Abstract
Background When women from families with a known BRCA1 or BRCA2 mutation test negative for the family mutation, it is assumed that they will transition their personal cancer risk perception from high to average risk. However, there are scant data regarding the experience of mutation‐negative women after genetic testing disclosure, particularly related to the shift of risk perception from assumed mutation‐positive to actual mutation‐negative. This study was designed to explore cancer risk perception and the experience of being a mutation‐negative woman within a known BRCA1/2 mutation‐positive family.
Methods We employed a qualitative descriptive design and convened a sample of 13 women who contributed in‐depth, semi‐structured telephone interviews (audio‐recorded and transcribed verbatim) and performed qualitative content analysis with NVivo 2.0 software.
Results Six major content areas emerged from interview data: (i) rationale for initial involvement in the breast imaging study, (ii) rationale for continued participation, (iii) experience of living in a multiple‐case family, (iv) risk perception: the personal meaning of mutation‐negative status, (v) opinions regarding cancer aetiology and (vi) communication patterns between mutation‐negative and mutation‐positive family members.
Conclusions Living in a hereditary breast and ovarian cancer family is a complex experience that affects cognitive, emotional and social functioning. Our findings indicate that mutation‐negative women may have unmet psychosocial needs that must be addressed by health‐care professionals, particularly in the primary‐care setting following genetic disclosure of a potentially reassuring result regarding their lack of the very high cancer risks associated with BRCA1/2 mutations.
Keywords: cancer, genetics, hereditary breast/ovarian cancer, mutation‐negative, psychosocial aspects
Introduction
For the last decade, families prone to breast and ovarian cancer have been referred for genetic testing for mutations in cancer‐susceptibility genes, such as BRCA1 and BRCA2. 1 , 2 When women from families with a known BRCA mutation opt to undergo genetic testing and test negative, we assume that they begin to transition their personal cancer risk perception from high to average risk. However, there are scant data related to their shifting risk perception from assumed mutation‐positive to actual mutation‐negative. Individuals from families with deleterious BRCA1/2 mutations have frequently witnessed family members struggle with hereditary breast and ovarian cancer (HBOC) syndrome‐related cancers. Prior to personal genetic testing, most of these women assumed their breast and ovarian cancer risks were equivalent to their cancer‐affected relatives. Women who are ‘true negatives’ are generally advised that their personal lifetime breast cancer risk is closer to the 13% risk of women from the general population, rather than the 50–85% risk of a mutation carrier. 3 , 4 , 5 , 6 , 7 , 8 Similarly, a mutation‐negative women has a much lower risk of developing ovarian cancer (2% lifetime) when compared with a BRCA1/2 mutation carrier (10–63% lifetime). 3 , 4 , 9 However, recent data have questioned the exact level of breast cancer risk for mutation‐negative members of mutation‐positive families, suggesting it may be higher than previously appreciated. 10
Regardless of the actual magnitude of risk among mutation‐negative women, there is no question that women without BRCA1/2 mutations were counselled that they had substantially lower breast and ovarian cancer risks than women with mutations. 11 Whether this information leads to a transition in cancer risk perception and the adoption of preventive and screening recommendations appropriate to the general population has not been studied adequately. Therefore, we conducted a qualitative descriptive study to explore the experience of being a mutation‐negative woman from a known BRCA1/2 mutation‐positive family.
Background
Our interest in this special population came from our experience working with these women before and after genetic testing in a research‐based clinical setting (JL, JP, RG, MHG). Mutation‐negative women were recruited, as healthy volunteers, to participate in a Breast Imaging Study (BIS) comparing mammographic density and MRI fibroglandular volume in mutation‐positive and mutation‐negative women in search of a breast imaging phenotype related to BRCA1/2. We speculated that subjects’ desire to participate in an intensive breast cancer screening study was driven by altruism. They understood that, as members of HBOC families, their participation in the BIS as true negative controls helped achieve the study’s primary research objectives, as well as simplified their routine preventive health care. As clinicians, we were surprised and concerned that they continued to express a persistent belief that their cancer risk was elevated. Furthermore, some reported feeling isolated from other family members during their annual BIS study visits. Our interactions with these women caused us to contemplate this phenomenon which subsequently became the basis of our investigation.
