Abstract
Herein, we report on an in vitro kinetic activity analysis that demonstrates that the protein known as the Akt C-terminal modulator protein is a broad-range, high-activity acyl-CoA thioesterase. In vitro tests of possible activity regulation by product inhibition or by Akt1 binding gave negative results. Truncation mutants confined the thioesterase activity to the C-terminal domain, consistent with our threading model. The N-terminal domain of unknown fold and function was found to contribute to solubility.
In this report, we examine the in vitro properties of the human protein hTHEM4 (NP 444283), also known as “Akt C-terminal modulator protein” (CTMP). Our attention was first drawn to hTHEM4 by reports that hTHEM4 mRNA expression is weakened in primary glioblastomas and in glioblastoma cell lines (1), and that hTHEM4 can revert the phenotype (cell morphology, growth rate, and in vivo turnorigenesis) of Akt1-transformed cell lines (2). Akt1 is a serine-threonine protein kinase that plays a key role in cancer by stimulating cell proliferation and inhibiting apoptosis (3). Reports from in vivo (cell culture or whole animal) studies of interactions of hTHEM4 with Akt1 are seemingly contradictory. The earliest work (2001) with transfected human cells provided evidence that hTHEM4 inhibits Akt1 phosphorylation and activity (2). This result is consistent with the studies carried out with K-ras null mice in 2007, which showed that lentivirus-based hTHEM4 delivery inhibited the Akt1 activity through selective repression of Akt1 phosphorylation at S473 (4). On the other hand, another 2007 study found that hTHEM4 facilitates translocation of Akt1 to the membrane of transfected human cells for activation via phosphorylation (5). Two recently reported studies (2009) identify hTHEM4 as a mitochondria protein (6, 7). One of these (6) provides evidence that hTHEM4 is released from the mitochondria into the cytosol early upon apoptosis and that its over-expression delays Akt1 phosphorylation upon treatment with actinomycin-D and sensitizes the cell to apoptosis. The other study suggests that hTHEM4 binds to an inner mitochondrial membrane protein (7). A most recent report (8) claims that neuronal hTHEM4 is a cytoplasmic protein that binds to Akt1 and plays a causal role in ischemia-induced neuronal death. In the work described in this paper, we demonstrate that in vitro, hTHEM4 exhibits a broad-range, highly efficient thioesterase activity.
Bioinformatic analysis suggested that hTHEM4 belongs to the Arthrobacter 4-hydroxybenzoyl-CoA thioesterase clade of the hotdog-fold thioesterase family (9). A typical thioesterase of this clade is ~140 amino acids in length and consists of a five-stranded β-sheet that wraps around a long α-helix. Two subunits associate β-strand to β-strand to form an “extended β-sheet” with active sites located at the subunit–subunit interface. The dimer associates back to back to form tetrameric or hexameric structures. hTHEM4 is 240 amino acids long and consists of an N-terminal domain of unknown fold and a C-terminal domain whose closest known counterpart is the 140-amino acid fatty acyl-CoA thioesterase known as “human hotdog-fold thioesterase 2” (hTHEM2)(10). A threading model of the hTHEM4 thioesterase C-domain (residues 113–240) made in Phyre (11) (E value of 3.390004 × 10−14) is shown in Figure 1 superimposed on the hTIIEM2 template bound with the substrate analogue inhibitor undecan-2-one-CoA. The model is missing residues 233–240 and thus does not depict the short helical C-terminus that in the hTHEM2 contributes to the substrate acyl-binding channel (10). Nevertheless, this model strongly suggested to us that hTHEM4 possesses thioesterase activity and thus directed our efforts toward obtaining enzyme for in vitro catalytic activity tests.
Figure 1.
