Abstract
Objectives
To quantify acute myocardial retention of cardiac-derived stem cells (CDCs) and evaluate different delivery methods using Positron Emission Tomography (PET).
Background
Success of stem cell transplantation for cardiac regeneration is partially limited by low retention/engraftment of the delivered cells. A clinically applicable method for accurate quantification of cell retention would enable optimization of cell delivery.
Methods
CDCs derived from syngeneic, male Wistar Kyoto (WK) rats were labeled with 18FDG and injected intramyocardially into the ischemic region of female WK rats following permanent left coronary artery ligation.
The effects of fibrin glue, bradycardia (adenosine) and cardiac arrest were examined. 18FDG PET was performed for quantification of cell retention. Quantitative PCR for the male-specific SRY gene was performed to validate the PET results.
Results
Myocardial retention of cells suspended in PBS 1 hr after delivery was 17.6±11.5% by PCR and 17.8±7.3% by PET. When CDCs were injected immediately following induction of cardiac arrest, retention was increased to 75.6±18.6%. Adenosine slowed the ventricular rate and doubled CDC retention (35.4±5.3%). A similar increase in CDC retention was observed following epicardial application of fibrin glue at the injection site (37.5±8.2%). PCR revealed a significant increase in 3 week cell engraftment in the fibrin glue animals (22.1±18.6% vs 5.3±3.1%, for fibrin glue and PBS respectively).
Conclusions
In vivo PET permits accurate measurement of CDC retention early after intramyocardial delivery. Sealing injection sites with fibrin glue or lowering ventricular rate by adenosine may be clinically translatable methods for improving stem cell engraftment in a beating heart.
Keywords: cardiac stem cells, PET, fibrin glue, adenosine
Introduction
Stem cell transplantation is a promising new treatment modality for chronic ischemic cardiomyopathy (1). Several different types of stem and progenitor cells have been used for this purpose(2,3). Experimental and small-scale clinical studies have provided encouraging, albeit inconsistent and modest, functional improvement. There are still many issues to be resolved, related to the optimal cell type, mechanism of efficacy, timing and route of cell delivery. Low cell retention and engraftment are major obstacles to achieving a significant functional benefit irrespective of the cell type or model used (4,5).
In order to address this problem, several methods for quantification of stem cell engraftment have been used, namely direct cell radiolabeling, genetic labeling with reporter genes and real time quantitative PCR) (4–14). All these techniques have revealed that acute cell retention is <10%, after intramyocardial, intracoronary or retrograde transvenous cell delivery, emphasizing the inefficiency of the currently used methods for intracardiac stem cell transplantation (5). There is a clear need for a noninvasive, accurate and readily translatable technique for quantification of cell engraftment that would allow rapid assessment of the efficacy of any cell delivery strategy. Within this context, in vivo imaging can play a major role (15,16).
Here we used Positron Emission Tomography (PET) for in vivo quantification of retention of [18F]-fluoro-deoxy-glucose (18FDG) labeled cells after direct intramyocardial delivery, in a rat myocardial infarction model. We validated the quantitative data derived noninvasively by PET using one of the most sensitive laboratory techniques (quantitative PCR). Using PET as a tool, we investigated the role of cardiac contraction and perfusion on cell retention, and we tested various interventions to optimize cell delivery, namely a mechanical plug at the site of injection, and drugs to lower heart rate or to suppress contractility. Sealing the injection site with fibrin glue and lowering heart rate by adenosine significantly improved cell retention. Importantly, the former intervention translated into a longer-term boost of engraftment and functional improvement in the same model.
Methods
Cells
CDCs were cultured from tissue samples derived from explanted hearts from male 3-months old Wistar Kyoto (WKY) rats (Harlan, Indianapolis, Indiana, USA), as previously described (17,18). WKY are inbred, syngeneic rats, therefore appropriate for use in cell transplantation studies, without the need for immunosuppression (19,20).
In vitro 3H-FDG labeling
105 cells were incubated in glucose-free medium for 1 hr and exposed to 2µCi/ml of 3H-FDG with or without insulin (0.1 U/ml) for 30 or 60 min. Tracer uptake was measured by beta-counting.