Participants and methods
Participants
Participants in this qualitative sub‐study were drawn from the on‐going, prospective National Cancer Institute (NCI) Breast Imaging protocol which enrolled women from BRCA mutation‐positive families in a breast cancer screening pilot study, consisting of annual evaluation for 4 years, including:
-
•
Physical examination, including the breast and pelvis;
-
•
Standard four‐view mammogram;
-
•
Breast MRI;
-
•
Breast duct lavage (BDL);
-
•
CA125 and transvaginal colour flow Doppler ultrasonography (TVUS) and
-
•
Questionnaires and interviews regarding the psychosocial impact of being a member of a family at high genetic risk of cancer.
All mutation‐negative participants in this study had undergone post‐genetic test counselling during which they were advised that they were non‐carriers of their family’s mutation or ‘BRCA negative’. Despite what we assumed was a reassuring test result, 15 mutation‐negative women voluntarily enrolled in this time‐consuming breast cancer screening study, between June 2001 and October 2002, tailored to the surveillance needs of high‐risk women. All subjects provided written informed consent at the time of enrolment in the BIS and were later invited to participate in the qualitative sub‐study.
Study design
We used a qualitative‐descriptive design to explore the experience of risk among BRCA1/2 mutation‐negative women from HBOC families. This approach is often implemented to produce knowledge situated in the intra‐ and inter‐personal realm, such as the origin of perceptions of health and illness experiences. 12 Qualitative description is a widely accepted design strategy, particularly in the context of psychosocial questions about which there is little information. 13 A semi‐structured interview guide was developed by the nurse researchers (AB and SH) based on the experience of the clinicians who provided care to the study participants (JL, RG, JP, MG). The interview guide focused on three domains that were believed to influence a woman’s decision to participate in the BIS:
-
•
Social Domain: Altruism and motivating factors;
-
•
Affective Domain: Emotional impact and experience of being a BRCA mutation‐negative woman;
-
•
Cognitive Domain: Risk perception and communication.
The middle‐range Theory of Transition was chosen as the analytical framework for this study (Fig. 1). 14 , 15 , 16 Transition theory offers a framework to identify the nature of transition, facilitators and inhibitors of transition and the patterns of response as individuals attempt to integrate new health information into personal health care decisions. By identifying areas within the transition framework in which patients struggle to adapt to new health‐care information, specific interventions to assist in the uptake of recommendations may be developed. 14 Genetic information regarding cancer risk has the potential to affect patients’ concept of the nature of individual health. If, prior to genetic testing, a woman considers herself to be at the equivalent risk of developing cancer as a mutation carrier, what interventions will facilitate her transition to a lower cancer risk perception? Genetic information also has the potential to affect risk perception about children and other close relatives. Will the effect of genetic information have a long‐lasting effect over her lifespan? Are there times in a woman’s life when genetic information is more critical for health‐care decision‐making than at other times? Are there differences in how people of different social, economic and cultural backgrounds understand and use genetic information? Are there facilitators or inhibitors of the integration of genetic information into personal health decision making? Are there social or community barriers to the access and uptake of genetic information which delay transition in risk perception?
Figure 1.
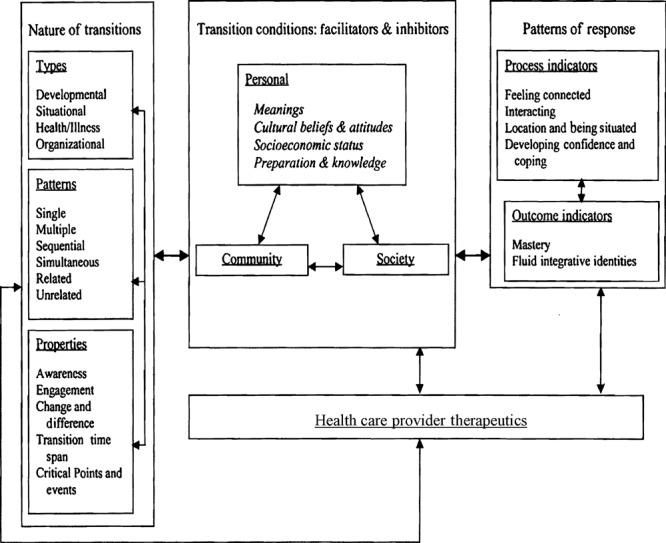
Theory of transition. Reprinted with permission from Meleis AI, Sawyer LM, Im E, Hilfinger‐Messias D, Schumacher K. Experiencing transitions: an emerging middle‐range theory. Advances in Nursing Science, 2000; 23: 12–28.
