for a week before each experiment.
An equilibration chamber was used for anesthesia delivery prior to fear conditioning. The chamber was similar to the rat's home cage. It was covered with a sheet of plastic pierced with two small conduits that allowed inflow and sampling of gases. A 17 cm diameter hole in the sheet was capped by a 20 cm high × 17 cm diameter plastic cylinder. This chimney-like structure allowed the escape of excess gases, but more importantly allowed rapid movement of rats into and out of the equilibration chamber with minimal disturbance to the isoflurane concentration.
We used a Gow-Mac gas chromatograph (Gow-Mac Instrument Corp., Bridgewater, NJ) equipped with a flame ionization detector to measure concentrations of isoflurane. The 4.6 meter-long, 0.22 cm (ID) column was packed with SF-96. The column temperature was 100°C. The detector was maintained at approximately 50°C warmer than the column. The carrier gas flow was nitrogen at a flow of 15-20 mL/min. The detector received 35-38 mL/min hydrogen and 240-320 mL/min air. Primary standards were prepared, and the linearity of the response of the chromatograph was determined. We also commonly used secondary (cylinder) standards referenced to primary standards.
Fear conditioning took place in four acrylic training chambers (27 cm L × 24.5 cm W × 20 cm H). The lid to each chamber had acrylic sidewall guides that fit inside the chamber and an 8 cm diameter hole fitted with a rubber stopper that was transiently removed during the transfer of a rat from the equilibration to the training chamber. Two 2 cm diameter conduits on opposing sides of the chamber allowed inflow and the outflow of gases to all four chambers. Gas inflow was delivered to the circuit in a 5-l/min oxygen flow through an isoflurane vaporizer (Isotec 4, Ohmeda; Los Angeles, CA) set to the desired concentration.
The chambers were used in two different procedures, and they were altered between procedures to maximize the animals' ability to differentiate between them. Pre-exposure to the stress took place in Context A, and training to a single shock took place in Context B. The contexts differed in texture, lighting, odor, and floor. In Context A, the walls were wiped with a pine-scented cleaner (5% Pine Scented Disinfectant, Midland, Inc.; Sweetwater, TN) before and after each session. The grid floor used to deliver shocks was composed of 14 stainless steel bars, each bar 5 mm in diameter, all bars spaced 13 mm from center to center. These floors were connected to a shock delivery system (San Diego Instruments; San Diego, CA). Overhead fluorescent room lights were left on, and a squirrel fan supplied background noise (65 db).
In Context B, rubber mats with suction backing covered the clear acrylic walls and ceiling. These mats were wiped with acetic acid (1%, Fisher Scientific; Fair Lawn, NJ) before and after each session. The grid floors used to deliver a single shock in this context were composed of 31 stainless steel bars, each 3 mm in diameter, spaced 7 mm center to center, which were also wired to the shock generator. Context B appeared dark to the rats, being lit by a single red 30 W red bulb placed at the opposite end of the room. Before each set of animals entered the room, an aerosol (Amphyl Disinfectant Deodorant Spray, Reckitt Benckiser Inc.; Wayne, NJ) was sprayed in the room. A compact disc player played a disc of white noise (65 db). In both Context A and B, the front walls of each chamber were clear and allowed a video camera and corresponding VCR to record the animals' behavior for analysis.
Experiment 1
Table 1 illustrates the design and procedures for this experiment. One hundred and four rats were used in this experiment. On Day 1, rats were transported in their home cages from their housing area to a small dark room in the laboratory. Sets of four animals were transported from the dark room to the study room and placed in the equilibration chamber at a time where they received 0%, 0.2%, 0.4%, 0.6%, 0.8%, or 1% isoflurane exposure for 30 minutes.
Table 1.
Experimental Design and Procedure for Determination of the Concentration-dependent Effect of Isoflurane on Stress-enhanced Fear Learning (Experiment 1)
| Day 1, Context A | ||||||
|---|---|---|---|---|---|---|
| Group | No. of Rats | Concentration of Isoflurane, % | Preexposure Treatment, No. of Shocks | Day 2, Context B | Day 3, Context B | Day 4, Context A |
| 1 | 12 | 0 | 0 | 1 Shock | Context Test | Context Test |
| 2 | 12 | 15 | ||||
| 3 | 8 | 0.2 | 0 | |||
| 4 | 8 | 15 | ||||
| 5 | 8 | 0.4 | 0 | |||
| 6 | 8 | 15 | ||||
| 7 | 8 | 0.6 | 0 | |||
| 8 | 8 | 15 | ||||
| 9 | 8 | 0.8 | 0 | |||
| 10 | 8 | 15 | ||||
| 11 | 8 | 1.0 | 0 | |||
| 12 | 8 | 15 | ||||
After equilibration, animals were removed via the chimney and, within 5-10 seconds, were placed into the Context A chambers, which were pre-equilibrated with an isoflurane concentration equivalent to the concentration in the equilibration chamber. Rats were randomly assigned to either receive 0 or 15 shocks (1 mA, 1 s shocks with a variable intershock interval of 240-480 s; producing a total pre-exposure time of 93 min) during Context A pre-exposure. Animals receiving no shock during pre-exposure were placed in Context A for the same time as shocked animals. After the pre-exposure, the animals were removed from the chambers, and placed in their home cages. The rats were returned to their housing area upon completion of the last group.