Radiotoxicity of 18FDG
To assess toxicity of 18FDG, 1000 cells were incubated with 2µCi /ml of media 18FDG. Similar number of cells in regular media served as controls. Cell viability and proliferation were examined by a WST-8 colorimetric assay (Cell Counting Kit-8, Dojindo Molecular Technologies, MD, USA), as per manufacturer’s protocol.
Animal model
Female WKY rats (n=85 total) underwent left thoracotomy under general anesthesia and myocardial infarction was produced by permanent ligation of the left anterior descending coronary artery. CDCs (2 million, suspended in 150µl of PBS) were injected directly into the myocardium, at two sites into the infarct. Subsequently, the chest was closed and the animals were transported to the PET scanner. Animal care was in accordance to Johns Hopkins University guidelines (Details in Data Supplement).
Cell injections (Table 1)
Table 1.
Description of the study groups
| Experimental Group |
N | Protocol of cell injection |
|---|---|---|
| Cardiac arrest | 4 | Cells were injected intamyocardially after the induction of cardiac arrest |
| Lysed cells | 2 | Cells were lysed with sonication after labeling and before injection |
| Cells in PBS | 8 | Cells were suspended in PBS and injected intramyocardially |
| BDM | 6 | Cells were suspended in PBS containing 100µM of BDM to locally suppress contractility at the injection site |
| Fibrin glue | 8 | After intamyocardial cell injection, the epicardial side of the injection site was sealed by fibrin glue |
| Adenosine | 4 | Intramyocardial delivery of cells was performed during slowing of ventricular rate by IV injection of adenosine (1mg) |
| Fibrin Glue + adenosine | 8 | Cell delivery was performed during IV adenosine injection and subsequently the injection site was sealed epicardially by fibrin glue |
Two million CDCs were labeled with 18FDG immediately before injection. Subsequently, cells were pelleted by centrifugation for removal of labeling media and washed twice in PBS.
In order to explore the role of cardiac contraction and coronary blood flow on CDC retention, cardiac arrest was induced in 4 animals by intravenous thiopental injection through the tail vein. After cardiac arrest was confirmed by visual inspection, cells were injected at two sites of the left ventricle. Subsequently the chest was closed and the animals underwent PET imaging one hour after the injection.
In 8 animals, (FG group) cells were injected intramyocardially, in two sites, within the infarct border zone. While the needle tip was still in situ, one or two drops of fibrin glue (Tisseel VH-Baxter Healthcare Corp., Glendale, California) were applied directly over each injection site, in order to provide a seal and prevent backwash of the cells. In 8 animals (cells in PBS, or control), similarly-labeled cells were injected without application of fibrin glue.
To determine if transient suppression of myocardial contraction locally at the site of cell injection could improve retention, cells were resuspended in 150µl of PBS containing 100µM 2,3-butanedione-2-monoxime (BDM, Sigma-Aldrich, St.Louis, MO, USA), an excitation-contraction uncoupler(21), and then injected in the myocardium of infracted rats (n=6).
Since rats have a heart rate of 300–400bpm (22), we sought to determine if the slowing of ventricular rate by the intravenous injection of adenosine (1mg) immediately before cell delivery, would lead to an increase of acute cell retention, by improving the accuracy of the cell injection (n=4).
Additionally, in 8 animals, IV adenosine was combined with epicardial FG, to investigate any potential synergistic effect of these two interventions.
Finally, in order to investigate if radioactivity derived from dead cells could confound quantification, 2 million CDCs were radiolabeled and subsequently lysed by sonication. The lysate was then injected intramyocardially at 2 sites of the infarct border zone, in similarly infarcted Wistar Kyoto rats (n=2).
In vivo imaging
PET images were acquired on a GE VISTA (GE Healthcare, Piscataway, New Jersey, USA) small animal PET system. Features of this system have been published before (23). Details about the imaging protocol can be found on the Data Supplement.
A static PET acquisition of the syringe containing the labeled cells (5min) was obtained immediately before cell injection. After cell injection, the same syringe was imaged again (same imaging parameters), to calculate the net injected radioactivity (that corresponds to the exact cell number delivered in every animal).
After the completion of the 18FDG acquisition, a perfusion PET study using 13NH3 (ammonia) was performed for myocardial delineation (Data Supplement).