The application of transition theory to individuals who have undergone genetic testing in families with a known BRCA1/2 mutation is both novel and appropriate. We chose transition theory to assist us in the identification of areas in a woman’s transition from high genetic risk to low genetic risk which were successful or unsuccessful and to discover commonalities in transition among mutation‐negative women. We also sought to describe outcome indicators for successful transition of genetic risk perception. Specifically, we wished to know whether mutation‐negative women would adopt cancer screening guidelines for lower risk women or continue with high‐risk surveillance. Further, we wanted to explore whether women were comfortable speaking with family members about cancer risk. Transition theory provides a highly relevant framework to identify issues related to changes in risk perception during and after genetic testing and developing specific mitigating interventions.
Data collection
BRCA‐negative women participated in a single, in‐depth, semi‐structured telephone interview regarding their experience of being mutation‐negative. Following a brief introduction and review of the study purpose, the interviewer (ADB) obtained verbal consent for participation. A semi‐structured interview guide facilitated the interview process, and was modified to incorporate more focused questions and new themes as they emerged during data collection, as is standard in this study design. Interviews lasting from 90 to 120 min were audio‐recorded and transcribed verbatim. The interviewer recorded field notes from each interview and created case summaries to highlight the most relevant aspects and contextual observations. These documents served as the study’s audit trail and were particularly useful for intra‐ and inter‐case data analysis.
Data analysis
Qualitative content analysis was used to inductively analyse interview data. Codes which represented specific features of the data were derived in a dynamic process, allowing the investigator to continuously modify the treatment of the data, to accommodate new insights as data collection progressed. NVivo 2.0 software (QSR International, Doncaster, Victoria, Australia) was used to facilitate data management. All data were independently coded by two investigators (AB and SH) to enhance reliability of concepts which were divided in an iterative process based on defining narrative units of text and organizing those units into descriptive categories. Ultimately, categories were grouped and elevated to a conceptual level. In sum, 1056 verbatim passages from the interviews were classified using 151 data driven codes and then categorized into six major content areas which are described here. Following each illustrative quotation is the age range of the participant from whom it was obtained.
Results
The final sample consisted of 13 BRCA‐negative women from nine families (Table 1). Experiential domains related to being mutation‐negative for hereditary breast/ovarian cancer centred around six major content areas: (i) experience of living in a multiple‐case HBOC family, (ii) communication patterns between mutation‐negative and mutation‐positive family members, (iii) risk perception: the personal meaning of mutation‐negative status, (iv) opinions regarding cancer aetiology, (v) rationale for initial involvement in the BIS and (vi) rationale for continued participation in the BIS.
Table 1.
Participant characteristics
| Characteristic | Number of subjects | % |
|---|---|---|
| Age, years | ||
| Mean | 49 | – |
| Range | (43–57) | |
| Education, years | ||
| Mean | 15.5 | – |
| Range | (13–18) | |
| Race | ||
| White | 10 | 77 |
| Other | 3 | 23 |
| Family mutation | ||
| BRCA1 | 12 | 92 |
| BRCA2 | 1 | 8 |
| Family breast cancer cases | ||
| Mean | 8 | – |
| Range | (1–15) | |
| Family ovarian cancer cases | ||
| Mean | 3 | – |
| Range | (1–7) | |
Experience of living in a multiple‐case family
To better understand the choices made by BIS participants, we chose to explore their experiences growing up in multiple‐case BRCA families and providing care for relatives. The vigilance that compels these women to seek early diagnostic care is rooted in their heightened awareness of death; proactive measures are taken to ensure the very best health. One participant stated, ‘I was pretty good at having mammograms done, and being that we are from a high‐risk family, I requested my doctor to do transvaginal ultrasounds to look at my ovaries’ (49–51 years).
The effects of having seen so many female relatives die from breast and ovarian cancer continued to have a profound impact on all participants: ‘I think it’s overwhelming, because when they find out that there is one half of the family that is dying at age 27, and it’s just a real big wakeup call that you actually have the potential for that, and you can actually see a whole line of the entire family wiped out and it is really clear’ (46–48 years).
All but one participant expressed the fear that cancer would eventually cause their demise: ‘Before we got into the National Institutes of Health (NIH) study, I just assumed that I would get cancer because my grandmother, my mother, cousin and my sister had all developed breast or ovarian cancer. I just thought “Well this is just what is going to happen to me”. And I didn’t realize until I enrolled into the study that there was a chance that I wouldn’t. I mean I didn’t even know that was possible. I just figured it was definite’ (55–57 years).
The sadness and social isolation triggered by watching older family members die created a void related to familial mentoring relationships: ‘…I didn’t have a lot of women in my lifetime, family members in my lifetime that I was talking to about [cancer]. I wished I had a woman there I could talk with but that just wasn’t the case. I mean I had girlfriends and stuff, but we weren’t talking about that kind of thing’ (43–45 years).
