Abstract
Evidence exists that protein kinase c (PKC) and the mammalian target of rapamycin (mTOR) are important regulators of cardiac hypertrophy. We examined the contribution of these signaling kinases to cardiac growth in Spontaneously Hypertensive Rats (SHR). Systolic blood pressure (mmHg) was increased (p<0.001) at 10 weeks in SHR vs. Wistar Kyoto controls (WKY) (162±3 vs.128±1), and further elevated (p<0.001) at 17 weeks in SHR (184±7). Heart: body weight was not different between groups at 10 weeks, but was 22% greater (p<0.01) in SHR vs. WKY at 17 weeks. At 10 weeks, activation of Akt and S6 ribosomal protein was greater (p<0.01) in SHR, but returned to normal by 17 weeks. In contrast, SHR had PKC activation only at 17 weeks. To determine if mTOR regulates the initial development of hypertrophy, rats were treated with rapamycin (2 mg/kg/day i.p.) or saline-vehicle from 13 to 16 weeks of age. Rapamycin inhibited cardiac mTOR in SHR as evidenced by reductions (p<0.001) in phosphorylation of S6 ribosomal protein and eukaryotic translation initiation factor-4E binding protein-1. Rapamycin treatment also reduced (p<0.001) heart weight and hypertrophy by 47% and 53%, respectively, in SHR in spite of increased (p<0.001) systolic blood pressure vs. untreated SHR (213±8 vs. 189±6). Atrial natiuretic peptide, brain natiuretic peptide, and cardiac function were unchanged between SHR treated with rapamycin or vehicle. These data show that mTOR is required for development of cardiac hypertrophy evoked by rising blood pressure in SHR.
Keywords: Heart, blood pressure, signal transduction, hypertrophy
INTRODUCTION
Over the last decade, much research has focused on identifying the signaling pathways that regulate cardiac hypertrophy. Among these pathways, protein kinase c (PKC) and the mammalian target of rapamycin (mTOR) have emerged as potentially important regulators of cardiac hypertrophy1.
PKC is a family of serine-threonine kinases consisting of 11 isoforms in the heart2. Studies using transgenic mice with cardiac specific overexpression of PKC βII or ε, and mice with overexpression of peptide activators of PKC δ and ε, have reported that these isoforms regulate pathological (PKC βII) and/or physiological cardiac hypertrophy (PKC δ and ε) 3–6. Similarly, humans with hypertrophy and heart failure exhibit activation of PKC α and βII7.
Signaling through components of the mTOR pathway is an important regulator of normal cardiac growth and pathological hypertrophy. For example, overexpression of phosphoinositide 3-kinase (PI3K) in mice results in Akt activation and increased heart size, while overexpression of dominant negative PI3K leads to decreased Akt activation and reduced heart size8. Other studies using cardiac specific overexpression of Akt report that development of both physiological and pathological hypertrophy are correlated with the degree of Akt activation9, 10. Human studies examining components of mTOR signaling during hypertrophy or heart failure are scarce, and what data exists is conflicting. For example, it has been reported that implantation of a left ventricular assist device (LVAD) in patients with heart failure resulted in cardiac improvements (reduced left ventricular end diastolic dimensions and apoptosis), associated with a reduction in phospho (p)-Akt11. In contrast, a more recent study stated that hypertensive patients without heart failure had higher p-Akt than patients with heart failure12. Therefore the role of the mTOR signaling pathway during human hypertrophy and heart failure remains unclear.
In the present study we tested the hypothesis that PKC and mTOR contribute to cardiac hypertrophy that develops in spontaneously hypertensive rats (SHR). We chose the SHR because these animals model human hypertension and cardiac growth. In this regard, these rats are normotensive at 6 weeks of age, but develop hypertension and cardiac hypertrophy at ~ 12 weeks of age, and heart failure by ~ 24 months13, 14. Data provided herein show that signaling via mTOR, but not PKC is increased in SHR during the development of cardiac hypertrophy (i.e., at 10 weeks). Furthermore, when mTOR was inhibited using rapamycin, cardiac hypertrophy was attenuated independent of changes in blood pressure. These data show clearly that mTOR is required for the initiation and full development of cardiac hypertrophy evoked by rising blood pressure in SHR.