After the perfusion scan, [18F]-fluoride was injected in order to facilitate the co-registration of PET and CT images obtained with the different scanners as previously described (Data Supplement) (13). The use of micro CT for attenuation correction of micro PET images has recently been shown to be the most accurate technique for this purpose and has been applied in integrated PET/CT scanners (24).
Image analysis
All images were analyzed using AMIDE software (Data Supplement) (25).
Quantification of engraftment by real time PCR
Quantitative PCR was performed 1 hr after cell injection in 6 animals (cells in PBS group) and in 16 at 21 days after cell injection (8 of the FG and 8 of the cells in PBS group) in order to validate the results obtained by PET but also compare medium term engraftment in these groups. We injected cells isolated from male donor WK rats into the myocardium of female recipients and quantified engrafted donor cell numbers, as a function of time, by real-time PCR, using the SRY gene located on the Y chromosome as target (Data Supplement) (4).
Echocardiography
To assess global cardiac function in 39 rats [cells in PBS (n=11), FG (n=11), control group where PBS only was injected (n=9) and control group where FG was applied epicardially after PBS injection (n=8)], echocardiography was performed with the Vevo 770 system (Visualsonics, Toronto, Canada) on day 2 and 21 after the induction of myocardial infarction. The fractional area change (%FAC) was measured on the parasternal long axis view and changes from baseline (day 2 post MI) are reported.
Histology
In 6 animals (3 of the cells in PBS group and 3 of the FG group) eGFP labeled cells were injected to allow detection by immunocytochemistry (Data Supplement).
Statistical analysis
Values are reported as mean ± SD. The Student’s t-test was used to compare cell retention rates, engraftment and ejection fractions, when comparisons were performed between two independent groups. The t-test with the Welch’s correction was used when the assumption of equal variances was not satisfied. One way Analysis of variance (ANOVA) was used when the groups were three or more, and the Dunnett’s test was applied for post hoc comparisons between the baseline group and the intervention groups. P<0.05 was chosen for statistical significance.
Results
Radiolabeling of CDCs with 18FDG
Accumulation of 3FDG in CDCs was 2.2 ± 1.3% of the administered dose after 30 min of exposure and did not show any significant change after 60 min or after addition of insulin in the labeling medium (data not shown). Using 18FDG for labeling, 2 µCi/ml had no effect on cell viability and proliferation for up to 7 days after labeling (data not shown). Based on these findings, radiolabeling CDCs with a dose of 2 µCi/ml of media, for 30 min, was selected for in vivo experiments.
In vivo PET imaging
Normally-perfused myocardium was delineated by 13NH3 perfusion imaging. The infarct region appeared as a large anterolateral perfusion deficit. Injected cells were easily identified as intramyocardial bright spots by PET, localized within the infarct area and infarct border zone (Figure 1A–C). Co-registration with CT was successful in all animals (Figure 1D–F), allowing accurate in vivo quantification of cell retention.
Figure 1. Detection of 18FDG labeled cells injected in the myocardium by micro-PET.
A: transverse, B:coronal, C: sagittal image orientation. In a different experiment, fusion of CT and micro-PET images provides more detailed anatomical information. D: transverse, E:coronal, F: sagittal image orientation
Retention of intramyocardially-injected cells
When non-viable (lysed) cells were injected, %ID was 6.4±4.3%, indicating low re-uptake of the released radiolabel by the myocardium.
In the animals that received cells resuspended in plain PBS, retention 1 hour post-injection was 17.8±7.3%, a low retention rate that is in accordance with previous studies that quantified efficiency of cell delivery by ex vivo methods (4,5).
When cells were injected in the arrested heart, retention increased to 75.8±18.3% (P<0.01 vs cells in PBS). The arrested heart does not contract, nor does it sustain perfusion. Thus, cardiac contraction and/or coronary perfusion are major potential culprits in the early washout of cells from the injection site.
One way that contraction might affect cell retention is by active extrusion of the injectate during each heartbeat. We tested this notion by creating a mechanical plug with FG at the site of injection, so as to minimize backwash. In the FG group, retention was significantly increased to 37.5±8.2 % (P<0.01 vs cells in PBS), revealing a dramatic effect of sealing the injection site on cell retention.
