Abstract
During progression to an androgen-independent state following androgen ablation therapy, prostate cancer cells continue to express the androgen receptor (AR) and androgen-regulated genes, indicating that AR is critical for the proliferation of hormone-refractory prostate cancer cells. Multiple mechanisms have been proposed for the development of AR-dependent hormone-refractory disease including changes in expression of AR coregulatory proteins, AR mutation, growth factor-mediated activation of AR, and AR protein upregulation. The most prominent of these progressive changes is the upregulation of AR that occurs in greater than 90% of prostate cancers. A common feature of the most aggressive hormone-refractory prostate cancers is the accumulation of cells with neuroendocrine characteristics that produce paracrine factors, and may provide a novel mechanism for the regulation of AR during advanced stages of the disease. In this study, we demonstrate that neuroendocrine-derived PTHrP-mediated signaling through the EGF receptor (EGFR) and Src pathways contributes to the phenotype of advanced prostate cancer by reducing AR protein turnover. PTHrP-induced accumulation of AR depended on the activity of Src and EGFR and consequent phosphorylation of the AR on Tyrosine 534. PTHrP-induced tyrosine-phosphorylation of AR resulted in reduced AR ubiquitination and interaction with the ubiquitin ligase carboxy-terminus of HSP70-interacting protein (CHIP). These events result in increased accumulation of AR and thus enhanced growth of prostate cancer cells at low levels of androgen.
Keywords: Androgen Receptor, neuroendocrine differentiation, PTHrP, EGFR, Src
Introduction
Expression of the Androgen Receptor (AR) plays an integral role in the progression of prostatic adenocarcinoma to hormone-refractory disease (1, 2). Several mechanisms have been described to account for the development of AR-dependent, androgen-refractory prostate cancer including AR mutational activation, coactivator overexpression, and activation via crosstalk with growth factor signaling pathways. The most frequent mechanism however, involves increases in AR protein levels.
Increases in AR are both necessary and sufficient to convert prostate cancer cells from a hormone-sensitive to a hormone-refractory state (3). This finding is consistent with reports demonstrating that high levels of AR are associated with aggressive clinicopathologic features and with decreased recurrence-free survival in prostate cancer patients treated with radical prostatectomy (4). In addition, immunohistochemical studies have shown that the AR is expressed in essentially all human prostate cancers, including those that have become hormone-refractory following hormone ablation therapy, and that the level of AR expression is often increased relative to untreated tumors (5, 6).
One explanation for the elevated levels of AR is gene amplification, reported to occur in approximately 22% of advanced prostate tumors (7). However, increased levels of AR observed in the majority of prostate cancers may also result from increased AR protein stability. As seen in recurrent CWR22 and LNCAP xenograft tumors (8, 9), increases in AR are sufficient to allow continued androgen signaling under conditions of low circulating ligand. Chen and colleagues (3) have shown that increasing the concentration of AR in prostate cancer cells using an AR-expressing lentivirus reduces the latency period for the development of LNCaP and LAPC4 xenograft tumors in castrate mice, supporting the hypothesis that increased AR protein promotes growth and survival of prostate tumors in low levels of androgen.
Another mechanism postulated to promote the progression of androgen-refractory prostate cancer is the appearance of cells with a neuroendocrine phenotype. Neuroendocrine-like cells are more prevalent in androgen-refractory disease, occurring in 30-100% of tumors studied (10, 11). The low proliferative capacity of neuroendocrine cells allows them to resist treatment with most chemotherapeutic agents, as well as endocrine and radiation treatments (12, 13). Neuroendocrine-like cells are thought to provide growth and survival signals to surrounding tumor cells promoting the progression of hormone-refractory prostate cancer (14, 15). This hypothesis is supported by work demonstrating that neuroendocrine-like cells enhance the growth of LNCaP xenografts, with the greatest effects seen under conditions of androgen deprivation (16, 17).
Parathyroid hormone-related protein (PTHrP) is one of the secreted products of prostatic neuroendocrine cells, and its expression in the human prostate is a manifestation of abnormal growth regulation. In culture, PTHrP cooperates with low levels of androgen to promote growth of androgen-dependent prostate cancer cells, but the molecular mechanisms responsible for these effects remain unclear (18). In particular, it is not known whether the effects of PTHrP on growth are dependent on the AR.
In the present study, we describe a novel mechanism by which neuroendocrine-derived factors, such as PTHrP, provide a proliferative advantage to prostate cancer cells. Our studies reveal that LNCaP cells at low androgen concentration proliferated more rapidly when stimulated with PTHrP. Under these conditions steady state levels of AR protein were increased and this increase, like PTHrP-induced proliferation, required the activation of epidermal growth factor receptor (EGFR) or Src and the subsequent phosphorylation of the AR on Tyr 534. We found that targeting of AR for proteasomal-dependent degradation via the chaperone-associated ubiquitin ligase carboxy-terminus of Hsp70-interacting protein (CHIP) was decreased, resulting in the accumulation of AR in response to PTHrP. The phosphorylation of AR on Tyr534 reduced the interaction of AR with CHIP. Together these findings demonstrate that neuroendocrine-derived PTHrP stimulation of cancer cells results in the upregulation of AR protein, thereby promoting growth under low androgen conditions and progression of hormone-refractory disease.
Materials and Methods
Cell Culture, Transfections, and Reagents
LNCaP cells were maintained in T-medium with 5% (v/v) fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) in a humidified chamber at 37 °C with 5% CO2. Transfections were performed using TransIT-prostate transfection kit (Mirus, Madison, WI) according to manufacturer's instructions. The plasmid encoding FLAG-tagged AR has been previously described (19). The FLAG-AR-Y534F expression vector was constructed by sub-cloning AR-Y534F (gift of Dr. Young E. Whang, University of North Carolina, Chapel Hill, NC) into pCDNA3.1-FLAG plasmid (20). For experiments with cycloheximide (Calbiochem), cells were treated with cycloheximide (10μg/ml) throughout the period of R1881 and PTHrP stimulation.