Watching the premature deaths of so many young family members contributed to the sense that life was short and needed to be lived to its fullest: ‘…when my mom passed away, I was relatively young…and I think you just grow up a lot faster when you are around that, especially when there is more than one [death] in your family. So, I’ve always kind of made every day count’ (43–45 years).
Longevity and life expectancy meant something different for women from multiple‐case families: ‘Honestly, at that time, I was thinking, I was probably going to die young, say in my 40s’ (55–57 years). Another participant explained her lack of long‐term planning: ‘I think I kind of lived my life in my 20s and 30s to that extent, you know, not saving for my 401K (retirement plan). All those things that you kind of figure, “Hey, why bother?”’ (43–45 years).
Spirituality helped make sense of living in a multiple‐case family and dealing with loss for many participants: ‘My faith makes all of this to a certain degree a whole lot less significant. Because to me the big thing in my life is God and serving him, and when this whole messed up world is over I’m going to heaven to a better place…’ (49–51 years). Another remarked, ‘I would say that families that have a strong faith in God probably, it doesn’t affect them as much’ (55–57 years).
Communication patterns between mutation‐negative women and mutation‐positive family members
We explored communication patterns between mutation‐negative and mutation‐positive family members. Study participants reported a strategy of silence pertaining to disclosure and discussion of genetic test results: ‘We made a pact we wouldn’t talk about [our mutation status]…because one of my cousins constantly feared the health insurance [ramifications]’ (55–57 years). There appeared to be two levels of avoidance regarding this issue. First, participants described avoiding disclosure of genetic test results to extended family members, to protect their relatives from guilt related to transmitting an inherited mutation and to avoid provoking anxiety. Second, they specifically described revealing their negative status to immediate family members following disclosure, but then avoiding the topic in future conversations, especially among women with cancer‐affected family members: ‘I think at first when I got my results it was very hard for my sister. It was just very hard. And I couldn’t be too happy [around] her because she still had this cancer. It was really hard for me to go around saying “Oh my God I have been spared!” My younger sister has never been tested. She didn’t want to hear one word about anything…so within my family, I just don’t want to talk about it’ (55–57 years).
Several participants reported that their negative test result seemed to damage relationships with mutation‐positive relatives: ‘Sadly, my poor cousin is positive. She is just struggling terribly with the results because she is the only one and she feels very, very alone. And there is no amount of talking that’s going to calm her. We don’t relate, and in fact it has created a wedge. I felt like we were on an even playing field before…’ (46–48 years).
Another important communication issue was a behaviour we characterize as ‘inverse jealousy’, in which mutation‐positive family members expressed a desire to have another mutation‐positive relative with whom to share their experience, rather than wishing to be mutation‐negative themselves. Mutation‐positive family members were described as isolated: ‘And, she [her cousin] said, “I hate to even say the words that I wish somebody else was positive, but I wish so bad I had somebody else to share these feelings with that totally understood”’ (49–51 years). These perceptions may lead to intensified guilt feelings for mutation‐negative women and could create a need for psychosocial intervention among mutation‐negative and mutation‐positive women.
Risk perception: the personal meaning of mutation‐negative status
We tried to understand what being mutation‐negative meant for these women and whether they had any lingering concerns related to their familial risk. Although a few participants stated they had an average risk of developing breast or ovarian cancer, 67% of the women (n = 10) felt that, although their risk was not as high as mutation‐positive carriers, they continued to have a slightly higher risk than the general population. These women used phrases such as ‘a bit above average’ and ‘elevated but not high risk’. For example: ‘Well, I feel like I have the same risks [as those in the general population] but at the same time, in the back of my mind, I think I’m always going to feel that I might have a little higher risk’ (43–45 years). This impression may reinforce cancer worry.
Although participants seemed hesitant to state that their breast/ovarian cancer risks were at general population levels, knowing that they were BRCA negative was a great relief: ‘As I approach the age where my mother first detected her lump, I become more aware of the relief, that is, to know that I am negative’ (43–45 years). Participants who are mothers spoke poignantly of their relief vis‐a‐vis their own children, especially in terms of not passing on the mutation and in not leaving their children motherless: ‘When my sister died, my daughter was obviously upset about losing her, but she was just sixteen thinking “this could happen to my mother”. Well, I tried to tell her that that is not going to happen to me in that same way, I may develop cancer, but not at age 46 because I am past that. So I was enormously relieved for her and to be able to tell her that the same thing would not happen to me’ (55–57 years).