Another approach to minimize the effects of cardiac contraction is to slow the heart rate. Adenosine injection lowered the heart rate in all animals of the group and exerted a favorable effect on cell retention (35.4±5.3%, P<0.05 vs cells in PBS). When IV adenosine was combined with FG use, retention was 39.3±11.6% (P<0.01 vs cells in PBS). The fact that no significant increase of cell retention over the levels achieved by either technique alone was observed probably indicates that mean retention rates approximately of 40% are probably the limit in our model. The difference between 40% and the ~75% seen in the arrested heart likely reflects the contribution of myocardial perfusion on cell washout from the injection site.
Yet another approach to decrease cardiac contraction is to paralyze the heart locally using a drug that uncouples excitation from contraction. Local injection of BDM along with the CDCs did not result in any improvement of cell retention (14.9±6.9%, P=ns vs cells in PBS), despite the fact that contractility was transiently suppressed by visual inspection at the injection site. The inadequacy of this intervention was probably due to its very transient nature: normal contraction resumed visually a few seconds after BDM injection, reflective of quick washout of the drug from the injection site.
Comparisons of acute cell retention between the different experimental groups are summarized in Figure 2 (One-way ANOVA, P<0.0001).
Figure 2. Retention of intramyocardially injected cells at 60 min after cell injection, in the different experimental groups.
P values correspond to comparisons between the cells in PBS and the intervention groups. Values are reported as mean±SD.
Validation of PET quantitative results by qPCR
In 6 animals of the PBS group, retention at 1hr post cell injection, measured by quantitative real time PCR, was 17.6±11.5%, almost identical to the values obtained by in vivo PET, underscoring the accuracy of quantification with this imaging modality (Figure 3).
Figure 3. Retention of intramyocardially injected cells 60 min after cell injection measured by in vivo PET and Real Time quantitative PCR.
Cells in PBS group-values are reported as mean±SD.
Longer-term engraftment
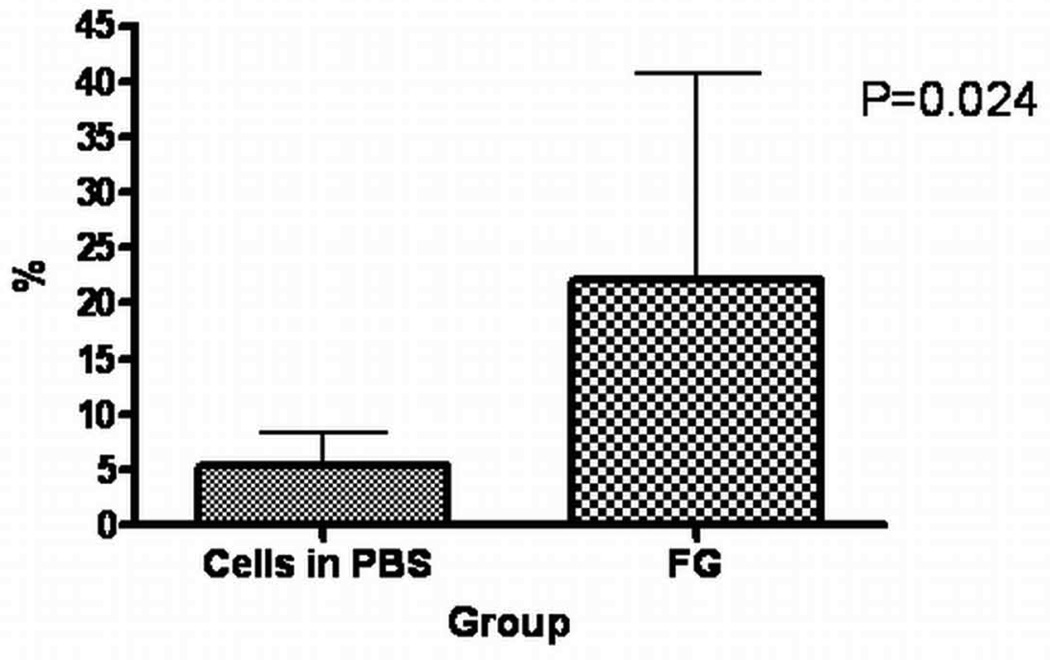
To determine if the increase of acute cell retention would have an impact on cell engraftment three weeks after cell transplantation, we compared engraftment between the cells in PBS and FG groups, using quantitative real time PCR. Indeed, in the FG group, cell engraftment was significantly augmented (22.1±18.6% vs 5.3±3.1%, P=0.039), indicating that an effective early intervention aimed at improving retention of cells in the infarcted myocardium could have important implications for the sustained presence of these cells in the heart (Figure 4). In addition, at 3 weeks, GFP+ cells were identified by immunocytochemistry in the infarct border zone of two out of three animals of FG group only, confirming their stable engraftment (Figure 5).