Antibodies and reagents were obtained from the following sources: anti-AR PG-21 (Upstate, Lake Placid, NY); anti-FLAG antibody, M2, (Sigma, St. Louis, MO); anti-phosphorylated Src (Tyr418), anti-Src, anti-phosphorylated EGFR (Tyr992), and anti-EGFR (Biosource, Camarillo, CA, USA); anti-PTH/PTHrP receptor, anti-ubiquitin, and anti-CHIP (Santa Cruz, CA, USA); anti-tubulin (Calbiochem); anti-Hsp90 (StressGen, Ann Arbor, MI); goat-anti-rabbit and goat-anti-mouse antibodies (GE Healthcare, Buckinghamshire, England); Alamar Blue (Biosource); PTHrP (1-34aa), PP2, AG1478 (Calbiochem); and PTHrP receptor antagonist (Nle8,18, Tyr34)-pTH(3-34)amide (Bachem, Torrance, CA)
The anti-phospho-Tyr534 antibody was raised against a peptide spanning the Tyr534 phosphorylation site in human AR by standard methods. The peptide (DSYSGPpYGDMRLETC) was synthesized with phospho-tyrosine and an N-terminal cysteine (Anaspec, San Jose, CA), it was then coupled to keyhole limpet hemocyanin, and used for antibody production in rabbits (Cocalico Biologicals, Reamstown, PA). Antibody titers were monitored by immunoblotting, and the terminal bleeds were affinity purified using Sepharose-immobilized peptide. The specificity of the antibody was verified by immunoblot analysis against wt and Y543C mutant AR. The increase in immunoreactivity observed with the phospho-Tyr534 antibody in response to EGF was similar to that reported in published studies (21).
Cell Proliferation Assays
Growth of cells in a 96-well plate format (2500 cells/well), maintained for 2 days in various concentrations of R1881 and 1% charcoal-stripped serum (CSS) with or without PTHrP, was monitored with the Alamar blue assay according to the manufacturer instructions. Cell numbers were determind by seeding 5×104 cells/mL in triplicate onto 6-well plates in RPMI-1640 without phenol red, supplemented with 0.1nM R1881 and 1% CSS. The cells were treated with PTHrP alone or together with PTHrP receptor antagonist (10nM), PP2 (10uM), AG1478 (5uM), or DMSO control, and counted using a hemacytometer.
Western Blotting and Immunoprecipitation
LNCaP cells were serum starved overnight in RPMI-1640 and stimulated with R1881 (0.1nM) or PTHrP (10nM) for the indicated times. The cells were lysed in radioimmunoprecipitation assay buffer [137mM NaCL, 20mM Tris (pH 7.5), 10% glycerol (v/v), 1% Triton-X100, 0.5% (w/s) deoxycholate, 0.1% (w/s) SDS, 2mM EDTA, 1mM phenylmethylsulfonyl fluoride, 1mM Na3VO4, and protease inhibitors]. The lysate protein concentration was determined using the BCA-containing assay (Pierce, Rockford, IL).
Endogenous AR was immunoprecipitated from LNCaP cells with 5μg anti-human AR per 100-mm dish, and immunoprecipitates were captured on protein A-conjugated sepharose beads (Roche, Indianapolis, IN). FLAG-AR was immunoprecipitated with M2 anti-FLAG antibody coupled to agarose (Sigma). Precipitates were resuspended in sample buffer [10% (v/v) glycerol, 62.5mM Tris (pH 6.8), 2% (w/v) SDS, 0.01mg/ml bromophenol blue], resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting.
RNA Interference
Silencing of Src was performed with Validated Stealth RNAi DuoPak (Invitrogen) according to manufacturer instructions. Transfection of Src siRNA was done in LNCaP cells using Oligofectamine (Invitrogen) in Opti-MEM medium. A non-specific control duplex-XIII (Dharmacon Research, Lafayette, CO) was used as a siRNA silencing control. Maximal knockdown of Src was observed on day 3 after transfection.
Real-time RT-PCR
Real-time RT-PCR was performed as previously described (22). Briefly, total RNA was isolated from LNCaP cells treated for 6 hours with PTHrP, R1881, or both using Qiagen Rneasy kit (Qiagen, Chatworth, CA). RNA quantity was determined with RiboGreen RNA assay kit (Invitrogen). First-strand cDNA was produced following the iScript™cDNA synthesis protocol (Bio-Rad, Hercules, CA). cDNA amplification was conducted on an iCycler optical system, in the presence of iQ SYBR Green mastermix (Bio-Rad). The PCR primers used were: AR-forward, 5'-CCTGGCTTCCGCAACTTACAC-3'; AR-reverse, 5'-GGACTTGTGCATGCGGTACTC-3'; PSA-forward, 5'-TGGTGCATTACCGGAAAGTGGATCA-3' ; P S A-reverse 5'-GCTTGAGTCTTGGCCTGGTCATTTC-3' ; F K B P 5 1-f o r w a r d , 5'-AGGAGGGAAGAGTCCCAGTG-3' ; F K B P 5 1-r e v e r s e , 5'-TGGGAAGCTACTGGTTTTGC-3'; NKX3.1-forward, 5'-GCACATATTTGCATGGAAGG-3'; N K X 3 . 1-reverse, 5'-ACAGCGAGTGCATCTTGTTC-3'; DKK1-forward, 5'-CCTTGGATGGGTATTCCAGA-3'; DKK1-reverse, 5'-CAGTCTGATGACCGGAGACA-3'; GUS-forward, 5'-CCGACTTCTCTGACAACCGACG-3'; GUS-r e v e r s e , 5'-AGCCGACAAAATGCCGCAGACG-3'.