The following day, the animals were transported in cages that were the same size as the home cage, but outfitted with opaque plastic dividers such that each cage housed 3 rats separated from one another. These cages were placed in the laboratory housing area, now illuminated with a fluorescent bulb. Rats in each squad were then transferred to the Context B test area in separate plastic pots (23 cm high × 25 cm diameter) containing woodchip bedding and covered with opaque lids. In Context B, the rats were given a single shock (1 mA, 1 s) 192 s after placement in the chamber and then were removed from the chamber after an additional 30 s. Freezing, defined as the absence of all movement except that necessary for respiration was measured during this time.10
We assessed freezing for the 192 s pre-shock period to provide a baseline measure prior to shock in Context B. This indicated whether animals generalized between Context A and Context B. Generalization may occur when details of the two contexts are not sufficiently different. If the contexts were too similar for the rats to distinguish, rats pre-exposed to the 15 shocks in Context A would show higher baseline freezing in Context B (before shock) compared to rats receiving no shock in Context A (that is, they would be unable to distinguish that they were in a different place). On day 3, animals were returned to Context B for 8 min 32 s for a test of fear conditioned to the B context (the context test). On day 4, rats were returned to Context A and given another 8 min 32 s context test. Freezing was scored during each context test. Each rat's behavior was scored every 8 s during the observation period, and a percentage of freezing calculated from these observations (number of freezing observations counted/total number of freezing observations in the session). Four animals were scored at a time. To ensure that each rat had equivalent observations in each scoring period, we used the 192 s baseline period (24 observations/rat) and the 8 min 32 s context test (64 observations/rat).
Experiment 2
A question of substantial clinical relevance is whether anesthesia given after trauma exposure during treatment could be useful in suppressing the development of PTSD symptoms. Often times, individuals exposed to traumas such as a car accidents or assault must go to the hospital for treatment of injuries.11,12 Is it possible that anesthetics given in this type of situation could actually help to suppress the development of post-trauma symptoms? Given that isoflurane suppressed SEFL in Experiment 1, we tested whether its administration after the stressor could also suppress SEFL.
Thirty-two rats were used in this experiment. Rats were assigned to one of four conditions: 0 shocks during Context A pre-exposure/0% isoflurane, 15 shocks during Context A pre-exposure/0% isoflurane, 15 shocks during Context A pre-exposure/0.6% isoflurane given during the 15 shocks, 15 shocks during Context A pre-exposure/0.6% isoflurane given after the 15 shocks. In all groups, rats were first equilibrated as described in Experiment 1. After equilibration and Context A pre-exposure to 0 or 15 shocks (as described in Experiment 1), all groups were returned to the equilibration chamber for an additional two hours. Here, rats receiving post-shock administration were exposed to 0.6% isoflurane (all other groups received 0% isoflurane). In total, all groups received approximately two hours (either 30 min equilibration + 93 min pre-exposure or 2 h and 3 min post Context A pre-exposure) of isoflurane exposure. In Experiment 1, 0.4% isoflurane and higher suppressed conditional fear and SEFL. In this experiment and subsequent experiments, we chose to use 0.6% isoflurane [or 0.4 minimum alveolar concentration (MAC), MAC in rats is 1.4913] because animals given 0.6% and 15 shocks were statistically different from animals given 0% isoflurane and 15 shocks for the dependent measure of Context A freezing. Animals given 15 shocks and 0.4% isoflurane were not statistically different from animals given 15 shocks and 0% isoflurane. On Day 2, rats received a single shock in Context B. Rats were tested in Context B on Day 3 and in Context A on Day 4. This design and procedure are detailed in Table 2.
Table 2.
Experimental Design and Procedure for Examining the Effect of Poststressor Administration of 0.6% Isoflurane (Experiment 2)
| Day 1, Context A | |||||||
|---|---|---|---|---|---|---|---|
| Group | No. of Rats | Concentration of Isoflurane During Equilibration, % | Preexposure Treatment, No. of Shocks | Concentration of Isoflurane after Preexposure, % | Day 2, Context B | Day 3, Context B | Day 4, Context A |
| 1 | 8 | 0 | 0 | 0 | 1 Shock | Context Test | Context Test |
| 2 | 8 | 0 | 15 | 0 | |||
| 3 | 8 | 0.6 | 0 | 0 | |||
| 4 | 8 | 0 | 15 | 0.6 | |||
Experiment 3
PTSD is a long-lasting phenomenon. In fact, one of the diagnostic criteria for PTSD is that symptoms persist for at least one month after the trauma.2 Accordingly, Experiment 2 tested whether isoflurane suppressed SEFL for a substantial duration of time. Previous studies found that rats show SEFL when pre-exposed to the footshock stressor three months previously.5 We used this same time separation in the present experiment. Table 3 illustrates the design and procedures for Experiment 3.
Table 3.
Design and Procedures for Examining the Long-term Effect of 0.6% Isoflurane on Stress-enhanced Fear Learning (Experiment 3)
| Day 1, Context A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | No. of Rats | Concentration of Isoflurane, % | Preexposure Treatment, No. of Shocks | Days 2–90 | Day 91, Context B | Day 92, Context B | Day 93, Context B | Day 94, Context A |
| 1 | 8 | 0 | 0 | Wait | Extinction | 1 Shock | Context Test | Context Test |
| 2 | 8 | 15 | ||||||
| 3 | 8 | 0.6 | 0 | |||||
| 4 | 8 | 15 | ||||||
We used 32 rats in this experiment. Animals were randomly assigned to receive either 0 or 15 shocks and 0% or 0.6% isoflurane on Day 1 in Context A. After pre-exposure, which occurred as in Experiment 1, animals were returned to their housing area where they remained for 90 days.