Figure 4. Comparison of engraftment at 3 weeks between the cells in PBS and FG groups.
Assessment by Real Time quantitative PCR-values are reported as mean±SD.
Figure 5. Immunocytochemistry of frozen cardiac sections at 3 weeks after cell injection.
The presence of eGFP+ cells (green) at the infarct border zone (red: Troponin I), in an animal of the FG group, was revealed.
Functional effect of CDC transplantation
Baseline FAC was 62±10% in the experimental animals. On day 2 after induction of myocardial infarction, FAC was 39.3±7.1%, 39.1±9.9%, 42.5±9.7% and 41.6±11% in the PBS only, FG only, cells in PBS and cells with FG groups, respectively, indicating that infarct sizes were comparable in all groups. In animals injected with vehicle only (PBS without or with FG), FAC decreased from day 2 to day 21 post MI (−25.9±23.5% and −7.6±37.2%, respectively). In contrast, in both cells in PBS and FG groups, FAC was significantly improved from day 2 to day 21, when compared to the placebo group of PBS injection only (+7.9±15.6% and 24.3±31%, P<0.05 and P<0.01 vs PBS only, respectively). Although there was a trend for greater increase in the FG group, in comparison to cells in PBS (indicating that optimizing acute cell retention and engraftment translates to a superior functional benefit, in this model), this did not reach statistical significance, probably because of the large variability (Figure 6).
Figure 6. % change in Fractional Area Change (FAC) between day 2 and 21 post-MI, in animals treated with cells in PBS, cells and FG, PBS only and FG only.
One-way ANOVA, P=0.002. P values on the figure correspond to post-hoc comparisons between the PBS only and the intervention groups. Values are reported as mean±SEM.
Discussion
Acute retention of cells delivered directly into the myocardium is low, compromising the potential of stem cell therapy for myocardial regeneration. In the present study, we applied a safe, non-toxic method for cardiac stem cell radiolabeling, we quantified cell retention by in vivo PET imaging and developed methods for significantly improving the efficiency of intramyocardial injections, using clinically approved compounds.
Many studies in the past acknowledged the problem of low engraftment as one of the main hurdles to a significant functional improvement after stem cell transplantation (4,5,11,26). In order to address this issue, an easily applicable and potentially translatable method is required for quantitative assessment of cell delivery. PET imaging is particularly attractive for this purpose, since it is non-invasive, sensitive, readily quantifiable and widely used in clinical practice.
In vivo PET imaging presents a significant advantage over the other available techniques for assessing engraftment, since it allows relative quantification of acute cell retention as a percentage of the net injected activity, thus eliminating errors resulting from cell counting or from residual cells in the dead space of the syringe. In addition, direct radiolabeling presents significant advantages over a reporter gene technique for quantification purposes, since no systemic tracer injections are needed. Thus, the background is minimal, very small numbers of cells can be detected and the dose of the radiolabel can be kept to very low levels, without sacrificing sensitivity.
Single Photon Emission Tomography (SPECT) has also been used for short term tracking of injected cells (7,9,10,12). The inherent lower sensitivity of this modality necessitates the use of relatively large doses of radioactivity to label the cells and obtain adequate images within reasonable time, raising the risks of radiotoxicity (reduced viability or proliferation rates), particularly in sensitive cell types. Several reports have reported substantial toxicity from 111-indium on labeled stem cells (7,12,27). In addition, quantification of SPECT images remains challenging, mainly due to the significant confounding effect of scattering (28).