Statistical Analysis
All data were expressed as the mean ± standard error of mean from three or more independent experiments. Statistical analysis was performed by Student's t-test. Significance was determined with P<0.05.
Results
PTHrP Promotes Growth of Prostate Cancer Cells in Low Androgen
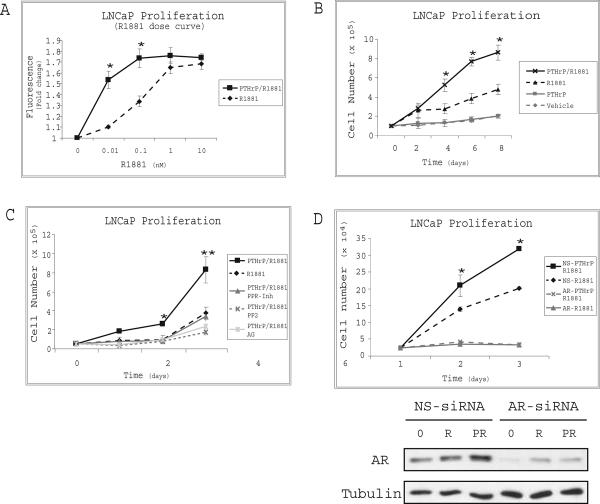
The correlation of hormone-refractory prostate cancer with increased neuroendocrine differentiation and up-regulation of neuropeptide signaling, such as PTHrP (23, 24), prompted us to investigate a potential involvement of PTHrP in androgen-dependent prostate cancer cell proliferation. We performed a dose-response study of the effects of the synthetic androgen R1881 alone or R1881 together with recombinant PTHrP (10nM) on LNCaP cells. After 48 hours, maximal proliferation induced by R1881 alone occurred at 1nM. However, a significant growth increase was seen when cells were maintained in media containing 1% CSS supplemented with as little as 0.01nM R1881 and PTHrP (Fig.1A) indicating that PTHrP reduces the androgen requirement of LNCaP prostate cancer cells for growth. As indicated in Figure 1B, the proliferative advantage provided by PTHrP occurs only in the presence of R1881.
Figure 1. PTHrP Enhances Proliferation of LNCaP Cells.
(A) Growth of cells, maintained for 2 days in the indicated concentration of R1881 and 1% CSS with or without PTHrP (10nM), was monitored with the Alamar blue assay. Results represent the mean fluorescence ±SEM of three independent experiments. Asterisks indicate a significant difference of PTHrP/R1881 compared to R1881 treatment based on a Student's t-test (*P<0.05). (B) 5×104 cells were grown for 24 hrs in T-medium containing 5% FBS, transferred to RPMI-1640 medium supplemented with 1% CSS and treated with R1881 (0.1nM), PTHrP (10nM), or both. Cells were counted by hemacytometer, and the results are expressed as the mean cell number ±SEM of three independent experiments (*P<0.05). (C) PTHrP/R1881 stimulated cells were treated with inhibitors of the PTHrP receptor (PPRInh.), Src (PP2), EGFR (AG1478), or vehicle (DMSO). Cell number was determined by hemacytometer counting. Results are expressed as the mean cell number ±SEM of three experiments (*P<0.05, **P<0.01). (D) Cells were transfected with either nonspecific siRNA (NS) or siRNA targeting the AR transcript (AR). Two days after transfection the cells were treated as in panel B. Cell number was determined by hemacytometer counting. Immunoblotting of cell lysates was performed to confirm knockdown of AR (lower panels). Results are representative of three experiments and error bars depict SEM (* P<0.05).
It has previously been documented that EGFR and Src pathways are induced upon GPCR activation (25-28). To gain insight into the role of these pathways in PTHrP-induced proliferation, we evaluated effects of EGFR or Src inhibitors on proliferation of the prostate cancer cells. PTHrP-induced proliferation of the cells was completely abolished by treatment with, AG1478, or PP2, the EGFR or Src inhibitors respectively. Similarly, PTHrP-induced LNCaP cell proliferation was abrogated upon treatment with PTHrP receptor antagonist (Fig. 1C). These observations indicated that transactivation of both EGFR and Src by the PTHrP receptor is necessary for the proliferation of LNCaP cells in response to PTHrP.
Growth of castrate-resistant prostate cancer occurs in low levels of circulating androgens but requires AR (2). Figure 1D shows that growth of LNCaP cells in lowandrogen and low-serum medium was significantly increased by PTHrP, and this effect could be blocked by AR siRNA. Together these data suggest that PTHrP is driving AR-dependent cell growth in this experimental model.
PTHrP Stabilizes Androgen Receptor Protein
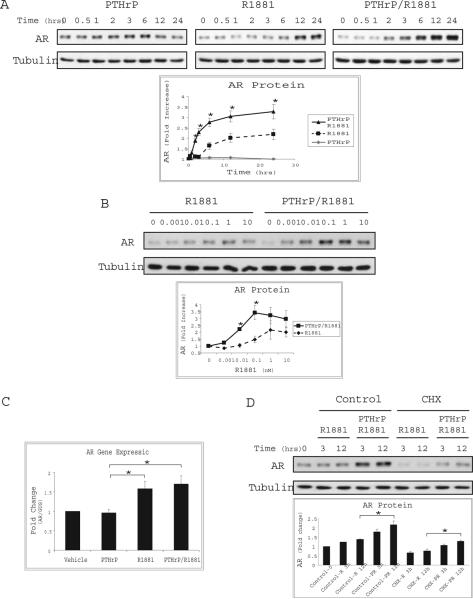
We next analyzed effects of the neuropeptide on AR protein levels. Serum deprived LNCaP cells were treated with R1881 (0.1nM), PTHrP (10nM), or both, and AR expression was determined by immunoblotting. Androgen was found to increase the steady-state expression of AR protein as previously reported, but this effect was further enhanced in the presence of PTHrP. Treatment of LNCaP cells with PTHrP alone did not increase the AR protein level, indicating that the effect of PTHrP signaling on AR is dependent on the presence of androgen (Fig. 2A). A dose-response analysis of R1881 alone or together with PTHrP showed that AR protein accumulates to a greater extent at low androgen concentrations (0.01-0.1nM) when PTHrP is present (Fig. 2B). Thus, PTHrP signaling leads to increased AR protein under androgen-limiting conditions, suggesting that PTHrP-induced LNCaP cell growth results from its modulation of AR levels.