METHODS
Please see http://hyper.ahajournals.org for a detailed description of the methods and experimental groups.
Animals
All protocols were approved by the University of Utah Institutional Animal Care and Use Committee. Six week old male, spontaneously hypertensive rats (n=42) and Wistar Kyoto rats (WKY, n=24) were purchased from Harlan (Indianapolis, IN) and housed in the University of Utah Comparative Medicine Center under standard conditions (12h light: 12h dark cycle) and free access to food and water. Rapamycin was purchased from LC Laboratories (Woburn, MA).
Blood pressure
Blood pressure was measured using a fluid filled catheter placed into the caudal artery of rats anesthetized with 2–5% isoflurane15, 16. After rats regained consciousness, blood pressure was measured over 20 cardiac cycles.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from LV using Trizol reagent (Invitrogen, Carlsbad, California) and purified using the RNAeasy total RNA isolation kit (Qiagen, Valencia, California). RT-PCR was done as detailed by Boudina et al17.
Tissue homogenization and Western blotting
Homogenization of the LV, electrophoresis, and transfer of proteins to PVDF membranes were done as we have previously described18, 19. Western blots were verified in duplicate if no significant differences were observed, or triplicate if significant differences were present.
Myocardial Function
Cardiac function was determined in a subset of SHR after 3 weeks of rapamycin (2mg/kg i.p., n=5) or vehicle (saline, n=5) treatment using echocardiography15.
Statistical Analysis
An analysis of variance was used to detect differences among groups using SPSS v11 for Macintosh. When a significant P value was obtained (P < 0.05), post hoc procedures were performed using LSD (Least Significant Difference) analyses to identify individual group differences. Results are presented as mean ± standard error (SE).
RESULTS
PKC, mTOR, cardiac mass, and blood pressure in 10 week and 17 week-old SHR
At 10 weeks of age, heart: body weight was similar in WKY vs. SHR (Table 1). In contrast, cardiac hypertrophy was present in 17 week-old SHR as evidenced by increased heart to body weight ratio vs. WKY (Table 1). Systolic blood pressure was 26% higher in 10 week-old SHR compared to WKY rats, and 33% greater in 17 weeks SHR vs. WKY (Table 1).
Table 1.
Characteristics of WKY and SHR rats.
| Group | 10 week | 10 week | 17 week | 17 week |
|---|---|---|---|---|
| WKY | SHR | WKY | SHR | |
| (n) | 6 | 6 | 6 | 6 |
| Heart Weight (mg) | 1015±54 | 1183±29* | 1048±35 | 1297±43† |
| Body Weight (g) | 245±5 | 297±5* | 319±10 | 325±10† |
| Heart:Body Weight (mg:g) | 3.95±0.11 | 3.99±0.09 | 3.32±0.14 | 3.99±0.10† |
| Heart Rate | 398±10 | 410±8 | 422±14 | 480±18† |
| Caudal blood pressure (mm Hg) | ||||
| Systolic | 128±1 | 162±3* | 138±2 | 184±7† |
| Diastolic | 109±2 | 148±2* | 114±3 | 159±5† |
| Mean Arterial Pressure | 116±1 | 152±2* | 122±2 | 167±4† |
Data are means ± SE. WKY, normotensive control rats. SHR, spontaneously hypertensive rats,
P<0.05 vs. 10 week WKY.
P<0.05 vs. 17 week WKY.