Several groups in the past have used three-dimensional injectable scaffolds as vehicle for cell implantation and have reported improved results(29). Within this context, fibrin ‘glue’, appears as a particularly attractive option, since it has already been approved for clinical use (30–34). It consists of two components, a thrombin/CaCl2 and a fibrinogen/aprotinin solution that are mixed immediately before application. In previous reports, fibrin glue was used to facilitate delivery of endothelial cells, skeletal myoblasts or bone marrow mononuclear cells in the infracted myocardium (31,32,34). This approach led to an improved functional outcome that was attributed to a higher engraftment rate, although the latter was not documented directly by any quantitative technique. In addition, in all these studies, cells and the two components of the glue were mixed, creating an environment that may promote cell clumping or intravascular thrombus formation. In all intramyocardial injections, a fraction of the cells will be either inadvertently injected in the left ventricular cavity and end up in the microcirculation of peripheral organs or will migrate through the cardiac venous system to the right heart and eventually reach the lungs. Fibrin glue solidifies fast after application enabling the encapsulated cells to create large aggregates within the three dimensional scaffold. These aggregates have the potential to embolize, a risk that could preclude clinical application of this material for cell delivery. In addition, the risk of inducing intravascular thrombosis when the thrombin component is injected intravascularly has been recognized in clinical applications, further raising concerns about the safety of the practice of mixing the cells with the compound and injecting it in the myocardium (35,36).
In the present study, we applied the fibrin glue exclusively on the epicardium, in order to either prevent back leak of injected cells due to myocardial contraction or washout by bleeding caused by the injection trauma. We were able to confirm a significant increase in cell retention by PET, and importantly, we showed a benefit in cell engraftment at 3 weeks and a trend towards larger functional benefit. Our modified protocol maintained efficacy while minimizing potential risks, suggesting potential for future clinical applications.
Optimization of cell retention by fibrin glue should however be confirmed in more clinically-relevant models, before use in clinical trials of stem cell therapy is attempted. In larger models, the relative volume of the injectate in relation to the myocardial mass is smaller, therefore back-leak of cells after intramyocardial injection might be less pronounced and the need for sealing the injection site may be smaller.
Adenosine was also proven effective in increasing acute CDC retention in the rat model. Although cell injection during adenosine infusion could be easily attempted in the clinical setting, it is difficult to predict the impact of this approach, since heart rates are significantly lower in humans and injections are less demanding technically. Adenosine, in the doses that can be used safely in clinical applications, may lead to a significant increase in coronary blood flow, exaggerating the washout of cells from the myocardium. Adenosine has been recently reported to increase the adhesion of endothelial progenitor cells in the coronary microcirculation and increase their cardiac retention, through up-regulation of P-Selectin on endothelial cells (37). This effect is rapid and thus might have played a role in the improved CDC retention we observed with adenosine, although our initial rationale was purely to effect negative chronotropy.
Nevertheless, both fibrin glue and adenosine should be tested in large animal models that are more clinically relevant, in order to determine their true potential. PET appears to be the method of choice to reliably quantify cell retention and to evaluate these and any other cell delivery techniques.
Limitations
Despite the numerous advantages of PET, the short half life of most of the available tracers significantly diminishes the potential applications. 18FDG PET can only be used for tracking the cells within the first few hours after delivery, since this tracer has a half life of 110min. However, successful labeling of cells with the PET tracer 64Cu-PTSM has been reported (half life of 12.7hr), allowing longer term cell detection(38). In addition, PET availability remains limited to larger centers, although this situation is rapidly changing. Despite these facts, the quality and reproducibility of the results are favorable aspects of this technology for accurate quantification of acute cell retention. Finally, the impact of increased acute retention on long term engraftment and cardiac function in the adenosine group was not assessed in the present study and should be demonstrated in future experiments.
Conclusion
In vivo PET imaging is an effective, accurate, clinically translatable approach for measurement of acute cell retention after intramyocardial cell injection. It permits assessment of different delivery methods and can contribute to optimization of cellular therapies and functional benefits of transplantation. Using PET, we have compared several methods for improving intramyocardial cell delivery and shown that, sealing of the epicardial injection site with fibrin glue and reduction of ventricular rate by intravenous adenosine can significantly improve cell retention.
Supplementary Material
Acknowledgments
We would like to thank Dr R. Smith, PhD, and Dr D. Davis, MD, for their suggestions concerning histology
The work was supported by the Donald W. Reynolds Foundation, the NHLBI, the LeDucq Foundation, the WW Smith Foundation (MRA) and NIH U24 CA92871 (MP)
Abbreviations
- PET
Positron Emission Tomography
- CT
Computed Tomography
- PCR
Polymerase Chain Reaction
- 18FDG
[18F]-fluoro-deoxy-glucose
- CDC
Cardiac Derived Stem Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to disclose for other authors.