Figure 2. PTHrP Stabilizes AR Protein.
(A) LNCaP cells were serum starved for 16 hrs and stimulated for the indicated times with PTHrP (10nM, left panel), R1881 (0.1nM, center panel), or together (right panel). The lysates were analyzed by immunoblotting with anti-AR or anti-tubulin antibodies. Densitometric quantitation of the immunoblot bands was performed. Results are expressed as mean fold increase ±SEM of five independent experiments. Asterisks indicate a significant difference of PTHrP/R1881 compared to R1881 treatment (*P<0.05). (B) Following serum starvation LNCaP cells were treated with increasing concentrations of R1881 alone or together with PTHrP (10 nM) as indicated. Lysates were analyzed as described in (A). Results are representative of three experiments (*P<0.05). (C) RNA extracted from LNCaP cells treated for 6 hrs with PTHrP, R1881, alone or in combination, were analyzed by quantitative real-time PCR using primers specific to AR. Experiments were repeated three times. The results were normalized to β-glucuronidase reference gene and are expressed as fold change from vehicle treatment (*P<0.05). (D) Extracts of LNCaP cells stimulated as in (A) and maintained in cycloheximide (CHX) or vehicle (DMSO) for the indicated time were immunoblotted as described above. Results represent mean AR fold change ± SEM analyzed after 3 or 12 hour treatment (*P<0.05).
Significant accumulation of AR protein was observed following treatment of LNCaP cells with PTHrP and R1881 for six hours. To determine whether transcriptional upregulation of the AR gene occurs under these conditions quantitative real-time PCR was utilized to measure AR mRNA. Induction of AR gene expression was observed upon treatment of LNCaP cells with R1881 alone but no significant change was detected relative to treatment with R1881 and PTHrP (Fig. 2C). To ascertain whether PTHrP-induced increases in AR are a result of changes in protein turnover, LNCaP cells were treated with the protein synthesis inhibitor, cycloheximide (CHX, 10ug/ml). As shown in Figure 2D, treatment with CHX reduced the steady state level of AR but did not inhibit the stabilizing effect of PTHrP on AR protein, indicating that PTHrP-mediated signaling influences AR levels posttranslationally. Together these results suggest that the production of PTHrP by neuroendocrine cells hypersensitizes prostate cancer cells to androgen by decreasing AR turnover and thus increasing the level of AR protein.
PTHrP-induced Modulation of AR protein Involves EGFR and Src Signaling
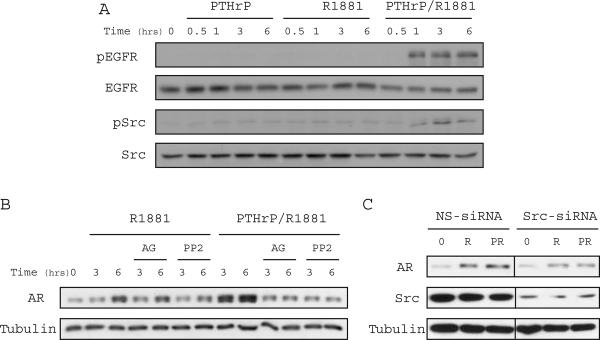
It is well documented that upregulation of growth factor signaling correlates with AR activation and progression of prostate cancer (29-31). In addition, stimulation of GPCRs has been demonstrated to result in the transactivation of the EGFR and Src pathways, prompting us to examine whether PTHrP signaling stabilizes AR through the activation of these pathways. To determine whether PTHrP stimulation is capable of activating EGFR in LNCaP cells, phosphorylation of Tyr992 was measured by immunoblotting with phosphospecific antibodies. Phosphorylation of Tyr992 was increased after one hour stimulation with PTHrP in the presence of androgen, but was not induced by PTHrP or R1881 alone. Similarly, phosphorylation of the activating Tyr418 residue of Src was shown to increase in response to PTHrP and R1881 treatment (Fig. 3A).
Figure 3. PTHrP Modulation of AR Protein is Mediated by EGFR/Src Signaling in LNCaP Cells.
(A) LNCaP cells were treated with either R1881 (0.1nM) alone or together with PTHrP (10nM) for the indicated time. Immunoblotting was performed with anti-phospho-EGFR (Tyr992), anti-EGFR, anti-phospho-Src (Tyr418), or anti-Src antibodies. (B) Serum-starved LNCaP cells were treated with R1881 (0.1nM), R1881 together with PTHrP (10nM), as well as the small molecule EGFR (AG1478, 5uM) or Src (PP2, 10uM) inhibitors. Cell extracts were prepared for immunoblotting with anti-AR or anti-tubulin antibodies. (C) LNCaP cells were transfected with either non-specific siRNA or siRNA targeting the Src gene as described in Materials and Methods. Two days post-transfection the cells were serum starved and treated with R1881 (0.1nM) alone or together with PTHrP (10nM) for 12 hrs. Immunoblotting of cell extracts was performed using anti-AR, anti-Src, or anti-tubulin antibodies.