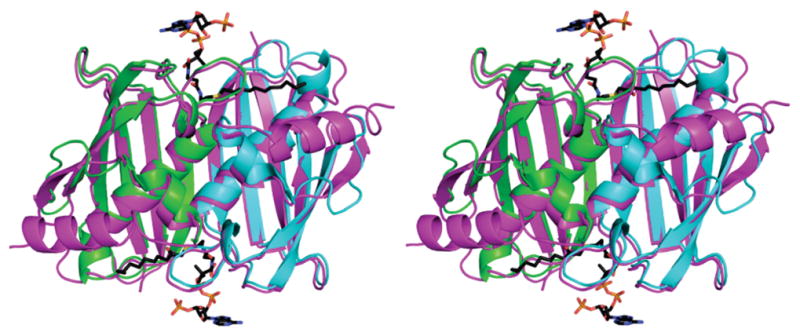
Stereo representation of the superposition of the hTHEM4 model (residues 113–232 with monomers colored lime and cyan) with the template hHTEM2 dimer (colored magenta) bound with undecan-2-one-CoA (shown as sticks with carbons colored black, oxygens red, and nitrogens blue).
To this end, the encoding gene was cloned and expressed in Escherichia coli to produce the N-terniinal His6-tagged protein, which was isolated using a Ni-NTA agarose column (see the Supporting Information for preparation of full-length and truncated recombinant hHTEM4). SDS–PAGE analysis of the product revealed three proteins that migrated as ~29 kDa (major), ~27 kDa (trace), and ~23 kDa (minor) proteins (Figure 2A). The “29 kDa” and “23 kDa” proteins were extracted from the gel and subjected to N-terminal sequence analysis to reveal the (fl)hTHEM4 (GSSH6S) (theoretical molecular mass of 29293 Da) and an N-terminal truncate (FSSEEVILKD) missing the His6 tag and the first 35 amino acids (theoretical molecular mass of 23317 Da).
Figure 2.
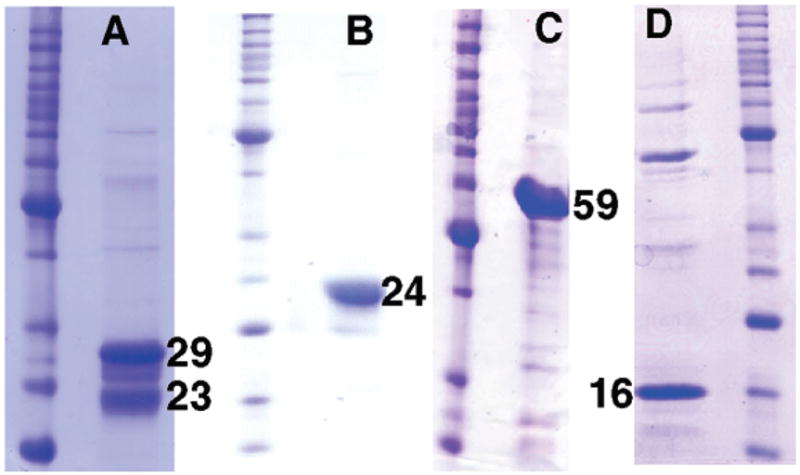
SDS–PAGE gels of molecular mass markers and recombinant His6-tagged (A) 29 kDa (fl)hTHEM4, (B) 24 kDa (Δ39)hTHEM4, (C) 59 kDa MBP-(Δ99)hHTEM4, and (D) 16 kDa (Δ99)hHTEM4. Numbers represent the approximate mass in kilodaltons. Ladders are not labeled.
As the first ~36 residues are predicted (6) to be targeted for proteolytic removal by the mitochondrial signal protease, we experimented with preparing the (C-terminal His6-tagged) hTHEM4 N-terminal truncates [viz. (Δ35)hTHEM4, (Δ39)-hTHEM4, and (Δ50)hTHEM4]. Of these, (Δ39)hTHEM4 was obtained in the best yield and homogeneity (Figure 2B). The ES-MS-determined mass is 24062 Da (theoretical molecular mass of 24063 Da). The commercial SEC-LS/RI/UV analysis of native (Δ39)hTHEM4 revealed a major protein peak corresponding to a mass of 50 kDa and a minor peak of a 102 kDa protein indicative of a dimer in a mixture with a trace amount of tetramer (Figure SI1). To examine the viability of a putative splice variant, we prepared the gene encoding the N-terminal domain truncate (Δ99)hTHEM4. Translation in E. coli resulted in an insoluble protein aggregate, and therefore, the His6-tagged maltose binding domain protein (MBP), in fusion with the N-terminus of (Δ99)-hTHEM4, was prepared (Figure 2C).