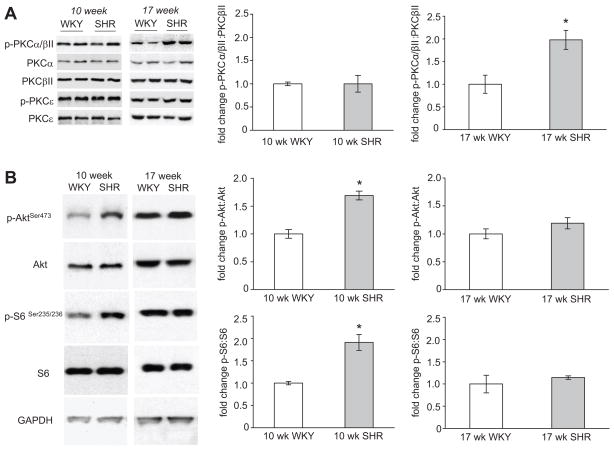
There were no differences detected in total protein expression of PKCα, PKCβII, PKCδ, or PKCε in SHR vs. WKY rats at either age (Figure 1A). Levels of p-PKCε were also similar at both ages of SHR and WKY (Figure 1A). p-PKCδ was not detected in the myocardium of SHR or WKY rats. This might be due to a lack of specificity of the primary antibody against rat heart p-PKCδ rather than an absence of p-PKCδ. There was no change in p-PKCα/βII in 10 week-old SHR compared to their age matched controls. However, 17 week SHR had an ~ 80% increase in p-PKCα/βII vs. WKY (Figure 1A). To control for any possible protein loading differences, p-PKCα/βII: total PKCβII was determined and found to be significantly greater in 17 week-old SHR vs. WKY rats (Figure 1A). Similar results were obtained with p-PKCα/βII: total PKCα (data not shown). After these initial experiments, we also examined PKC status at 14.5 weeks in a subset of SHR and WKY, but found no change in p-PKCα/βII at this age (data not shown).
Figure 1.
A. Western blot analysis of PKCα, βII, δ, and ε in hearts of 10 week-old SHR during the development phase of cardiac hypertrophy and 17 week-old SHR with established cardiac hypertrophy. Bar graphs represent fold change of p-PKCα/βII: total PKCβII in SHR vs. WKY. B. Western blots of Akt, and S6 in hearts of 10 week-old SHR during the development phase of cardiac hypertrophy and 17 week-old SHR with established cardiac hypertrophy. Bar graphs represent fold changes in p-AktSer473: total Akt and p-S6: total S6 in SHR vs. age matched WKY. For all experiments GAPDH was used as a loading control. SHR; spontaneously hypertensive rats, WKY; Wistar Kyoto rats. For all bar graphs, data presented as mean±SE, n=6 in all groups. * Denote significant difference at P<0.05.
p-AktSer473 and the ratio of p-AktSer473 to total Akt was ~ 70% greater in 10 week SHR, but unchanged in 17 week-old SHR compared to age matched WKY rats (Figure 1B). GAPDH protein expression, used as a loading control, was similar among all groups (Figure 1B).
mTOR signaling and cardiovascular variables in rats treated with rapamycin
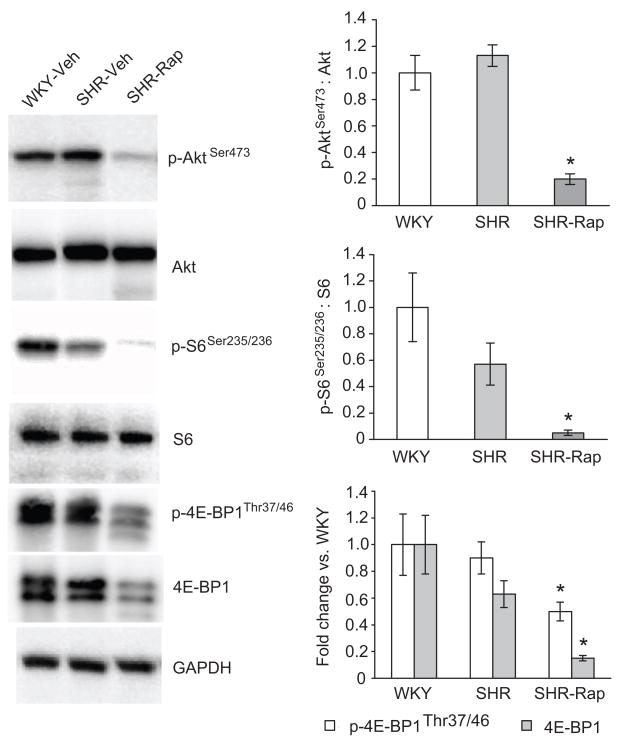
We reasoned that mTOR signaling might regulate the development of hypertrophy because both Akt and S6 phosphorylation were greater at 10, but not 17 weeks in SHR vs. WKY. To address this, 13-week old SHR were treated with rapamycin or vehicle for 3 weeks, a duration similar to those used in pressure overload experiments20. In this experiment we did not employ a rapamycin treated WKY group since cardiac mass has been reported to be unaffected by this drug in control animals20, 21. Thirteen-week old SHR were chosen because our preliminary studies indicated that cardiac hypertrophy is minimal at this time, and p-AKT is still elevated while p-PKCα/βII is normal in 14.5 week old SHR. Inhibition of mTOR signaling by our rapamycin treatment regimen was confirmed by ~4-fold reduction in levels of p-S6Ser235/236 and p-4E-BP1Thr37/46 in SHR-Rap vs. SHR-Veh and WKY-Veh (Figure 2). p-AktSer473 was reduced almost 80% in SHR-Rap (Figure 2). Taken together, signaling through mTOR in the heart was markedly reduced in rapamycin treated SHR.