To determine if the PTHrP-induced activation of EGFR and Src mediates the increase in AR protein, these pathways were blocked pharmacologically. Treatment of LNCaP cells with either AG1478 or PP2 abrogated the AR stabilizing effect of PTHrP (Fig. 3B). Reduction of Src protein in cells transfected with specific siRNA also resulted in a decrease in AR protein level relative to LNCaP cells transfected with control siRNA even in the presence of PTHrP (Fig. 3C), further implicating Src in mediating the PTHrP-induced increase in AR protein. Together, these observations suggest that both EGFR and Src signaling are critical to PTHrP-induced regulation of AR.
PTHrP Signaling Decreases AR Interaction with Hsp90 and the E3-ubiquitin Ligase CHIP
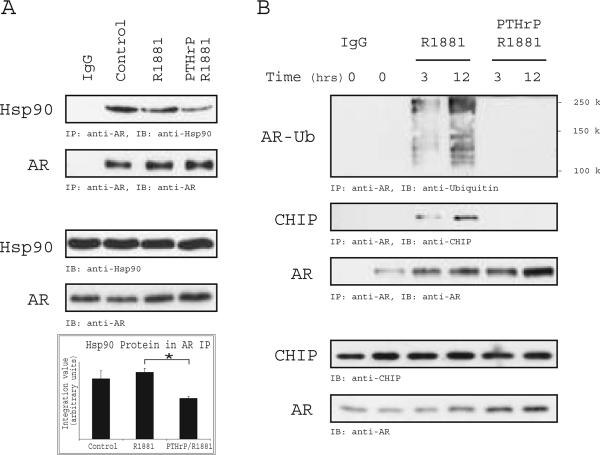
AR protein turnover is regulated in part by association with chaperones, such as Hsp70 and Hsp90, and occurs via ubiquitin-dependent proteolysis (32, 33). To examine whether the interaction of AR with chaperones is altered in the presence of PTHrP, AR was immunoprecipitated from LNCaP cells deprived of serum and treated with R1881 alone or in combination with PTHrP. As shown in Figure 4A, less Hsp90 was in complex with AR when the cells were treated with R1881 and PTHrP. Analysis of AR ubiquitination following immunoprecipitation also indicated that PTHrP treatment reduced AR ubiquitinated species (Fig. 4B). This observation indicated that AR is subject to proteasome-dependent degradation under normal physiological conditions and that the presence of PTHrP can stabilize the AR protein by interfering with its ubiquitination.
Figure 4. PTHrP Signaling Decreases AR Interaction with Hsp90 and the E3-Ubiquitin Ligase CHIP.
(A) LNCaP cells were serum-starved and stimulated for 12 hrs with R1881 (0.1nM) or PTHrP (10nM) and R1881. Cell extracts were subjected to immunoprecipitation using anti-AR antibodies, immunoblotting with anti-Hsp90 and anti-AR antibodies and densitometric quantitation of Hsp90 protein co-immunoprecipitated with AR was determined (lower panel). Results are expressed as the mean ±SEM of 3 independent experiments (*P<0.05). (B) LNCaP cells were deprived of serum and stimulated with R1881 or R1881/PTHrP for the indicated times. AR immunoprecipitation of the cell extracts was performed, and an immunoblot was probed with anti-ubiquitin, anti-CHIP, and anti-AR, as indicated. Input levels of AR and CHIP were determined by immunoblotting 5% of the cell extracts.
AR is a target of the ubiquitin ligases Mdm2- or CHIP, leading to degradation by the 26S proteasome (34, 35). Based on the observation that PTHrP reduces the interaction of AR with the Hsp90 chaperone complex, we hypothesized that the reduction in AR ubiquitination may be due to decreased interaction with the CHIP E3-ligase. Analysis of the AR immunoprecipitate showed reduced CHIP protein upon treatment with PTHrP (Fig. 4B). These data suggest that upon PTHrP stimulation the interaction of AR with chaperone-associated ubiquitin ligases and subsequent proteasomal-directed degradation is reduced.
AR Tyrosine Phosphorylation Reduces CHIP Interaction and Prevents PTHrP-induced Accumulation of AR
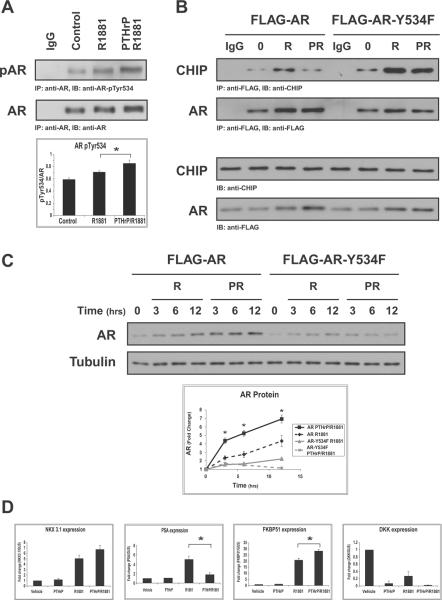
In previous work, we showed that EGF signaling results in the tyrosine phosphorylation of AR and modulates its interaction with Src (19). To determine whether PTHrP treatment influences the tyrosine phosphorylation of AR, LNCaP cells were deprived of serum and stimulated with R1881 or R1881 and PTHrP for 6 hours. Cell extracts were subsequently prepared and immunoblotted with phosphospecific antibodies to Tyr534, the primary site of AR tyrosine phosphorylation (21). As shown in figure 5A, phosphorylation of AR was increased upon treatment with R1881 and PTHrP relative to R1881 treatment alone. Similar phosphorylation levels were observed when AR was immunoprecipitated and immunoblotting with anti-phospho-tyrosine antibodies was performed (data not shown).