Previous results demonstrated that animals showed increased generalized freezing between Context A and Context B after a prolonged period of time between stress pre-exposure and single shock training.5 To ensure that all groups showed virtually no baseline freezing in Context B before the single shock, we extinguished baseline freezing in Context B on the day before the single shock (Day 91, 90 days after Context A pre-exposure on Day 1). For extinction, the rats were transported to Context B as described in Experiment 1, and placed in the context for 30 minutes with no shock. Freezing was observed during the first three minutes of the extinction session. When animals were returned to Context B on the next day (Day 92), they received a single shock in Context B as described in Experiment 1. On Day 93, animals were tested for freezing to Context B, and on Day 94, animals were tested for freezing to Context A.
Experiments 4 and 5
Experiments 3 and 4 assessed whether the intensity of the stressor affected the capacity of isoflurane to suppress SEFL. We used 30 rats in Experiment 3, and 24 rats in Experiment 4. Using the 0.6% isoflurane concentration that completely suppressed SEFL in Experiment 1, we compared SEFL resulting from 15 1 mA versus 15 3 mA shock intensities. The procedure was essentially the same as in Experiment 1, except that we applied different shock intensities. On Day 1, animals received 0 shocks, 15 1 mA shocks, or 15 3 mA shocks during pre-exposure in Context A. On Day 2, all animals received a single 1 mA shock in Context B. On Day 3, we tested freezing to context in B, and on Day 4 we tested freezing to context in A; all as described in Experiment 1. Experiment 4 was conducted using the same procedures as described in Experiment 3, but the isoflurane concentration during pre-exposure was increased to 1%.
Experiment 6
We wanted to determine whether the ability of inhaled anesthetics to suppress SEFL was unique to isoflurane. We tested another commonly used anesthetic, nitrous oxide, in the SEFL model.
Twenty-eight animals were used in this study. All rats were given 70%-80% nitrous oxide (or 0.34 MAC, MAC in rats is 2.21 atm14). Half the rats received 0 shocks during Context A pre-exposure on Day 1, and the other half received 15 shocks. The rest of the experiment (Days 2-4) was carried out as described in Experiment 1.
Statistical Analysis
One or two-way analysis of variance (ANOVA) were used to analyze freezing during the Context B training baseline and the context tests. Fisher protected least significance test (PLSD) was used for all planned group comparisons. An alpha level of less than 0.05 was accepted as significant. Statview statistical software (Berkeley, CA) was used to perform all analyses.
Results
Experiment 1
Baseline (Context B)
During the 192 s pre-shock (baseline) period in Context B, there was virtually no generalized freezing (Fig 1). Pre-exposure to shock did not affect this measure (P = 0.54).
Figure 1.
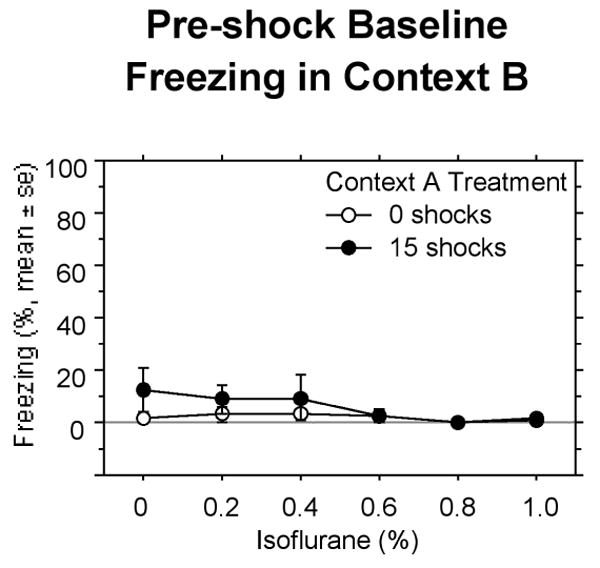
Experiment 1: Freezing measured during the baseline period prior to the single shock in Context B. Context A shock and isoflurane concentration did not significantly affect this measure.
SEFL (Context B)
Overall, increasing concentrations of isoflurane decreased SEFL in pre-shocked animals, and did not affect Context B freezing in non-pre-shocked animals (Fig. 2). The overall two-way ANOVA with isoflurane concentration as one factor (with six levels) and shock pre-exposure (0 or 15 shocks) as the other factor revealed a significant effect of isoflurane concentration, F(5,92) = 2.45 (P < 0.05.) on Context B freezing. A priori comparisons between animals receiving 0 or 15 shocks during pre-exposure at each dose showed that only concentrations of 0.4% isoflurane and greater suppressed SEFL. That is, shock pre-exposed animals breathing 0% or 0.2% isoflurane showed SEFL while shock pre-exposed animals breathing concentrations of 0.4% isoflurane and higher did not show enhanced freezing compared to their non-shock pre-exposed counterparts.
Figure 2.