Figure 2.
Impact of rapamycin treatment on mTOR regulated signaling kinases. Bar graphs represent fold changes in p-AktSer473: total Akt and p-S6: total S6. Bar graphs for 4E-BP1 represent fold change in p-4E-BP1 and total 4E-BP1 in SHR vs. age matched WKY. The ratio of p-4E-BP1 to total 4E-BP1 is not shown since it does not convey the severe reduction in both 4E-BP1 phosphorylation and total 4E-BP1 protein that is evident from the blots themselves. For all experiments GAPDH was used as a loading control. WKY; Wistar Kyoto rats treated with saline vehicle, SHR; spontaneously hypertensive rats treated with saline vehicle, SHR-Rap; spontaneously hypertensive rats treated with rapamycin. n=7 WKY, n=6 SHR, n=9 SHR-Rap * P<0.05 vs. WKY and SHR.
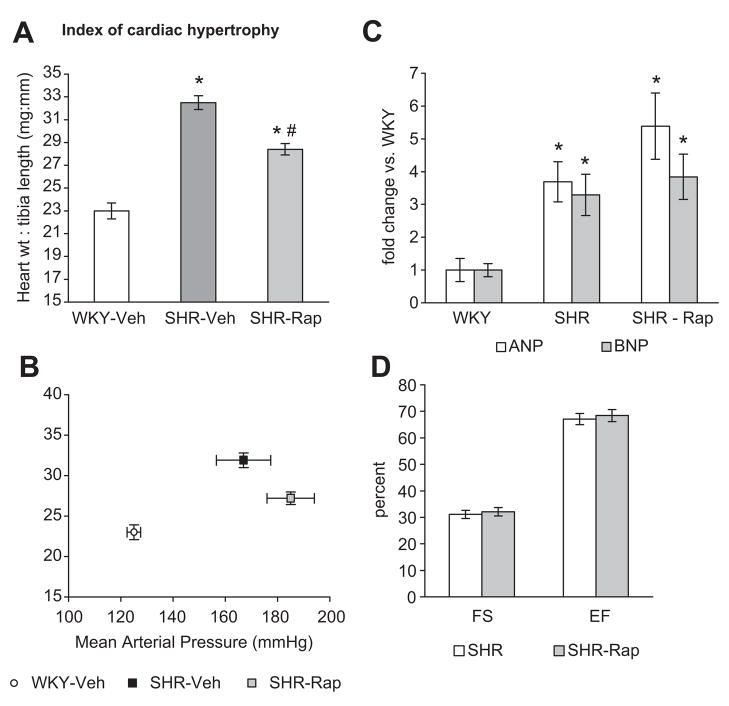
Heart weights were lower in SHR-Rap than SHR-Veh, but still greater than WKY-Veh (Table 2). As expected, blood pressure was greater in SHR vs. WKY, but surprisingly it was even higher in SHR-Rap vs. SHR-Veh (Table 2). Rapamycin treated SHR had lower body weight than WKY-Veh or SHR-Veh (Table 2). Heart mass was therefore normalized to tibia length for the rapamycin studies. In spite of increased blood pressure in the SHR-Rap, cardiac hypertrophy was attenuated (heart: tibia length) vs. SHR-Veh, but still greater than WKY-Veh (Figure 3A, B). Expression of ANP and BNP, markers of pathological cardiac hypertrophy and increased wall stress, was elevated in SHR-Veh vs. WKY-Veh. Although hypertrophy was attenuated in SHR-Rap, the expression of ANP and BNP remained similar to SHR-Veh (Figure 3C).