Figure 5. AR Tyrosine Phosphorylation Reduces CHIP Interaction and Enhances PTHrP-induced Accumulation of AR.
(A) LNCaP cells were serum-starved and stimulated with vehicle (ethanol), R1881 (0.1nM), or PTHrP (10nM) together with R1881 for 12 hrs. Cell extracts were immunoblotted with anti-AR or anti-phospho-AR (Tyr534). Densitometric quantitation of tyrosine phosphorylated AR was performed for 3 independent experiments. Results are expressed as the mean pTyr534/AR ±SEM (*P<0.05). (B) LNCaP cells were transfected with FLAG-wtAR or FLAG-AR-Y534F, and treated as described in (A). AR immunoprecipitates were probed with anti-CHIP or anti-AR antibodies. Input levels of AR and CHIP were determined by immunoblotting 5% of the cell extracts. (C) LNCaP cells as in (B) were stimulated for the indicated times with R1881 alone or together with PTHrP. Lysates were analyzed by immunoblotting with anti-AR and anti-tubulin antibodies. Densitometric quantitation was performed for three independent experiments. Results are expressed as the mean fold change ±SEM (*P<0.05). (D) RNA extracted from LNCaP cells treated for 6 hrs with PTHrP, R1881, alone or in combination, were analyzed by quantitative real-time PCR using primers specific to the indicated androgen-regulated genes. Experiments were repeated three times. The results were normalized to β-glucuronidase reference gene and are expressed as fold change from vehicle treatment (*P<0.05).
To assess whether the CHIP/AR interaction is modulated by AR tyrosine phosphorylation, LNCaP cells were transiently transfected with either FLAG-AR or with FLAG-AR-Y534F. Immunoprecipitation of exogenously expressed FLAG-AR complexes revealed that, similar to endogenous AR, the CHIP interaction is reduced in the presence of PTHrP. However, the phosphorylation-deficient AR-Y534F mutant showed enhanced association with CHIP in response to treatment with R1881 alone or in combination with PTHrP (Fig. 5B). This observation suggests that tyrosine phosphorylation of AR is required for decreasing the interaction between CHIP and AR, thus reducing its degradation.
To confirm that the phosphorylation of AR Tyr534 plays a role in the PTHrP-induced accumulation of the protein, LNCaP cells ectopically expressing FLAG-AR or FLAG-AR-Y534F were deprived of serum and stimulated with R1881 alone or PTHrP and R1881. Immunoblotting with anti-FLAG antibodies revealed that FLAG-AR, like endogenous AR, accumulated in the presence of PTHrP (Fig. 5C). FLAG-AR-Y534F failed to accumulate in response to PTHrP, indicating that the phosphorylation of this site is important for PTHrP-induced stabilization of AR.
PTHrP modulates expression of androgen-regulated genes
We examined AR transactivation in LNCaP cells stimulated with PTHrP, R1881 or both. Quantitative real-time PCR analysis of androgen-regulated genes demonstrated that PTHrP does not significantly modulate NKX3.1 expression in the presence of R1881, but antagonizes R1881 induction of PSA transcription and enhances mRNA levels of FKBP51. Downregulation of DKK was observed in the presence of R1881 or PTHrP (Fig. 5D).
Discussion
Increases in the neuroendocrine status of prostate tumors have been correlated with tumor size and progression to hormone-refractory stages. Existing evidence suggests that neuroendocrine-like cells arise within the tumor upon exposure to various differentiation factors, including β-adrenergic agonists, cytokines, or long-term androgen ablation (36-38). Although neuroendocrine-like cells are non-mitotic, they produce neuropeptides, such as PTHrP, neurotensin, and bombesin, that are thought to induce proliferative or survival signals to adjacent cancer cells (16, 39). Our data show that PTHrP-mediated signaling enhances the proliferation of prostate cancer cells by an alternative mechanism involving the regulation of AR protein stability.
Androgen ablation therapy reduces the circulating testosterone concentration from 4.5ng/ml (21nM) to 0.28-1.3ng/ml (1.3-6nM), resulting in apoptosis of androgen-dependent tissues and regression of prostate cancer (40). In comparing the growth properties of LNCaP cells, an androgen-dependent human prostate cancer cell line, in the presence of PTHrP a disparity was observed in the levels of androgen required for cell proliferation. LNCaP cells treated with PTHrP required androgen concentrations two orders of magnitude lower for growth stimulation (Fig. 1), suggesting that in the setting of low levels of androgen following pharmacological or surgical castration PTHrP may enhance the proliferation of prostate cancer cells. The proliferative advantage provided by PTHrP appears to be associated with a complex pattern of androgen-regulated genes, as indicated by the small sampling of AR targets we examined. Further analysis will be required to determine how the global pattern of gene expression contributes to enhanced prostate cancer cell proliferation in the presence of PTHrP and low androgen (Fig. 5D).
Under low androgen conditions, augmentation of LNCaP proliferation or AR protein concentration in response to PTHrP is mediated through EGFR and Src kinase activation (Figs. 1 & 3). Transactivation of this pathway in response to neuropeptide stimulation has been demonstrated by Ullrich and colleagues (41), and is consistent with our work in PC3 cells (25). In the current study, phosphorylation of EGFR was detected after one hour of PTHrP treatment corresponding with increased AR protein levels. Activation of Src appears to be an important step in the regulation of AR protein levels in the presence of PTHrP, as its inhibition or siRNA knockdown abrogates PTHrP-induced AR accumulation (Fig. 3). Src association with AR occurs either directly or in a ternary complex with the scaffold protein RACK1 (19, 42), resulting in AR phosphorylation, increased AR activity, and enhanced LNCaP cell proliferation (21). Our data agree with these findings showing that AR tyrosine phosphorylation is increased in response to PTHrP (Fig. 4), and that PTHrP-mediated cell proliferation requires AR as well as Src activation (Fig. 1). Src can also interact directly with Hsp90 chaperone complexes, and its ability to modulate client protein turnover further supports its involvement in the modulation of AR protein levels (43). Chaperone proteins maintain AR in a partially unfolded ligand-free state to prevent aggregation of the protein, but may also modulate AR protein degradation, nuclear translocation and transactivation (32). It is possible that Src could modify or recruit various components of the complex to influence multiple aspects of AR downstream signaling. Our work favors the hypothesis that phosphorylation of AR modulates its ability to associate with the chaperone complex. We observed that interaction of AR with Hsp90 as well as Hsp70 is decreased in the presence of PTHrP (Fig. 4 and data not shown).