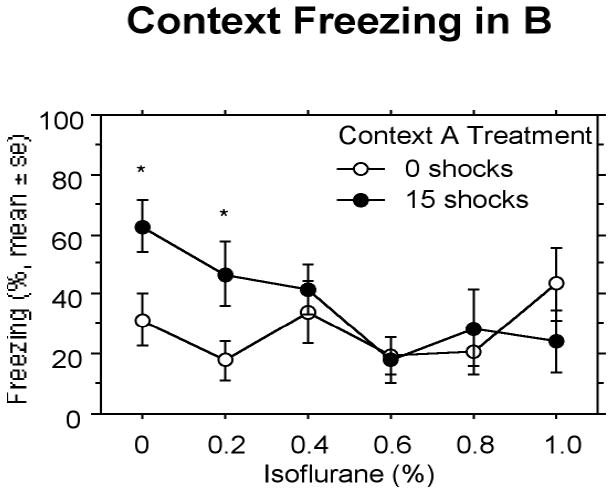
Experiment 1: Animals given 0 or 0.2% isoflurane and pre-exposure to 15 shocks in Context A show enhanced fear conditioned to the single shock given in B compared to animals given these concentrations of isoflurane and 0 shocks in Context A. Isoflurane concentrations of 0.4% and greater suppressed stress-enhanced fear learning. Significant differences (P<.05) between groups indicated by *.
Associative Fear (Context A)
Increasing concentrations of isoflurane decreased fear conditioning to the 15-shock context measured during the Context A test (Fig. 3). The overall two-way ANOVA conducted on these data revealed a reliable effect of isoflurane concentration, F(5,92) = 10.65, P < 0.0001, Context A treatment, F(5,92) = 21.99, P < 0.0001, and an interaction between the two, F(5,92) = 8.54, P < 0.0001. Shocked animals given 0% isoflurane and 0.2% isoflurane showed conditional fear in Context A compared to their non-shocked counterparts. Isoflurane concentrations of 0.4% and higher abolished contextual fear conditioning. However, rats receiving 0.6% isoflurane (but not 0.4% isoflurane) were significantly different that shocked controls receiving 0% isoflurane. Thus, 0.6% isoflurane (0.4 MAC) will be used in subsequent experiments.
Figure 3.
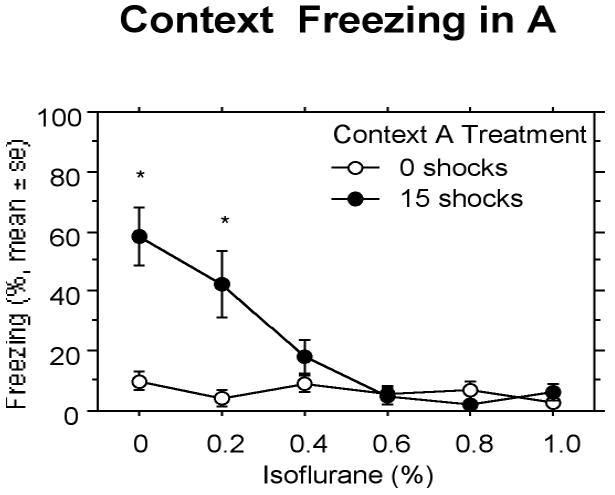
Experiment 1: Increasing isoflurane concentrations decreased fear conditioned to the 15 shocks in Context A. Relative to controls, animals receiving 0 and 0.2% isoflurane showed a substantial conditional fear in Context A, an effect that disappeared in animals receiving 0.4% and greater concentrations of isoflurane. Significant differences (P<.05) between groups indicated by *.
Experiment 2
In Experiment 2, we wanted to determine whether isoflurane given solely after pre-exposure to 15 shocks would produce suppression of freezing in both contexts. Three control groups that were run in Experiment 1 were included in this design: 0 shocks during Context A pre-exposure and 0% isoflurane, 15 shocks during Context A pre-exposure and 0% isoflurane, 15 shocks during Context A pre-exposure and 0.6% isoflurane. An additional group given 0.6% isoflurane only after Context A pre-exposure was included.
Baseline (Context B)
During the 192 s pre-shock (baseline) period in Context B, there was virtually no generalized freezing. All groups showed less than 1% baseline freezing.
SEFL (Context B)
The results from the three control groups replicated Experiment 1′ results. Rats receiving 0% isoflurane and 15 shocks showed enhanced freezing compared to rats receiving 0% isoflurane and 0 shocks, a replication of the SEFL effect. Rats receiving 0.6% isoflurane and 15 shocks showed suppressed SEFL and were indistinguishable from non-pre-shocked controls receiving 0% isoflurane. Post-shock exposure to isoflurane did not produce any suppression in SEFL (Fig. 4). A one-way ANOVA with each group as a level confirmed revealed a significant effect of treatment, F(3,28) = 16.536 (P < 0.0001). All post-hoc differences were significant with ps<.0001.
Figure 4.
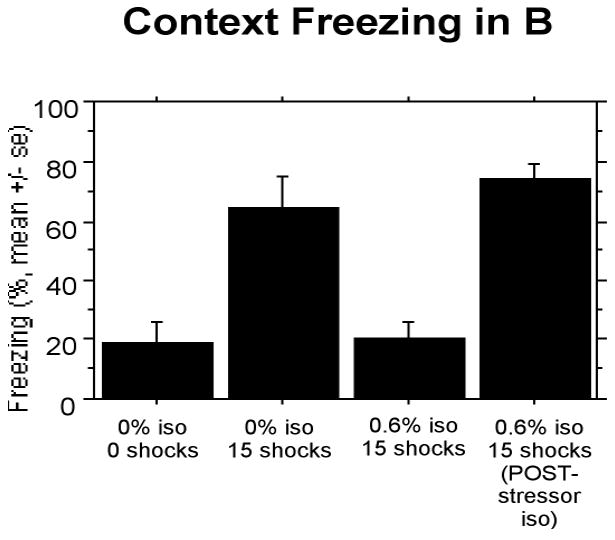
Experiment 2: Isoflurane given after pre-exposure to shock does not suppress stress-enhanced fear learning as when given during pre-exposure to shock.