Participants’ relief at being mutation‐negative was tempered by worries that they might still be at increased risk of breast/ovarian cancer, in part from believing that they might have an unidentified mutation: ‘I think [being negative] means that I am probably not in the mutation they found. And, I’ll stick with [the study] until they find what it is. What it really means to me is that there is probably something else they haven’t found’ (43–45 years). Perhaps our discussions of on‐going research about possible genetic and environmental modifiers contributed to this confusion.
Opinions about cancer aetiology
Every study participant expressed strong opinions concerning the terrifying effects of ovarian cancer and its poor prognosis: ‘I don’t know anybody who has survived ovarian cancer. So [breast and ovarian] are really different cancers in my mind’ (55–57 years). Participants expressed the opinion that there was a strong genetic link to ovarian cancer but, surprisingly, many seemed to believe that breast cancer was only minimally explained by genetics. Only one participant of 13 explicitly cited a connection between breast cancer and genetics.
Rationale for initial involvement in the breast imaging study
Interview participants offered three main reasons for joining the BIS: (i) the wish to help others, (ii) personal gain and (iii) the desire to advance science. Although distinct, these reasons are interrelated, forming a representational triptych characterizing the reasons for participating in clinical trials.
Explaining her altruism, one participant said, ‘that’s one of the primary reasons why I engaged in it all…to hopefully make a contribution or help somebody somewhere’ (43–45 years).
Although altruism was a universal theme, participants also reported that they discovered their own personal needs were being fulfilled. Said another participant, ‘with the mammograms and all the other tests they were doing, I thought “geez, I could participate in that and they could catch cancer much sooner doing all these tests”. So then I was more selfish’ (55–57 years).
The desire to advance breast cancer research was an equally important reason for the women to become involved in this study. One participant opined, ‘giving back doesn’t always happen the day you give any kind of service…what we are doing to advance research may not pay off today or pay off 50 years from now or 100 years from now…it gives me comfort knowing that somewhere it will help’ (49–51 years). Participants were keenly aware that their role as study controls was essential to research success.
Rationale for continued participation
All BRCA mutation‐negative women gave the same response when asked why they continued BIS participation, despite time away from their families and potentially painful study procedures (e.g. BDL): participants believed they were receiving cutting edge, state‐of‐the‐art screening and diagnostic care that they might not receive elsewhere. One participant quickly clarified what could be misconstrued as criticism of community‐based medicine, ‘I’m not criticizing my gynecologist. He is really excellent. Please don’t misunderstand me, but I can get really good diagnostic healthcare [in the study] in terms of mammograms, MRIs and ultrasounds. So I went through that selfish part that there would be good, safe diagnostic care from NIH during that period’ (55–57 years).
Participants believed that the diagnostic tests offered in the BIS would detect cancer at an earlier than usual stage, thus offering greater secondary prevention. One participant remarked, ‘At a very irrational level, I feel like I am safe…if something happens they will find it much earlier at NIH and they will take care of me’ (55–57 years). Several women reported that study participation reinforced their need to stay vigilant because of the devastation that could result if they ‘let their guard down’. Said one participant, ‘it has caused me to relax a little more, but it has also made me realize that you still need to be proactive in your healthcare…you can’t [relax] unless you really want to sign your own death warrant’ (43–45 years).
Another reason cited for continued participation was the assurance it provided regarding the mutation status of their children: ‘my mom died of breast and ovarian cancer and there was a lot in my family…so, I really did most of this for my children, but now I don’t have to worry about them as much…’ (55–57 years).
Discussion
Our findings reveal that women who have tested negative for a known familial BRCA mutation continue to carry a heavy burden related to being a member of an HBOC family. Major findings in this small sample of women were persistent sense of loss, isolation, lack of female mentorship and fear of death from cancer, despite being mutation‐negative. They described issues in family communication that can develop when mutation‐negative family members share genetic test results. Participants were extremely sensitive to the feelings of other family members and often avoided discussion of their status. The revelation of a mutation‐negative test result often fractured relationships with mutation‐positive family members creating a situation in which some mutation‐positive women longed for the camaraderie of a mutation‐positive individual.
Participants expressed great relief in knowing they could not pass on the family mutation to their children and yet continued to suspect that they, personally, carry a mutation that has not yet been identified. Participation in the BIS was an expression of altruism in support of breast cancer research; it streamlined their personal health care and may be an expression of their elevated cancer risk perception.