Molecular chaperones regulate the proteasome-dependent degradation of several classes of proteins including steroid receptors, membrane proteins, and kinases. One link between chaperones and the proteasome is CHIP. CHIP contains tetratricopeptide repeats for interaction with Hsp70 and Hsp90 and a modified form of the Ring finger domain found in some ubiquitin ligases (44). In conjunction with the UBCh5 group of E2 enzymes CHIP promotes ubiquitination of glucocorticoid receptor, CFTR, and Raf kinase (45-47). Overexpression studies have revealed that CHIP prevents the accumulation of AR and stimulates its ubiquitination in LNCaP or HeLa cells (34, 48). Our observations in LNCaP cells with endogenous CHIP levels are consistent with these studies and show that AR polyubiquitination is reduced in response to PTHrP stimulation and correlates with reduced CHIP binding.
The interaction of CHIP with AR has been characterized in vitro and was shown to depend on both unphosphorylated and serine/threonine phosphorylated peptides of AR (49). In this study we find that the interaction of CHIP with the phosphorylation-deficient AR-Y534F is enhanced, suggesting that unlike serine/threonine phosphorylation, tyrosine phosphorylation may disrupt the AR/CHIP complex. Serine phosphorylation of AR has also been described to enhance its proteasomal degradation. Serine phosphorylation by Akt is postulated to increase AR ubiquitination by Mdm2 and lead to its degradation (49). Further analysis will be required to elucidate the relationship between these apparently antagonistic phosphorylation events in the regulation of AR/CHIP interaction and their role in the regulation of AR protein turnover.
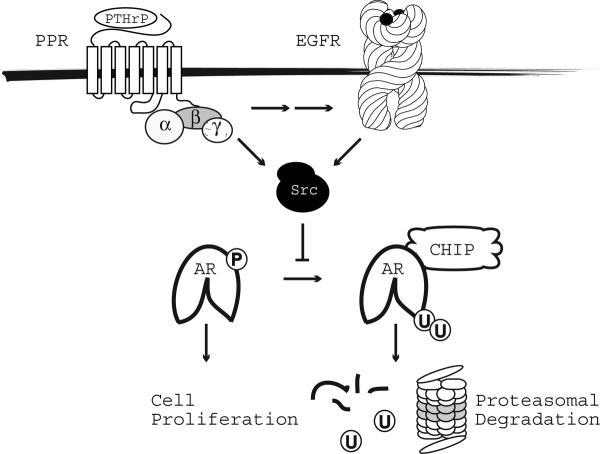
In summary, PTHrP enhances the proliferation of LNCaP cells at low concentrations of R1881 by modulating androgen-induced stabilization of AR. Under low androgen PTHrP functions as a compensatory signal to modulate the AR protein life cycle through pathways that involve the activation of EGFR and Src. We show that PTHrP-induced transactivation of EGFR, Src and consequent AR tyrosine phosphorylation sequesters a subpopulation of AR protein from chaperone/CHIP complexes that normally regulate the proteasomal-dependent degradation of the protein (Fig. 6). This study provides one mechanistic explanation whereby increased neuroendocrine differentiation promotes the progression of prostate cancer through the production of factors that stabilize AR protein. The findings reveal potential new sites of therapeutic intervention that can be combined with androgen ablation.
Figure 6. Model Delineating the Signaling Pathways Mediating PTHrP Enhanced AR Protein Stabilization.
Binding of PTHrP to its cognate receptor results in the transactivation of EGFR and Src. Tyrosine phosphorylation of AR catalyzed by Src results in the sequestration of AR from chaperone and CHIP complexes that mediate its proteasomal degradation. Increased AR protein levels enhance the ability of prostate cancer cells to proliferate.
Acknowledgments
We thank Vicki Gordon for preparation of the anti-AR phospho-Tyr534 antibody and Dr. Young E. Whang for providing the AR-Y534F construct. This work was supported by the Department of Defense grant W81XWH-07-1-0070 (DaSilva, J), National Institutes of Health-National Cancer Institute grant PO1 CA104106 (Parsons, S & Weber, M), National Institutes of Health grants T32 DK007646 and CA105402 (Weber, M).