Associative Fear (Context A)
A similar pattern of results as described for Context B was found for Context A (Fig 5). Of groups receiving 0% isoflurane, rats given 15 shocks showed a high level of freezing compared to non-shocked controls, and isoflurane given during shock suppressed freezing but not when given after shock. On this measure, there was also a reliable effect of treatment F(3,28) = 28.171, P < 0.0001, and post-hoc comparisons were significant at p<.0001.
Figure 5.
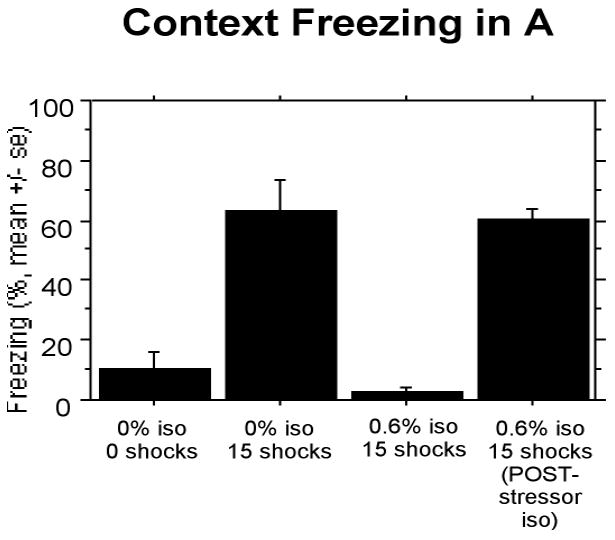
Experiment 2: Isoflurane given after pre-exposure to shock does not suppress associate fear to the 15 shocks as when given during pre-exposure to shock.
Experiment 3
Experiment 3 investigated whether isoflurane suppressed SEFL when the single shock was given three months after the stressor.
Baseline (Context B)
Animals given 15 shocks in Context A with 0% isoflurane showed increased freezing (44.27%±40.15%) during the first three minutes of the extinction session on day 91 compared to the other three groups (0%). A two-way ANOVA revealed a reliable effect of Context A treatment, F(1,27) = 9.26, P < 0.05, isoflurane, F(1,27) = 8.83, P < 0.05, and an interaction between the two, F(1,27) = 8.83, P < 0.05. When animals were returned to Context B the following day (Day 92), for the single shock, they showed virtually no freezing.
SEFL (Context B)
After 92 days, animals given 15 shocks and 0% isoflurane in Context A showed enhanced fear conditioned to the single shock compared to all other groups (Fig. 6). Isoflurane at 0.6% given during administration of the 15 shocks 92 days earlier suppressed SEFL. A two-way ANOVA revealed a reliable effect of Context A treatment, F(1,27) = 4.66, P < 0.05, a trend towards an effect of isoflurane, F(1,27) = 3.62, P = 0.068, and an interaction between the two, F(1,27) = 6.35, P < 0.05.
Figure 6.
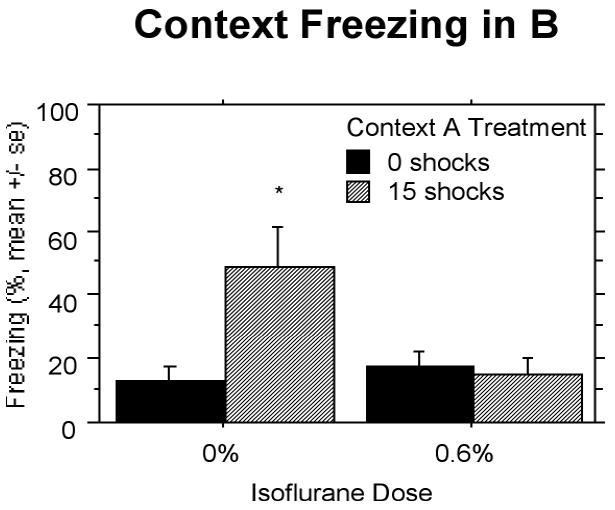
Experiment 3: Rats given 15 shocks and 0% isoflurane in Context A show enhanced fear conditioned to the single shock given in Context B three months later. Isoflurane at a concentration of 0.6% suppressed the enhancement. Significant differences (P<.05) between groups indicated by *.
Associative Fear (Context A)
When animals were returned to Context A for the 8 min 32 s test on Day 94, only animals given 15 shocks with 0% isoflurane showed fear to context (Fig. 7). Isoflurane at 0.6% suppressed conditional fear to the 15 shocks. The two-way ANOVA showed a significant effect of Context A treatment, F(1,27) = 8.14, P < 0.01, isoflurane concentration, F(1,27) = 11.34, P < 0.01, and an interaction between the two, F(1,27) = 10.38, P < 0.01.
Figure 7.
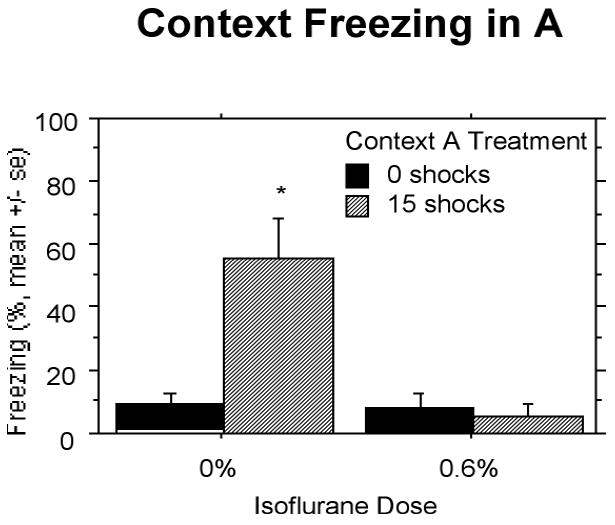
Experiment 3: When animals were returned to Context A for the test on Day 94, only animals given 15 shocks with 0% isoflurane showed fear to the context. Isoflurane at a concentration of 0.6% suppressed conditional fear to the 15 shocks. Significant differences (P<.05) between groups indicated by *.