As members of multiple‐case families, our participants detailed the devastating consequences of death from cancer. Many were preoccupied with fear of premature death, frequently assuming adult responsibilities while young. Necessity forced them to become well‐informed health‐care consumers and they lived with the persistent fear that their children could lose their mother prematurely. Spirituality reduces stress and increases coping ability for some women, while others develop familial relationships with surrogate females for personal support. None of these participants were clinically depressed; however, the majority expressed a pervasive sense of loss and sadness.
Although the psychosocial impact of a mutation‐negative genetic test result in HBOC women has not been directly evaluated, these women frequently serve as research controls, to whom mutation carriers and individuals with uninformative results are compared. 17 , 18 , 19 Meiser 20 reported that the impact of genetic testing on psychosocial functioning in unaffected non‐carriers is fairly consistent, with all but three 21 , 22 , 23 studies showing improvements in mood with time. 24 Previous publications regarding life as a mutation‐negative member of a mutation‐positive family have focused on the immediate post‐test counselling period and the ensuing 6–12 months. Our data suggest that being a ‘true‐negative’ member of a mutation‐positive family is more complex than previously described.
In our sample, the majority were worried about being at greater than average risk of cancer despite a ‘true negative’ mutation test result. Most study participants were greatly relieved that their children were not carriers, avoiding high cancer risk and the need for genetic testing. This sense of relief, however, did not extend to themselves. Most of the women in this study obtained informative genetic testing when they were in the fourth or fifth decade of life. Perhaps having lived so long with an elevated cancer risk perception, they are less able to relinquish their prior risk appraisal, especially when this belief has become integral to their identity. Specifically, there seemed to be difficulty in reframing the risk of ovarian cancer from high risk to average risk. Having seen family members suffer and die from ovarian cancer, women may resist ending ovarian cancer screening, despite the unequivocal evidence that periodic TVUS and CA‐125 testing is without evidence of benefit, for both high risk and population‐risk women.
Although several studies have demonstrated that the majority of carriers and non‐carriers adopt appropriate screening and preventive behaviours following genetic testing for breast/ovarian‐ and colon cancer‐related syndromes, this is not a consistent finding. Lerman et al. 25 found no change in breast cancer screening uptake after testing. In a study of hereditary colorectal cancer syndromes, more than one‐third of mutation‐negative individuals had bowel screening beyond that required for their risk level. 26 Others report that a significant proportion of mutation‐negative hereditary colorectal cancer family members expressed concerns about foregoing surveillance, despite feeling relief at no longer being required to have colonoscopies. 27 , 28 Similarly, a proportion of women undergoing cancer susceptibility genetic testing are not reassured by the negative test result.
Screening behaviour has also been found to be related to health professionals’ recommendations and country of origin, perhaps reflecting cultural differences in standards of medical practice and lay values. 20 This finding opens the door for communication and educational interventions, such as training health‐care professionals, offering supportive counselling, brief problem‐solving skills or communication tools designed to correct inflated risk perceptions to individuals who are undergoing genetic testing. 24 , 29
Our participants enrolled in the BIS for both altruistic and personal reasons, most stating that they enrolled to benefit future generations. This resembles previous reports of reasons for community volunteering. 23 Several participants believed that the care at NIH is meaningfully different from that received in their local communities. Participating in an NIH study seems to provide personal reassurance or perceived protection against cancer, a choice likely influenced by persistent perceived elevated cancer risk.
Transition theory offers a useful framework to evaluate the progress in adapting to mutation test results in individuals undergoing predisposition genetic testing for HBOC. The current standard of care is to consider genetic testing for HBOC syndrome no earlier than 18 years of age. It is unclear when an individual’s perception of their personal risk status is formed. Genetic information affects health/illness perceptions and can impact developmental goals of young adults as they start to contemplate careers, marriage, reproductive choices and cancer risk management. Many young adults from HBOC families have been exposed to the effects of multiple cases of cancer and therefore the type, pattern and properties of transition in the perception of individual mutation status are expected to be complex. It is likely to differ according to age, personal experience with the effects of cancer, maturity of the individual, current goals and how many close family members will be affected by the information. This group of women, having assumed that they were mutation carriers for 40–50 years, struggled to let go of prior perceptions of being mutation‐positive.
Transition theory also provides a framework to evaluate the conditions for transition in the perception of an individual’s mutation status. Individual adaptation to mutation test results will be influenced by an individual personal strength (meanings, beliefs, knowledge, economic status) and cultural/social supports available to them. In this study, participants described the benefits of spirituality in their adjustment to mutation test results. They poignantly described the isolation experienced by both some mutation‐positive and some mutation‐negative women within the family that developed after disclosing their personal mutation test results. Perhaps, the avoidance of discussion within the family inhibits the transition of an individual’s perception of being mutation‐positive to acceptance of being mutation‐negative.