All three constructs were tested for thioesterase activity against myristoyl-CoA at pH 7.5 and 25 °C (Table 1). (fl)hTHEM4 and (Δ39)hTHEM4 are both highly active (kcat/Km = 1 × 106M−1s−1) at 25 °C, and a test of the activity of (Δ39)hTHEM4 at 37 °C revealed a modest increase in kcat(Table 1). We thus conclude that hTHEM4 is a functional acyl-CoA thioesterase and that the N-terminal region purported to be removed by the MLS peptidase (6) is not required for folding or for thioesterase activity. The His6-MBP-(Δ99)hTHEM4 construct showed 100-fold less activity, yet with a kcat/Km of 2 × 104M−1s−1, it is still considered to be a competent catalyst. This result is consistent with the threading model presented in Figure 1, which predicts that the hotdog-fold thioesterase unit is formed by residues 113–240. Proteolytic removal of the His6-MBP from His6-MBP-(Δ99)hTHEM4 (Figure 2D) resulted in greatly diminished solubility. This finding suggests that the N-terminal domain of the full-length enzyme contributes to its solubility.
Table 1.
Steady-State Kinetic Constants for the Catalyzed Hydrolysis of Myristoyl-CoA Measured at pH 7.5 and 25 °C for the hTHEM4 Constructsa
| hTHEM4 | kcat(s−1) | Km(μM) | kcat/Km(M−1s−1) |
|---|---|---|---|
| fl | 2.26 ± 0.04 | 1.17 ± 0.08 | l.0 × 106 |
| Δ39 | 4.2 ± 0.1 | 2.4 ± 0.1 | 1.7 × 106 |
| Δ39(37 °C) | 6.6 ± 0.6 | 2.8 ± 0.1 | 2.4 × 106 |
| Δ99b | 0.12 ± 0.01 | 6 ± 1 | 2.0 × 104 |
Experimental details are provided in the Supporting Information.
N-Terminal maltose binding protein fusion construct.
The hTHEM4 substrate profile was measured using (Δ39)-hTHEM4. The rate constants reported in Table 2 indicate physiologically significant catalytic efficiency over a broad substrate range. The medium to long chain fatty acyl-CoAs constitute the most active substrates that have been found (kcat/Km = 1 × 105 to 1 × 106M−1s−1). The aromatic substrates tested, 4- and 2-hydroxybenzoyl-CoA and 3-hydroxyphenylacetyl-CoA, are ~ 10-fold less active. The activity toward short chain, nonpolar acyl-CoA metabolites is even lower, however still within a physiologically relevant range. The polar acyl-CoA thioester succinyl-CoA was the least active substrate tested (kcat/Km = 6.5 × 102 M−1 s−1).
Table 2.
Steady-State Kinetic Constants for the hTHEM4-Catalyzed Hydrolysis of Acyl-CoA Thioesters Measured at pH 7.5 and 25 °Ca
| acyl group | kcat(s−1) | Km (μM) | kcat/Km (M−1s−1) |
|---|---|---|---|
| 4-HBb | 0.29 ± 0.02 | 29 ± 4 | 1.0 × 104 |
| 2-HBb | 0.34 ± 0.02 | 42 ± 2 | 8.2 × 103 |
| 3-HPAb | 4.1 ± 0.1 | 48 ± 3 | 8.5 × 104 |
| succinyl | 0.10 ± 0.01 | 150 ± 10 | 6.5 × 102 |
| acetyl | 0.55 ± 0.03 | 23 ± 3 | 2.4 × 104 |
| n-propionyl | 0.24 ± 0.01 | 88 ± 1 | 2.8 × 103 |
| n-butyryl | 0.22 ± 0.01 | 16 ± 2 | 1.4 × 104 |
| isobutyryl | 0.5 ± 0.03 | 103 ± 8 | 4.9 × 103 |
| hexanoyl | 1.2 ± 0.3 | 13 ± 1 | 9.4 × 104 |
| octanoyl | 2.4 ± 0.03 | 8.3 ± 0.3 | 2.9 × 105 |
| decanoyl | 2.2 ± 0.1 | 6.1 ± 0.4 | 3.6 × 105 |
| lauroyl | 4.7 ± 0.2 | 3.3 ± 0.5 | 1.4 × 106 |
| myristoyl | 4.2 ± 0.1 | 2.4 ± 0.1 | 1.7 × 106 |
| palmitoyl | 3.9 ± 0.1 | 2.6 ± 0.1 | 1.5 × 106 |
| oleoyl | 2.8 ± 0.03 | 5.2 ± 0.1 | 5.3 × 105 |
| arachidonyl | 1.0 ± 0.05 | 8.6 ± 0.2 | 1.1 × 105 |
Experimental details are provided in the Supporting Information.