Experiment 4
When animals were given 15 1 mA shocks during pre-exposure, isoflurane concentrations of 0.6% and greater suppressed SEFL (Experiment 1). In this experiment, we investigated whether the same concentration suppressed SEFL when 15 3 mA shocks were given during pre-exposure.
Baseline (Context B)
There was virtually no baseline freezing during the 192 s pre-shock period in Context B, with all groups below 2% baseline freezing.
SEFL (Context B)
Animals receiving 15 3 mA shocks in Context A showed more freezing compared to the other two groups during the 8 min 32 s test in Context B (Fig. 8). A one-way ANOVA showed a reliable effect of treatment F(2,27) = 3.391, P < 0.05.
Figure 8.
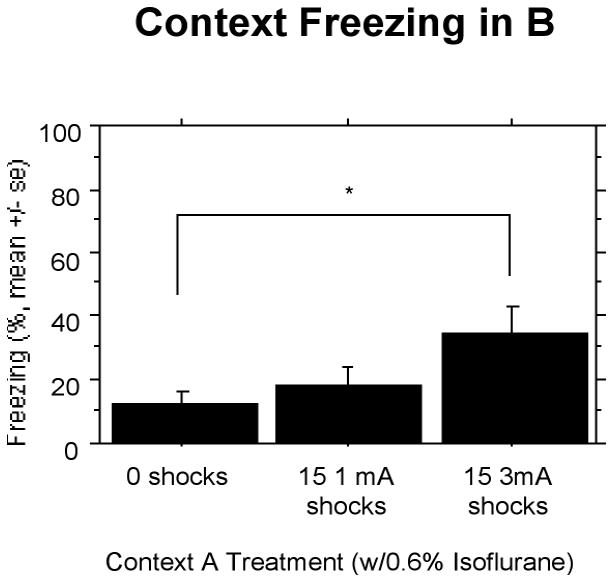
Experiment 4: Rats receiving 15 3 mA shocks and 0.6% isoflurane showed more freezing compared to control rats. Significant differences (P<.05) between groups indicated by *.
Associative Fear (Context A)
Animals receiving 15 3 mA shocks showed more freezing in Context A (20.31%±20.58%) compared to animals receiving 15 1 mA shocks (14.06%±14.778%) and animals receiving no shock (7.62%±5.38%), but this difference was not significant.
Experiment 5
Since 0.6% isoflurane did not suppress SEFL with 15 3 mA shocks during pre-exposure in Experiment 3, we increased the concentration to 1% in the present experiment.
Baseline (Context B)
There was virtually no baseline freezing in Context B during the 192 s pre-shock period, with all groups below 1% baseline freezing.
SEFL (Context B)
The ANOVA conducted on percentage of freezing during the 8 min 32 s context test in B revealed no reliable effect of treatment (Fig 9).
Figure 9.
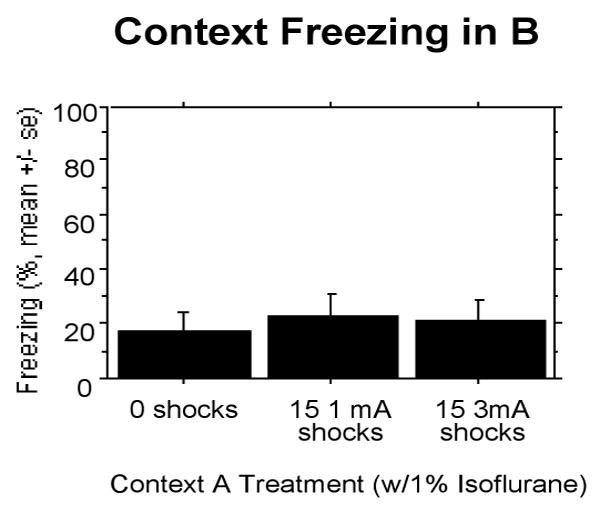
Experiment 5: Freezing in rats receiving 15 3 mA shocks and 1% isoflurane is completely suppressed and similar to control rats.
Associative Fear (Context A)
The ANOVA conducted on the percentage of freezing during the 8 min 32 s context test in context A also revealed no reliable effect of treatment (non-shocked rats: 6.25±10.95; rats receiving 15 1 mA shocks: 6.645±13.41; rats receiving 15 3 mA shocks: 9.96±12.13). Thus, 1.0% but not 0.6% isoflurane, suppressed SEFL when the footshock intensity during pre-exposure was 3 mA.
Experiment 6
In this experiment, we investigated whether a concentration of nitrous oxide (approximately 0.34 MAC) with similar potency to isoflurane (0.6% determined in Experiment 1, 0.4 MAC) would produce a suppression of associative fear and SEFL.
Baseline (Context B)
There was no difference in baseline freezing scores during the 192 s pre-shock period in Context B. Both groups showed less than 5% freezing.
SEFL (Context B)
Nitrous oxide completely suppressed the effect of the 15 shocks. Both groups showed fear proportional to the single shock given in this context. Animals receiving 0 and 15 shocks were indistinguishable from one another (Fig. 10).