Table 2.
Characteristics of WKY and SHR rats treated with vehicle or 2 mg/kg rapamycin.
| WKY-Veh | SHR-Veh | SHR-Rap | |
|---|---|---|---|
| Age in weeks | 16 | 16 | 16 |
| (n) | 7 | 11 | 14 |
| Heart Weight (mg) | 827±26 | 1244±21* | 1080±18*† |
| Body Weight (g) | 252±4 | 322±3* | 269±3*† |
| Tibia Length (mm) | 36.1±0.2 | 38.3±0.2* | 38.0±0.2* |
| (n) | 7 | 6 | 9 |
| Heart Rate (beats/min) | 376±11 | 390±10 | 405±13 |
| Caudal blood pressure (mm Hg) | |||
| Systolic | 139±6 | 189±7* | 213±6*† |
| Diastolic | 118±6 | 156±7* | 171±6* |
| Mean Arterial Pressure | 125±6 | 167±7* | 184±5*† |
Data are means ± SE. SHR, spontaneously hypertensive rats, and WKY, normotensive control rats vehicle, treated with saline vehicle (Veh) i.p. from 13–16 weeks of age. SHR-Rap treated with 2 mg/kg rapamycin from 13–16 weeks of age.
P<0.05 vs. WKY.
P<0.05 vs. SHR.
Figure 3.
A. Index of cardiac hypertrophy expressed as heart weight: tibia length in rats treated with rapamycin at 2 mg/kg (Rap) or vehicle (saline) from 13 to 16 weeks of age (n=7 WKY, n=7 SHR, n=9 SHR-Rap). Heart: body weight was not used due to weight loss that occurred in SHR after rapamycin treatment. B. Heart: tibia length plotted against mean arterial blood pressure (MAP) illustrates attenuation of cardiac hypertrophy in SHR-Rap in spite of significantly increased blood pressure compared to SHR-Veh (n=7 WKY, n=7 SHR, n=9 SHR-Rap). C. mRNA expression of atrial natiuretic peptide (ANP) and brain natiuretic peptide (BNP) (n=7 WKY, n=7 SHR, n=9 SHR-Rap). D. In vivo assessment of cardiac function using parameters of percent fractional shortening (FS) and ejection fraction (EF) (n=5 SHR, n=5 SHR-Rap). WKY; Wistar Kyoto rats treated with saline vehicle, SHR; spontaneously hypertensive rats treated with saline vehicle, SHR-Rap; spontaneously hypertensive rats treated with rapamycin. * P<0.05 vs. WKY. † P<0.05 vs. SHR-Veh.
It is possible that the combination of exaggerated hypertension and attenuated hypertrophy in SHR-Rap vs. SHR-Veh might lead to increased wall stress and cardiac dysfunction. However, there was no echocardiographic evidence for LV dysfunction as both ejection fraction and fractional shortening were similar in SHR-Veh and SHR-Rap (Figure 3D). Interventricular septal dimension, left ventricular diastolic dimension, and left ventricular posterior wall dimension were also similar in SHR-Rap vs. SHR-Veh (data not shown).
DISCUSSION
The contribution of mTOR signaling to cardiac hypertrophy that develops in response to a gradual increase in afterload, as occurs in the SHR, is unknown. In the present study we observed activation of mTOR signaling in hearts of young SHR during the developmental stage of cardiac hypertrophy. Pharmacologically inhibiting this pathway attenuated the extent of cardiac hypertrophy that ultimately occurs in this model. These are the first data indicating that mTOR signaling contributes importantly to cardiac hypertrophy in a clinically relevant model of hypertension.