References
- 1.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima S, Varela-Carver A, Coppen SR, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–2261. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 5.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 7.Brenner W, Aicher A, Eckey T, et al. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004;45:512–518. [PubMed] [Google Scholar]
- 8.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou R, Thomas DH, Qiao H, et al. In vivo detection of stem cells grafted in infarcted rat myocardium. J Nucl Med. 2005;46:816–822. [PMC free article] [PubMed] [Google Scholar]
- 10.Goussetis E, Manginas A, Koutelou M, et al. Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279–2283. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 12.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrovitis J, Kwok KF, Lautamaki R, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengel FM, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32 Suppl 2:S404–S416. doi: 10.1007/s00259-005-1898-5. [DOI] [PubMed] [Google Scholar]
- 16.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–3383. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 17.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 18.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Martinez OM, Krams SM, Burns W, Esquivel CO. Characterization of allograft rejection in an experimental model of small intestinal transplantation. J Gastrointest Surg. 1998;2:325–332. doi: 10.1016/s1091-255x(98)80071-1. [DOI] [PubMed] [Google Scholar]
- 20.Swanger SA, Neuhuber B, Himes BT, Bakshi A, Fischer I. Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 2005;14:775–786. doi: 10.3727/000000005783982594. [DOI] [PubMed] [Google Scholar]
- 21.Backx PH, Gao WD, Azan-Backx MD, Marban E. Mechanism of force inhibition by 2,3-butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross-bridge kinetics. J Physiol. 1994;476:487–500. doi: 10.1113/jphysiol.1994.sp020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith TL, Coleman TG, Stanek KA, Murphy WR. Hemodynamic monitoring for 24 h in unanesthetized rats. Am J Physiol. 1987;253:H1335–H1341. doi: 10.1152/ajpheart.1987.253.6.H1335. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Seidel J, Tsui BM, Vaquero JJ, Pomper MG. Performance evaluation of the GE healthcare eXplore VISTA dual-ring small-animal PET scanner. J Nucl Med. 2006;47:1891–1900. [PubMed] [Google Scholar]
- 24.Chow PL, Rannou FR, Chatziioannou AF. Attenuation correction for small animal PET tomographs. Phys Med Biol. 2005;50:1837–1850. doi: 10.1088/0031-9155/50/8/014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 26.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Kong H, Stodilka RZ, et al. Determining the minimum number of detectable cardiac-transplanted 111In-tropolone-labelled bone-marrow-derived mesenchymal stem cells by SPECT. Phys Med Biol. 2005;50:4445–4455. doi: 10.1088/0031-9155/50/19/001. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi H, Koral KF. Scatter modelling and compensation in emission tomography. Eur J Nucl Med Mol Imaging. 2004;31:761–782. doi: 10.1007/s00259-004-1495-z. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, El Haj AJ. Biodegradable scaffolds--delivery systems for cell therapies. Expert Opin Biol Ther. 2006;6:485–498. doi: 10.1517/14712598.6.5.485. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 31.Chekanov V, Akhtar M, Tchekanov G, et al. Transplantation of autologous endothelial cells induces angiogenesis. Pacing Clin Electrophysiol. 2003;26:496–499. doi: 10.1046/j.1460-9592.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ, et al. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 33.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 34.Ryu JH, Kim IK, Cho SW, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–326. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 35.Goerler H, Oppelt P, Abel U, Haverich A. Safety of the use of Tissucol Duo S in cardiovascular surgery: retrospective analysis of 2149 patients after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2007;32:560–566. doi: 10.1016/j.ejcts.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 36.Lamm P, Adelhard K, Juchem G, et al. Fibrin glue in coronary artery bypass grafting operations: casting out the Devil with Beelzebub? Eur J Cardiothorac Surg. 2007;32:567–572. doi: 10.1016/j.ejcts.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Ryzhov S, Solenkova NV, Goldstein AE, et al. Adenosine receptor-mediated adhesion of endothelial progenitors to cardiac microvascular endothelial cells. Circ Res. 2008;102:356–363. doi: 10.1161/CIRCRESAHA.107.158147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adonai N, Nguyen KN, Walsh J, et al. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci U S A. 2002;99:3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.