References
- (1).Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- (2).Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- (3).Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- (4).Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- (5).Pertschuk LP, Macchia RJ, Feldman JG, et al. Immunocytochemical assay for androgen receptors in prostate cancer: a prospective study of 63 cases with long-term follow-up. Ann Surg Oncol. 1994;1:495–503. doi: 10.1007/BF02303615. [DOI] [PubMed] [Google Scholar]
- (6).Prins GS, Sklarew RJ, Pertschuk LP. Image analysis of androgen receptor immunostaining in prostate cancer accurately predicts response to hormonal therapy. J Urol. 1998;159:641–9. [PubMed] [Google Scholar]
- (7).Bubendorf L, Kononen J, Koivisto P, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–6. [PubMed] [Google Scholar]
- (8).Gregory CW, Johnson RT, Jr., Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–8. [PubMed] [Google Scholar]
- (9).Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- (11).Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–9. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- (12).Cabrespine A, Guy L, Gachon F, Cure H, Chollet P, Bay JO. Circulating chromogranin a and hormone refractory prostate cancer chemotherapy. J Urol. 2006;175:1347–52. doi: 10.1016/S0022-5347(05)00640-3. [DOI] [PubMed] [Google Scholar]
- (13).Hvamstad T, Jordal A, Hekmat N, Paus E, Fossa SD. Neuroendocrine serum tumour markers in hormone-resistant prostate cancer. Eur Urol. 2003;44:215–21. doi: 10.1016/s0302-2838(03)00257-4. [DOI] [PubMed] [Google Scholar]
- (14).Bonkhoff H, Wernert N, Dhom G, Remberger K. Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991;19:91–8. doi: 10.1002/pros.2990190202. [DOI] [PubMed] [Google Scholar]
- (15).Noordzij MA, van Weerden WM, de Ridder CM, van der Kwast TH, Schroder FH, van Steenbrugge GJ. Neuroendocrine differentiation in human prostatic tumor models. Am J Pathol. 1996;149:859–71. [PMC free article] [PubMed] [Google Scholar]
- (16).Deeble PD, Cox ME, Frierson HF, Jr., et al. Androgen-independent growth and tumorigenesis of prostate cancer cells are enhanced by the presence of PKA-differentiated neuroendocrine cells. Cancer Res. 2007;67:3663–72. doi: 10.1158/0008-5472.CAN-06-2616. [DOI] [PubMed] [Google Scholar]
- (17).Jin RJ, Wang Y, Masumori N, et al. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64:5489–95. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- (18).Dougherty KM, Blomme EA, Koh AJ, et al. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 1999;59:6015–22. [PubMed] [Google Scholar]
- (19).Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–54. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- (20).Mahajan NP, Liu Y, Majumder S, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104:8438–43. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Guo Z, Dai B, Jiang T, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- (22).Gioeli D, Black BE, Gordon V, et al. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–15. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- (23).Abrahamsson PA, Falkmer S, Falt K, Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker”. Pathol Res Pract. 1989;185:373–80. doi: 10.1016/S0344-0338(89)80016-0. [DOI] [PubMed] [Google Scholar]
- (24).Iddon J, Bundred NJ, Hoyland J, et al. Expression of parathyroid hormone-related protein and its receptor in bone metastases from prostate cancer. J Pathol. 2000;191:170–4. doi: 10.1002/(SICI)1096-9896(200006)191:2<170::AID-PATH620>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- (25).Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26:745–56. doi: 10.1038/sj.onc.1209814. [DOI] [PubMed] [Google Scholar]
- (26).Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21:8385–97. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–78. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- (28).Schwindinger WF, Fredericks J, Watkins L, et al. Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [alpha-32P]GTP-gamma-azidoanilide photoaffinity labeling. Endocrine. 1998;8:201–9. doi: 10.1385/ENDO:8:2:201. [DOI] [PubMed] [Google Scholar]
- (29).Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–9. [PubMed] [Google Scholar]
- (30).Gregory CW, Fei X, Ponguta LA, et al. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–30. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- (31).Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- (32).Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–31. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- (33).Reddy GP, Barrack ER, Dou QP, et al. Regulatory processes affecting androgen receptor expression, stability, and function: potential targets to treat hormone-refractory prostate cancer. J Cell Biochem. 2006;98:1408–23. doi: 10.1002/jcb.20927. [DOI] [PubMed] [Google Scholar]
- (34).He B, Bai S, Hnat AT, et al. An androgen receptor NH2-terminal conserved motif interacts with the COOH terminus of the Hsp70-interacting protein (CHIP) J Biol Chem. 2004;279:30643–53. doi: 10.1074/jbc.M403117200. [DOI] [PubMed] [Google Scholar]
- (35).Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21:8471–82. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Jongsma J, Oomen MH, Noordzij MA, et al. Kinetics of neuroendocrine differentiation in an androgen-dependent human prostate xenograft model. Am J Pathol. 1999;154:543–51. doi: 10.1016/S0002-9440(10)65300-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yuan TC, Veeramani S, Lin FF, et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–67. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- (39).Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–30. [PubMed] [Google Scholar]
- (40).Sciarra F, Sorcini G, Di SF, Gagliardi V. Plasma testosterone and androstenedione after orchiectomy in prostatic adenocarcinoma. Clin Endocrinol (Oxf) 1973;2:101–9. doi: 10.1111/j.1365-2265.1973.tb00410.x. [DOI] [PubMed] [Google Scholar]
- (41).Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–36. [PubMed] [Google Scholar]
- (42).Migliaccio A, Varricchio L, De FA, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–29. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- (43).Duval M, Le BF, Huot J, Gratton JP. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell. 2007;18:4659–68. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ballinger CA, Connell P, Wu Y, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–45. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Connell P, Ballinger CA, Jiang J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–6. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- (46).Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–5. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- (47).Wang X, DeFranco DB. Alternative effects of the ubiquitin-proteasome pathway on glucocorticoid receptor down-regulation and transactivation are mediated by CHIP, an E3 ligase. Mol Endocrinol. 2005;19:1474–82. doi: 10.1210/me.2004-0383. [DOI] [PubMed] [Google Scholar]
- (48).Cardozo CP, Michaud C, Ost MC, et al. C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch Biochem Biophys. 2003;410:134–40. doi: 10.1016/s0003-9861(02)00680-x. [DOI] [PubMed] [Google Scholar]
- (49).Rees I, Lee S, Kim H, Tsai FT. The E3 ubiquitin ligase CHIP binds the androgen receptor in a phosphorylation-dependent manner. Biochim Biophys Acta. 2006;1764:1073–9. doi: 10.1016/j.bbapap.2006.03.013. [DOI] [PubMed] [Google Scholar]