Few of the participants in this qualitative sub‐study demonstrated outcome indicators of transition to being mutation‐negative. There were indications that these participants did not feel free to share information and elicit support from family members in relation to genetic test results. Confidence in coping with mutation test results was not well demonstrated in this small sample of women. If anything, there was a sense of disbelief in the test results and a persistent sense of being at high risk of cancer. Overall, framing these experiences with transition theory may identify obstacles to transition and guide future health care interventions.
Conclusion
We hypothesize that the particular situation and age at which individuals seek genetic testing will affect the nature, condition and patterns of response in their transition from perceived high cancer risk to perceived low cancer risk. In our patients, their perception of being at high cancer risk was fundamental to their self‐identity. Merely informing such women that they are not at high cancer risk may not suffice to lower their risk perception or change their self‐image. The transition required to redefine one’s self‐image requires time, emotional and social support, multiple opportunities for clarification and a profound change in awareness. It seems likely that within their family or social support systems, there are advantages and disadvantages to being either mutation‐negative or mutation‐positive. We recommend that providers who care for these women take a family‐centred approach incorporating both mutation‐positive and mutation‐negative women. This study suggests that we recognize that one cannot simply assume that mutation‐negative women immediately and unambiguously embrace their lower risk status. Mutation‐negative women may require additional help in redefining their cancer risk perception and in viscerally accepting this new reality. They require reassurance that their risk is not at the high level faced by their mutation‐positive relatives, that a reduced level of cancer screening is appropriate and safe and that their children are not going to inherit the family mutation or lose their mothers to the ravages of HBOC. This is not as simple as it seems, because they must simultaneously accept that they could develop sporadic breast or (less likely) ovarian cancer. In addition, these women may require additional psychosocial support related to grieving the loss of family members and the on‐going difficulty of living as a mutation‐negative member of an HBOC family, as their mutation‐positive relatives continue to develop HBOC‐related cancer. This qualitative study represents a first step towards an improved understanding of these dynamics, an understanding that may lead to substantial improvements in management strategies and quality of life for BRCA mutation‐negative members of mutation‐positive families.
Acknowledgements
The research activities of MH Greene, JA Peters and JT Loud were supported by funding from the Intramural Research Program of the US National Cancer Institute. The protocol patients described herein were consented participants in NCI Protocol 01‐C‐0009, which was supported in part by Westat (Contracts NO2‐CP‐11019‐50 and NO2‐CP‐65504‐50). We remain deeply grateful to the on‐going participation of the members of our cohort of hereditary breast/ovarian cancer families, whose selfless commitment to advancing our knowledge is absolutely essential to this and related HBOC projects. We are forever in their debt.
References
- 1. Miki Y, Swenson J, Shattuck‐Eidens D et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1 . Science, 1994; 266: 66–71. [DOI] [PubMed] [Google Scholar]
- 2. Wooster R, Bignell G, Lancaster J et al. Identification of the breast cancer susceptibility gene, BRCA2 . Nature, 1995; 378: 789–792. [DOI] [PubMed] [Google Scholar]
- 3. Thompson D, Easton DF, Breast Cancer Linkage Consortium . Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. American Journal of Human Genetics, 2001; 68: 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antoniou AC, Pharoah PPD, Narod SA et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series or unselected for family history: a combined analysis of 22 studies. American Journal of Human Genetics, 2003; 72: 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Easton DF, Hopper JL, Thomas DC, Antoniou AC, Pharoah PPD, Whittemore AS. Breast cancer risks for BRCA1/2 carriers. Science, 2004; 306: 2187–2188. [DOI] [PubMed] [Google Scholar]
- 6. Wacholder S, Streuwing JP, Hartage P, Greene MH, Tucker MA. Breast cancer risks for BRCA1/2 carriers. Science, 2004; 306: 2188–2191. [PubMed] [Google Scholar]