Abbreviations: 2-HB-CoA, 2-hydroxybenzoyl-CoA; 4-HB-CoA, 4-hydroxybenzoyl-CoA; 3-HPA-CoA, 3-hydroxyphenylacetyl-CoA.
To test whether hTHEM4 might also be active toward the fatty acyl-panthetheine moiety of the cytoplasmic fatty acid synthase acyl carrier protein (c-FAS-ACP) domain, a myristoylated holo-ACP substrate was prepared from the engineered domain of the human c-FAS (12) (details provided in the Supporting Information). The catalyzed hydrolysis was monitored at 37 °C and pH 7.5 by using ES-MS to quantitate the myristoylated holo-ACP and its hydrolysis product holo-ACP. A DTNB-based continuous spectroscopic assay for CoA formation was used to define the steady-state kinetic constants: kcat = 0.06 s−1, Km = 12 μM, and kcat/Km = 5 × 103 M−1 s−1 (details in the Supporting Information). This level of activity is significantly lower (~500-fold) than that observed toward myristoyl-CoA, yet it is still high enough to be of possible physiological relevance.
Indiscriminant hydrolysis of cellular thioester metabolites poses a potential threat to the cell, and therefore, some form of regulation is likely to be operative. One common form, which operates at the activity level, is feedback product inhibition. The broad substrate range, high activity, cytaplasmic acyl-CoA hotdog-fold acyl-CoA thioesterase YciA from bacteria appears to be regulated by CoA as suggested by its small Ki value ( < 1μM) compared to its acyl-CoA substrate Km values (2–40 μM) (13). Accordingly, the time course for the (Δ39)hTHEM4-catalyzed hydrolysis of 20 μM 4-HBA-CoA was monitored to complete conversion (see the Supporting Information for details). There was no sign of product inhibition (data not shown). For the purpose of defining its binding affinity, CoA was evaluated as a competitive inhibitor versus 4-HBA-CoA. The inhibition constant (Ki) was determined to be 43 ± 1 μM. As this value is too large to suggest regulation by CoA. we also tested inhibition for a range of carboxylate products: 4-hydroxybenzoate(Ki ~ 2mM), hexanoate (Ki > 2 mM), octanoate (K > 2 mM), and lauroate (Ki ~ 1 mM) (see the Supporting Information for details). Because these compounds exhibited very weak inhibition in vitro, we concluded that it is likely that hTHEM4 is not regulated by product inhibition.
There are two reports in the literature that claim that hTHEM4 binds to Akt1 (2, 8). In this work, we measured (fl)hTHEM4 and (Δ39)hTHEM4 (0.04 μM) catalytic activity toward 5 μM (~2Km level) myristoyl-CoA as a function of added Akt1 (0, 0.4, 0.8, 1.5, and 2.3 μM) to determine if Akt1 inhibits hTHEM4 in vitro. No inhibition was observed, and thus, there is no evidence at present that Akt1 partners with hTHEM4 in the regulation of acyl-CoA thioesterase activity.
Cellular compartmentalization of hTHEM4 might restrict its access to thioester metabolites. However, on the basis of the current literature (2, 5–8), we are unable to draw any firm conclusion regarding hTIIEM4’s location, which has been purported to include one or more of the following locales: cytoplasm, plasma membrane, inner mitochondrial membrane, and inner mitochondrial space. We suspect that variations in the cell line, gene expression, and protein construct employed in the respective investigations contribute to the seemingly conflicting findings. Nevertheless, hTIIEM4 is consistently observed in two molecular mass forms (2, 5, 6, 8): one that corresponds to the full-length protein (26–27 kDa) and one that corresponds to a truncate (22–25 kDa).
