Abstract
Specification of the appropriate neurotransmitter is a crucial step in neuronal differentiation because it enables signaling among populations of neurons. Experimental manipulations demonstrate that both autonomous and activity-dependent genetic programs contribute to this process during development, but whether natural environmental stimuli specify transmitter expression in a neuronal population is unknown. We investigated neurons of the ventral suprachiasmatic nucleus that regulate neuroendocrine pituitary function in response to light in teleosts, amphibia and in primates. We find that altering light exposure, which changes the sensory input to the circuit controlling adaptation of skin pigmentation to background, changes the number of neurons expressing dopamine in amphibian larvae in a circuit-specific and activity-dependent manner. Newly dopaminergic neurons then regulate changes in camouflage coloration in response to illumination. Thus physiological activity alters the numbers of behaviorally relevant aminergic neurons in the brain at postembryonic stages of development. The results may be pertinent to changes in cognitive states that are regulated by biogenic amines.
Keywords: dopaminergic neurons, neurotransmitter respecification, sensory stimulation, activity-dependent plasticity, ventral suprachiasmatic nucleus, retinohypothalamic projection, background adaptation
Electrical activity plays a key role in neurotransmitter specification1–4 and postsynaptic receptor expression5–7 in differentiating neurons. Moreover, postsynaptic receptors can be selected to match the transmitters expressed presynaptically8,9. L-type calcium channels have been implicated in the developmental expression of dopamine, because a loss-of-function mutation in tottering mutant mice10 leads to abnormal expression of tyrosine hydroxylase (TH), the rate-limiting dopamine-synthetic enzyme11. TH is ectopically co-expressed with GABA in cerebellar Purkinje neurons in these mutants. These studies led us to hypothesize that activity driven by environmental stimuli will cause neurons to express an additional transmitter, without changing the number of neurons or affecting their original transmitter phenotype. The neurons would be able to perform a new function in addition to their default role. Hijacking activity-dependent mechanisms regulating transmitter expression could be a useful step toward the development of clinical treatments that drive transmitter specification in selected classes of neurons.
Here we have examined the role of illumination in the specification of dopamine in neurons of the ventral suprachiasmatic nucleus (VSC) of Xenopus laevis. This is an attractive system to investigate during both pre- and post-embryonic development because of the small number of dopaminergic (DA) neurons, the clearly described retinohypothalamic circuitry to which they belong, and their well characterized inhibition of melanotrope cells that control skin pigmentation12,13. The neuroanatomy and behavior of the background adaptation circuit have been established in adult Xenopus12–15. We describe background adaptation in larvae and show that suppressing or enhancing electrical activity by misexpressing ion channels during embryonic development leads to widespread changes in the number of TH-expressing neurons in the brain. In contrast, changes in activation of the retinohypothalamic projection at larval stages of development alter the number of TH-expressing inhibitory neurons specifically in the VSC. We demonstrate that enhancing activity by brief light exposure stimulates a subclass of VSC neurons, characterized by neuropeptide Y (NPY) transmitter and Lim1,2 transcription factor expression, to acquire the additional DA phenotype. We find that these newly TH/NPY neurons retain their physiological target, the melanotrope cells, and drive dopamine-dependent, behaviorally appropriate changes in skin pigmentation.
Dopaminergic neurons regulate pigmentation
The regulation of pigmentation is a well conserved behavior among vertebrate species. Mammals undergo gradual changes in skin color under hormonal control16; many lower vertebrates including teleosts17 and amphibians18 display rapid physiological color changes in response to the same hormones. This behavior is mediated by simple neuronal circuitry in amphibia: glutamatergic retinal ganglion cells project to dopaminergic suprachiasmatic melanotrope inhibitory neurons (SMINs), which innervate cholinergic melanocyte-stimulating-hormone (MSH) releasing cells (melanotrope cells, MCs) (Fig. 1a)13,18. We find that epithelial pigmentation of amphibian larvae is regulated by the level of ambient illumination, either by incident light or by background light level, which leads to aggregation and dispersion of melanin granules within melanocytes19 as in teleosts. Pigmentation appears lighter with bright illumination or a white background and darker with dim illumination or a black background (Fig. 1b; Supplementary Fig. 1) after as little as 10 min exposure, and light on white background is most effective in inducing a decrease. The same circuit controls this behavior in the adult13,20.