Figure 10.
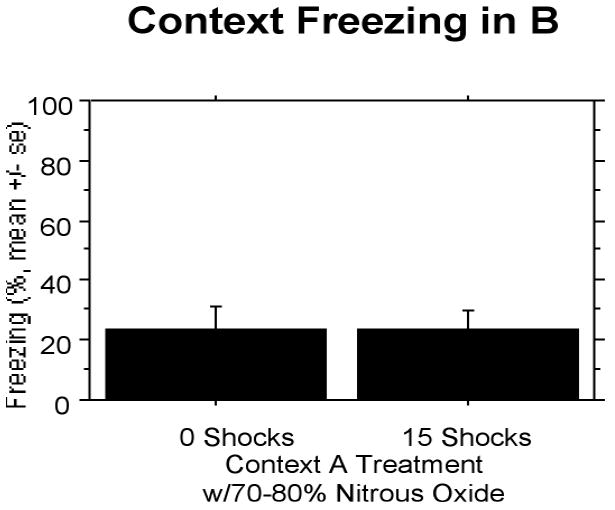
Experiment 6: Nitrous oxide at 70%-80% completely suppresses stress-enhanced fear learning. Both groups show fear proportional to a single shock.
Associative Fear (Context A)
Nitrous oxide also completely suppressed associative fear in Context A. Shocked rats were statistically indistinguishable from non-shocked rats (Fig 11).
Figure 11.
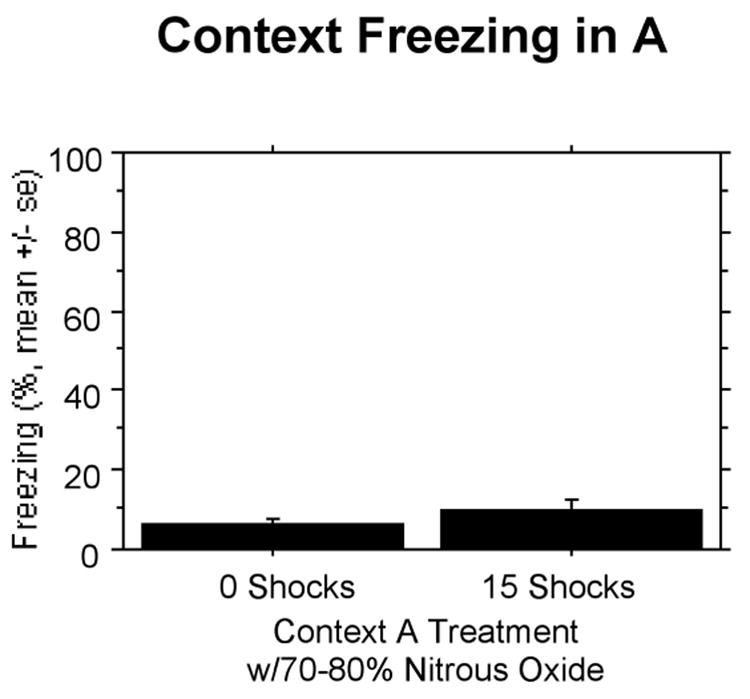
Experiment 6: Nitrous oxide at 70%-80% completely suppresses associative learning in the 15-shock context (A). Both groups show indistinguishable low levels of freezing.
Discussion
Preventing negative psychological sequellae, such as PTSD, from developing after surgery motivated the present research. SEFL allows us to study this issue and determine how such undesirable consequences might be prevented. SEFL produces two long lasting aversive reactions. One is associative fear conditioning to the neutral cues present at the time of shock. The other is a change in reactivity to future aversive reminder events (i.e. the single shock). Fear conditioned to the 15-shock stressor given in Context A provides a measure of what the animal has learned while under anesthesia, and allows a determination of the anesthetic concentration for its suppression. Fear conditioned to the single shock given in Context B allows a measure of how a reminder of the stressor may produce a sensitized reaction and the anesthetic concentration that suppresses it. Collectively, these studies allow a determination of how anesthesia might prevent the sensitized reactions shown in SEFL and perhaps PTSD.
Results from Experiment 1 show that increasing concentrations of isoflurane decrease conditional fear for the context in which it is given. Isoflurane concentrations of 0.4% and greater given during the 15 shocks suppress conditional freezing during a later anesthetic-free test, producing freezing comparable to that shown by non-shocked controls. This is consistent with previous results showing that 0.35% isoflurane and greater concentrations suppress contextual fear conditioned by three tone-shock (3 mA) pairings.8,9 Experiment 2 shows that in order for isoflurane to suppress associative learning, it must be given during the stressor, as isoflurane given after pre-exposure to 15 shocks in Context A did not suppress freezing during the Context A test. Results form Experiment 3 show that suppression by 0.6% isoflurane in Context A still occurs when the context test is given three months later. It is a long lasting suppression of learning.
The Context B test measures how pre-exposure to the stressor of 15 footshocks affects learning of new aversive events. As shown in Experiment 1, animals can distinguish Context B from Context A. That is, there is no generalization of fear to Context B after 15 shocks in Context A in control rats (i.e., those not given pre-exposure to the 15 footshock stressor). These control rats show 30% freezing when tested for the memory of the single shock received in Context B. Pre-exposure to the 15 shocks in the absence of isoflurane doubles that amount of freezing. Whether they do or do not receive shocks in Context A, animals given 0.4% and greater concentrations of isoflurane show an amount of fear conditioned by the single shock that is similar to that seen in controls.