Studies using pressure overloaded mice and guinea pigs reported that P70 S6 ribosomal kinase (S6K) phosphorylation is clearly correlated with cardiac hypertrophy21, 22, and that cardiac-specific Akt overexpression increases activation of mTOR and results in cardiac hypertrophy9, 10, 23. The in vivo importance of mTOR has also been demonstrated in pressure overloaded rodents where rapamycin treatment results in inhibition of mTOR as determined by downstream effectors such as S6K, and attenuates cardiac hypertrophy evoked by aortic constriction21, 24, 25. The studies using pressure-overloaded models are important, however, it should be noted that aortic constriction creates local hypertension, and does so in an abrupt manner. This process differs from the SHR that has gradually increasing systemic hypertension that eventually results in cardiac hypertrophy. The SHR may be considered to be more clinically relevant to the human experience where uncontrolled hypertension leads to cardiac hypertrophy. In the present study, rapamycin treated SHR demonstrated a clear and robust reduction in phosphorylation of S6 and 4E-BP1, both downstream targets of mTOR, and thus provided mechanistic evidence of the role of mTOR in the development of cardiac hypertrophy.
PKC isoforms have been reported to be mediators of cardiac function and hypertrophy. Overexpression or activation of PKCβII, ε and δ has been found to result in cardiac hypertrophy in mice26. Interestingly, mice with deletion of PKCβ still develop cardiac hypertrophy in response to pressure overload or phenylephrine27, and overexpression of PKCα does not cause cardiac hypertrophy, but results in diminished ventricular function 28. Similarly, inhibition of conventional PKC isoforms (α, β, γ) increases cardiac function in mice29, while adenoviral transfection of PKCα reduces cardiac contractility in the normally hypercontractile PKCα knockout mouse30. In contrast to our original hypothesis, we did not find activation of any PKC isoform in 10 week-old SHR during the developmental phase of cardiac hypertrophy. However, 17 week-old SHR had an increase in p-PKCα/βII. While no other studies have examined PKC during the developmental phase of hypertrophy in the SHR, others have found PKCα, δ, ε activation in 6 month-old spontaneously hypertensive heart failure rats (SHHF)31, and PKCβ activation in 16 week-old Dahl salt sensitive rats32. Given the lack of PKC activation in hypertensive 10 week old SHR without hypertrophy, one may speculate that the PKC alterations in SHHF and Dahl salt sensitive rats with established hypertrophy may be related to regulation of cardiac function rather than growth.
In the present study, we found that treating SHR with rapamycin for 3 weeks resulted in even greater blood pressure compared to vehicle treated SHR. This is consistent with previous studies reporting detrimental changes in kidney function along with tubular atrophy and vascular pathology after treating with 0.8 mg/g rapamycin for 2 weeks 33, 34. While hypertension has not been a reported side effect in human clinical trials using long term rapamycin treatment for immunosupression35, it should be noted that clinical use employs a lower dose of rapamycin compared to animal studies such as the present one. Another observed side effect of rapamycin treatment was the weight loss that occurred in the SHR-Rap group. While no other studies have used rapamycin to attenuate hypertrophy in genetically hypertensive models, several studies have used similar dosages of rapamycin in pressure overloaded mice and rats. Studies using mice have not observed changes in body weight after either one 21, 25 or four weeks 20 of treatment with rapamycin, while pressure overloaded rats show significant weight loss even after just three days of rapamycin24. To our knowledge the present study is the first to treat SHR with rapamycin.
It is seemingly contradictory that rapamycin treatment attenuated cardiac hypertrophy in SHR in spite of greater blood pressure, however, this underscores the importance of mTOR signaling in stimulating cardiac growth during the developmental phase of hypertrophy. Given the persistence of this pathological stimulus (hypertension) in SHR-Rap, it is also not surprising that expression of ANP and BNP, both markers of pathological cardiac hypertrophy or increased wall stress, remained increased. Therefore, it appears that the reduction in hypertrophy after rapamycin treatment is due solely to inhibition of mTOR, without fundamentally altering the pathological nature of the residual hypertrophy that develops in SHR-rap or the increase in wall stress. Given the disproportional degree of hypertrophy versus hypertension in rapamycin treated SHR, we used echocardiography to evaluate ejection fraction and fractional shortening as measures of cardiac function. We have previously reported that 16 week old SHR with cardiac hypertrophy have normal cardiac function compared to age matched WKY36. In the present study, our data indicated that both ejection fraction and fractional shortening were also similar in vehicle and rapamycin treated SHR. We conclude that short-term rapamycin treatment does not adversely affect cardiac function, however, the effect of an extended (greater than 3 weeks) period of rapamycin treatment on cardiac function remains unknown. A limitation, however, of the present study is the lack of histological analysis of hearts from rapamycin treated and untreated SHR. Histological analyses from previous studies indicate that myocyte size is increased in 14 week-old SHR 37 while cardiac fibrosis develops between 12 to 20 months of age38. However, in the present study, it is unknown to what degree rapamycin treatment may have altered cardiac structure with regard to myocyte size in 16 week-old SHR.