- 7. King MC, New York Breast Cancer Study Group . Breast cancer risks for BRCA1/2 carriers. Science 2004; 306: 2188–2191. [Google Scholar]
- 8. Risch HA, McLaughlin JR, Cole DEC et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin‐cohort study in Ontario, Canada. Journal of the National Cancer Institute, 2006; 98: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 9. Whittemore AS, Gong G, Itnyre J. Penetrance and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three U.S. population‐based case–control studies of ovarian cancer. American Journal of Human Genetics, 1997; 60: 496–504. [PMC free article] [PubMed] [Google Scholar]
- 10. Katki HA, Gail MH, Greene MH. Are BRCA mutation‐negative women from mutation‐positive families at increased risk of breast cancer? Lancet Oncology, 2007; 16: 1042–1043. [DOI] [PubMed] [Google Scholar]
- 11. Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 2007; 16: 241–260. [DOI] [PubMed] [Google Scholar]
- 12. Kearney MH. Levels and applications of qualitative research evidence. Research in Nursing & Health, 2001; 24: 145–153. [DOI] [PubMed] [Google Scholar]
- 13. Sandelowski M. Whatever happened to qualitative description? Research in Nursing and Health, 2000; 23: 334–340. [DOI] [PubMed] [Google Scholar]
- 14. Meleis AI, Sawyer LM, Im E, Hilfinger‐Messias D, Schumacher K. Experiencing transitions: an emerging middle‐range theory. Advances in Nursing Science, 2000; 23: 12–28. [DOI] [PubMed] [Google Scholar]
- 15. Schumacher K, Meleis AI. Transitions: a central concept in nursing. Image: The Journal of Nursing Scholarship, 1994; 26: 119–127. [DOI] [PubMed] [Google Scholar]
- 16. Kralik D, Visentin K, Van Loon A. Transition: a literature review. Journal of Advanced Nursing, 2006; 55: 320–329. [DOI] [PubMed] [Google Scholar]
- 17. Lynch HT, Snyder C, Lynch JF et al. Patient responses to the disclosure of BRCA mutation tests in hereditary breast‐ovarian cancer families. Cancer Genetics and Cytogenetics, 2006; 165: 91–97. [DOI] [PubMed] [Google Scholar]
- 18. Van Dijk S, Timmermans DR, Meijers‐Heijboer H, Tibben A, Van Asperen CJ, Otten W. Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. Journal of Clinical Oncology, 2006; 24: 3672–3677. [DOI] [PubMed] [Google Scholar]
- 19. Reichelt JG, Heimdal K, Moller P, Dahl AA. BRCA1 testing with definitive results: a prospective study of psychological distress in a large clinic‐based sample. Familial Cancer, 2004; 3: 21–28. [DOI] [PubMed] [Google Scholar]
- 20. Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psycho-oncology, 2005; 14: 1060–1074. [DOI] [PubMed] [Google Scholar]
- 21. Aktan‐Collan K, Haukkala A, Mecklin JP, Uutela A, Kääriäinen H. Psychological consequences of predictive genetic testing for hereditary non‐polyposis colorectal cancer (HNPCC): a prospective follow‐up study. International Journal of Cancer, 2001; 93: 608–611. [DOI] [PubMed] [Google Scholar]
- 22. Codori A, Zawacki KL, Petersen GM et al. Genetic testing for hereditary colorectal cancer in children: long‐term psychological effects. American Journal of Medical Genetics, 2003; 116a: 117–128. [DOI] [PubMed] [Google Scholar]
- 23. Omoto AM, Snyder M. Sustained helping without obligation – motivation, longevity of service, and perceived attitude‐change among aids volunteers. Journal of Personality and Social Psychology, 1995; 68: 671–686. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz MD, Lerman C, Audrain J et al. The impact of a brief problem‐solving training intervention for relatives of recently diagnosed breast cancer patients. Annals of Behavioral Medicine, 1998; 20: 7–12. [DOI] [PubMed] [Google Scholar]
- 25. Lerman C, Hughes C, Croyle R et al. Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Preventive Medicine, 2000; 31: 75–80. [DOI] [PubMed] [Google Scholar]
- 26. Michie S, Collins V, Halliday J, Marteau TM. Likelihood of attending bowel screening after a negative genetic test result: the possible influence of health professionals. Genetic Testing, 2002; 6: 307–311. [DOI] [PubMed] [Google Scholar]
- 27. Bleiker EMA, Menko FH, Taal BG et al. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology, 2005; 128: 280–287. [DOI] [PubMed] [Google Scholar]
- 28. Hadley D, Jenkins JF, Dimond E, DeCarvalho M, Kirsch I, Palmer CGS. Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. Journal of Clinical Oncology, 2004; 22: 39–44. [DOI] [PubMed] [Google Scholar]
- 29. Lobb EA, Butow PN, Meiser B et al. Tailoring communication in consultations with women from high risk breast cancer families. British Journal of Cancer, 2002; 87: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]