It is unlikely that the effect of isoflurane on SEFL is caused simply by blocking fear conditioning to Context A. This is because previous results show that when fear to the 15 shocks is extinguished with repeated presentations to the context or its acquisition is pharmacologically blocked, animals still show enhancement of conditioned fear to the single shock context.4 That is, eliminating the memory of the stressor experience does not block SEFL.
Experiment 4 shows that although 0.6% isoflurane blocks SEFL for a 1 mA stressor, it did not block SEFL for a 3 mA stressor. However, increasing the isoflurane concentration to 1% does block the SEFL for 3 mA. Whether a smaller increase in isoflurane concentration (e.g., to 0.8%) might have blocked the 3 mA SEFL remains to be determined. We also cannot say whether a further increase in the intensity of noxious stimulation would also increase the concentration of isoflurane needed to suppress SEFL. Our prediction is that it would not because the increase in concentration required to suppress the 3 mA stressor was considerably less than the increase in the intensity of the stimulation. We submit that we are at or approaching the maximum suppression with 1.0% isoflurane.
We conducted Experiment 6 to assess whether another commonly used anesthetic could suppress SEFL. Rats given 70-80% nitrous oxide and 15 shocks showed a complete suppression of SEFL. Rats pre-exposed to shock behaved like non-shocked controls. Moreover, suppression was observed at similar potencies for each compound (0.4 MAC for isoflurane; MAC in rats is 1.49%13 isoflurane and 0.34 MAC for nitrous oxide; MAC in rats is 2.21 atm14). Since nitrous oxide was not tested at lower concentrations, we cannot say with certainty that a slightly lower concentration of nitrous oxide would also suppress SEFL.
We also conducted Experiment 6 because nitrous oxide generally affects receptors differently from isoflurane. For example, it blocks NMDA receptors more completely than does isoflurane,15 but it is weaker in enhancing the response of GABAA or blocking acetylcholine receptors.16,17 Despite these disparate receptor effects, the suppression of SEFL appears to occur at comparable MAC fractions.
Our findings have several implications for PTSD. First, the anesthetic concentration given during a stressful event can suppress learning and memory of this stressful event, and potentially circumvent the development of PTSD symptomotology. Second, 0.4 MAC isoflurane and 0.34 MAC nitrous oxide suppresses SEFL and perhaps the development of PTSD. Third, stresses imposed during anesthesia can lead to PTSD symptoms in the distant future, and these can be prevented with sufficient concentrations of inhaled anesthetic.
Acknowledgments
This work was supported in part by grant 1P01GM47818 from the National Institutes of Health (Bethesda, MD).
Footnotes
Summary Statement: The inhaled anesthetics isoflurane and nitrous oxide suppress sensitization of fear responses in stress-enhanced fear learning, a rodent model of post-traumatic stress disorder.
References
- 1.Osterman JE, Hopper J, Heran WJ, Keane TM, van der Kolk BA. Awareness under anesthesia and the development of posttraumatic stress disorder. Gen Hosp Psychiatry. 2001;23:198–204. doi: 10.1016/s0163-8343(01)00142-6. [DOI] [PubMed] [Google Scholar]
- 2.American: Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. American Psychatric Press; Washington, DC: 1994. pp. 424–429. [Google Scholar]
- 3.Samuelsson P, Brudin L, Sandin RH. Late psychological symptoms after awareness among consecutively included surgical patients. Anesthesiology. 2007;106:26–32. doi: 10.1097/00000542-200701000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2008;1 doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- 6.Fanselow MaL LS. In: A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior, Evolution and Learning. Bolles RaB MD, editor. New Jersey: Erlbaum; 1988. pp. 185–211. [Google Scholar]
- 7.Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93:736–44. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 8.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., II The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology. 2001;94:514–9. doi: 10.1097/00000542-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., II Isoflurane causes anterograde but not retrograde amnesia for pavlovian fear conditioning. Anesthesiology. 2002;96:1223–9. doi: 10.1097/00000542-200205000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C. Psychiatric sequelae of disasters. J Burn Care Res. 2006;27:600–5. doi: 10.1097/01.BCR.0000235463.20759.A0. [DOI] [PubMed] [Google Scholar]
- 12.Mohta M, Sethi A, Tyagi A, Mohta A. Psychological care in trauma patients. Injury. 2003;34:17–25. doi: 10.1016/s0020-1383(02)00377-7. [DOI] [PubMed] [Google Scholar]
- 13.Eger EI, 2nd, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV, 2nd, Sonner JM. Contrasting roles of the N-methyl-D-aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesth Analg. 2006;102:1397–406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- 14.Gonsowski CT, Eger EI., 2nd Nitrous oxide minimum alveolar anesthetic concentration in rats is greater than previously reported. Anesth Analg. 1994;79:710–2. doi: 10.1213/00000539-199410000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Solt K, Eger EI, 2nd, Raines DE. Differential modulation of human N-methyl-D-aspartate receptors by structurally diverse general anesthetics. Anesth Analg. 2006;102:1407–11. doi: 10.1213/01.ane.0000204252.07406.9f. [DOI] [PubMed] [Google Scholar]
- 16.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Ueta K, Sugimoto M, Uchida I, Mashimo T. Nitrous oxide and xenon inhibit the human (alpha 7)5 nicotinic acetylcholine receptor expressed in Xenopus oocyte. Anesth Analg. 2003;96:443–8. doi: 10.1097/00000539-200302000-00028. table of contents. [DOI] [PubMed] [Google Scholar]