Perspectives
Recent studies have reported that sirolimus (rapamycin) treatment can reduce left ventricular (LV) hypertrophy in humans that is a side effect of kidney transplant39 and heart transplant40, 41. Not surprisingly these reports have led to suggestions that rapamycin may have therapeutic potential for treating LV hypertrophy in cardiac transplant patients, as well as those with other etiologies such as hypertension and myocardial infarction. However, our data indicates several factors that should be seriously considered in this regard. For example, transplant patients examined in previous studies did not have hypertension40, 41, or were under pharmacological blood pressure control39, whereas SHR used in our study were severely hypertensive. This point is noteworthy since our data indicates that rapamycin in the face of untreated hypertension may worsen the condition. Second, although we achieved more substantial reductions in cardiac hypertrophy in our study (~50% reduction in developed hypertrophy) compared to those reported in the kidney or heart transplant patients (10% and ~5% reduction in LV mass index, respectively), our rapamycin doses were much higher in SHR vs. patients (2 mg/kg i.p. / day in SHR vs. human total dose of 1 mg p.o. / day). Though our data suggests that a larger reduction of hypertrophy may be possible in clinical situations, safety and efficacy studies would be required to determine a dose of rapamycin that would strike a balance between optimal reductions in hypertrophy and reduced mortality without severe side effects. Finally, we have demonstrated in the SHR model that rapamycin markedly inhibits S6 and 4E-BP1, however, it is unclear whether this near total inhibition is actually required to attenuate cardiac hypertrophy. As such, the possibility exists that lower doses of rapamycin might be similarly efficacious in this regard. In light of this, we believe that further research is warranted to elucidate the degree of mTOR inhibition that is associated with LVH regression before any assumptions can be made as to the potential therapeutic use of rapamycin in patients with hypertrophy due to other causes such as hypertension MI.
Supplementary Material
Acknowledgments
TJ and JDS thank the Undergraduate Research Opportunities Program at the University of Utah for supporting Eric Hu.
Sources of funding
T.J. was supported by grants from the National Institutes of Health (1R15HL085226) and the University of Utah Technology Commercialization Office. J.D.S. was supported by an American Heart Association Grant in Aid (0655222Y), National Institutes of Health (1R15HL091493-01), and American Diabetes Association Research Grant (7-08-RA-164).
Footnotes
Conflicts of Interest Disclosure
None
References
- 1.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalili T, Takeishi Y, Walsh RA. Signal transduction during cardiac hypertrophy: the role of G alpha q, PLC beta I, and PKC. Cardiovasc Res. 1999;44:5–9. doi: 10.1016/s0008-6363(99)00211-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN, Yatani A, Robbins J, Dorn GW., 2nd Cardiotrophic effects of protein kinase C epsilon: analysis by in vivo modulation of PKCepsilon translocation. Circ Res. 2000;86:1173–1179. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 5.Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- 6.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 8.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. Embo J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 10.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, Takeda A, Wilhelm MJ, Scheld HH, Takeda N, Breithardt G, Levkau B. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59:390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez A, Ravassa S, Loperena I, Lopez B, Beaumont J, Querejeta R, Larman M, Diez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J Hypertens. 2007;25:2148–2157. doi: 10.1097/HJH.0b013e32828626e2. [DOI] [PubMed] [Google Scholar]
- 13.Rizzoni D, Castellano M, Porteri E, Bettoni G, Muiesan ML, Agabiti-Rosei E. Vascular structural and functional alterations before and after the development of hypertension in SHR. Am J Hypertens. 1994;7:193–200. doi: 10.1093/ajh/7.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Boluyt MO, Bing OH, Lakatta EG. The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. Eur Heart J. 1995;16(Suppl N):19–30. doi: 10.1093/eurheartj/16.suppl_n.19. [DOI] [PubMed] [Google Scholar]