Lastly, we mention that posttranslational phosphorylation of hTHKM4 in cell culture has been reported by two groups of investigators (2, 5). The phosphorylation site(s) has not been reported; however, we note that the “highly” probable phosphorylation sites predicted by NetPhos 2.0 (14) are located on the N-terminal domain and not on the thioesterase domain.
This work has shown that the protein known in the current literature as the Akt C-terminal modulator protein (CTMP) is in fact a broad-range, catalytically efficient acyl-CoA thioesterase with activity that extends to fatty acylated ACP. Its cellular location is uncertain and may in fact be dependent on cell type and prevailing cellular conditions. Furthermore, its mode(s) of regulation and its biological role(s) are unknowns at this time. Nevertheless, the accumulating evidence that hTHEM4(CTMP) is a regulator of cellular apoptosis, a regulator of Akt1, and a factor in the progression of cancer and neurodegeneration provides us with strong incentive to learn more about this curious enzyme. Our ongoing studies are focused on determining its three-dimensional structure and its biological function(s).
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant GM28688 and by NIMH RP.
SUPPORTING INFORMATION AVAILABLE
Detailed experimental protocols and tables and figures reporting experimental results. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Knobbe CB, Reifenberger J, Blaschke B, Reifenberger G. J Natl Cancer Inst. 2004;96:483–486. doi: 10.1093/jnci/djh064. [DOI] [PubMed] [Google Scholar]
- 2.Maira SM, Galelic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA. Science. 2001;249:374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 3.Brazil DP, Hemmiugs BA. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SK, Park SJ, Chang SH, Lee ES, Chung YS, Beck GR, Jr, Lee KH, Piao L, Park J, Cho MH. Gene Ther. 2007;14:1721–1730. doi: 10.1038/sj.gt.3303042. [DOI] [PubMed] [Google Scholar]
- 5.Ono H, Sakoda H, Fujishiro M, Anai M, Kushiyama A, Fukushima Y, Katagiri H, Ogihara T, Oka Y, Kamata H, Horike N, Uchijima Y, Kurihara H, Asano T. Am J Physiol. 2007;293:C1576–C1585. doi: 10.1152/ajpcell.00570.2006. [DOI] [PubMed] [Google Scholar]
- 6.Parcellier A, Tintignac LA, Zhuravleva E, Cron P, Schenk S, Bozulic L, Hemmings BA. Cell Signalling. 2009;21:639–650. doi: 10.1016/j.cellsig.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Piao L, Li Y, Kim SJ, Sohn KC, Yang KJ, Park KA, Byun HS, Won M, Hong J, Hur GM, Seok JH, Shong M, Sack R, Brazil DP, Hemmings BA, Park J. Cell Signalling. 2009 doi: 10.1016/j.cellsig.2009.01.020. in press. [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki T, Ofengeim D, Noh K-M, Latuszek-Barrantes A, Hemmings BA, Follenzia A, Zukin RS. Nat Neurosci. 2009 doi: 10.1038/nn.2299. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thodsn JB, Zhuang Z, Dunaway-Mariano D, Holden HM. J Biol Chem. 2003;278:43709–43716. doi: 10.1074/jbc.M308198200. [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Xu H, Zhao H, Gong W, Dunaway-Mariano D. Biochemistry. 2009;48:1293–1304. doi: 10.1021/bi801879z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley LA, Sternberg MJE. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Joshi AK, Smith S. J Biol Chem. 2003;278:40067–40074. doi: 10.1074/jbc.M306121200. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang Z, Song F, Zhao H, Li L, Cao J, Eisenstein E, Herzherg O, Dunaway-Mariano D. Biochemisiry. 2008;47:2789–2796. doi: 10.1021/bi702334h. [DOI] [PubMed] [Google Scholar]
- 14.Blom N, Gammeltoft S, Brunak S. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