- 15.Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC, Litwin SE, Symons JD. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol. 2006;47:531–541. doi: 10.1097/01.fjc.0000211746.78454.50. [DOI] [PubMed] [Google Scholar]
- 16.Symons JD, Stebbins CL, Musch TI. Interactions between angiotensin II and nitric oxide during exercise in normal and heart failure rats. J Appl Physiol. 1999;87:574–581. doi: 10.1152/jappl.1999.87.2.574. [DOI] [PubMed] [Google Scholar]
- 17.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 18.Jalili T, Takeishi Y, Song G, Ball NA, Howles G, Walsh RA. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. Am J Physiol. 1999;277:H2298–2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 19.Avelar E, Jalili T, Dong L, Arvizo J, Hu P, Litwin SE, Mattson JP. PKC translocation and ERK1/2 activation in compensated right ventricular hypertrophy secondary to chronic emphysema. BMC Physiol. 2005;5:6. doi: 10.1186/1472-6793-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens. 2006;24:1663–1670. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 21.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- 23.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 24.Boluyt MO, Li ZB, Loyd AM, Scalia AF, Cirrincione GM, Jackson RR. The mTOR/p70S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc Drugs Ther. 2004;18:257–267. doi: 10.1023/B:CARD.0000041245.61136.56. [DOI] [PubMed] [Google Scholar]
- 25.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 26.Sabri A, Steinberg SF. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem. 2003;251:97–101. [PubMed] [Google Scholar]
- 27.Roman BB, Geenen DL, Leitges M, Buttrick PM. PKC-beta is not necessary for cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H2264–2270. doi: 10.1152/ajpheart.2001.280.5.H2264. [DOI] [PubMed] [Google Scholar]
- 28.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 29.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW, 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 31.Johnsen DD, Kacimi R, Anderson BE, Thomas TA, Said S, Gerdes AM. Protein kinase C isozymes in hypertension and hypertrophy: insight from SHHF rat hearts. Mol Cell Biochem. 2005;270:63–69. doi: 10.1007/s11010-005-3781-x. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, Kihara Y. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–1385. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- 33.DiJoseph JF, Mihatsch MJ, Sehgal SN. Influence of rat strain on rapamycin’s kidney effects. Transplant Proc. 1993;25:714–715. [PubMed] [Google Scholar]
- 34.DiJoseph JF, Mihatsch MJ, Sehgal SN. Renal effects of rapamycin in the spontaneously hypertensive rat. Transpl Int. 1994;7:83–88. doi: 10.1007/BF00336467. [DOI] [PubMed] [Google Scholar]
- 35.Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, Schmidt J, Zeier M. Sirolimus in renal transplantation. Nephrol Dial Transplant. 2007;22(Suppl 8):viii61–viii65. doi: 10.1093/ndt/gfm652. [DOI] [PubMed] [Google Scholar]
- 36.Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in Spontaneously Hypertensive Rats. Journal of Nutrition. 2007;137:628–633. doi: 10.1093/jn/137.3.628. [DOI] [PubMed] [Google Scholar]
- 37.Der Sarkissian S, Marchand EL, Duguay D, Hamet P, deBlois D. Reversal of interstitial fibroblast hyperplasia via apoptosis in hypertensive rat heart with valsartan or enalapril. Cardiovasc Res. 2003;57:775–783. doi: 10.1016/s0008-6363(02)00789-7. [DOI] [PubMed] [Google Scholar]
- 38.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91:161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- 39.Paoletti E, Amidone M, Cassottana P, Gherzi M, Marsano L, Cannella G. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: a 1-year nonrandomized controlled trial. Am J Kidney Dis. 2008;52:324–330. doi: 10.1053/j.ajkd.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Kushwaha SS, Raichlin E, Sheinin Y, Kremers WK, Chandrasekaran K, Brunn GJ, Platt JL. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–2750. doi: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raichlin E, Chandrasekaran K, Kremers WK, Frantz RP, Clavell AL, Pereira NL, Rodeheffer RJ, Daly RC, McGregor CG, Edwards BS, Kushwaha SS. Sirolimus as primary immunosuppressant reduces left ventricular mass and improves diastolic function of the cardiac allograft. Transplantation. 2008;86:1395–1400. doi: 10.1097/TP.0b013e318189049a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.