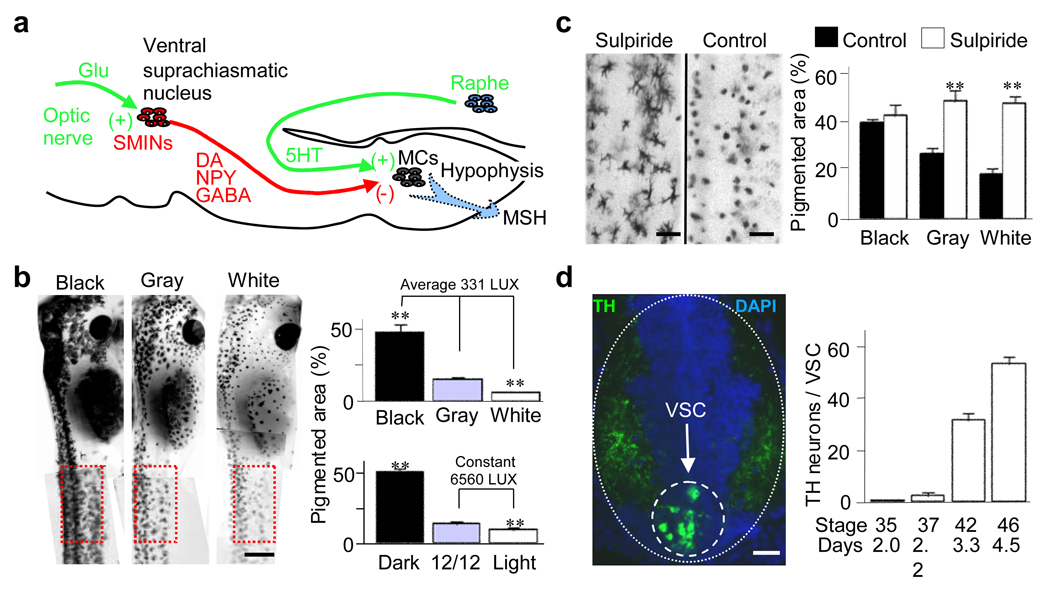
Figure 1.
Dopaminergic VSC neurons regulate skin pigmentation. a, The diagram illustrates the neuronal circuit controlling this behavior. Glu, glutamate; SMINs, suprachiasmatic melanotrope inhibitory neurons; MC, melanotrope cells; MSH, melanocyte stimulating hormone. b, Raising animals in different levels of light changes pigmentation. A constant level of incident light was tested with different backgrounds and different levels of incident light were tested on a gray background. Illumination changes pigmentation: left, phenotypes on different backgrounds; right, pigmentation adaptation assayed in boxed regions at left. **, significantly different from intermediate illumination, p<0.001. N≥6 St 42 larvae for each condition. c, Activity of DA SMINs is necessary for regulation of pigmentation: left, 2 hr white adaptation in a larva treated with 10 nM sulpiride for 30 min compared to control; right, pigmentation at various background light levels in sulpiride-treated larvae compared to controls. **, significantly different, p<0.001. N≥6 St 42 larvae raised in the dark for each condition. d, The number of VSC neurons increases during development of larvae raised 12 hr light/12 hr dark on a gray background: left, transverse section through the St 42 diencephalon (dotted oval) illustrating the VSC (dashed circle); right, appearance of TH neurons during development. N≥6 larvae for each stage. Scale bars: b, 1 mm; c, 200 µm; d, 50 µm.
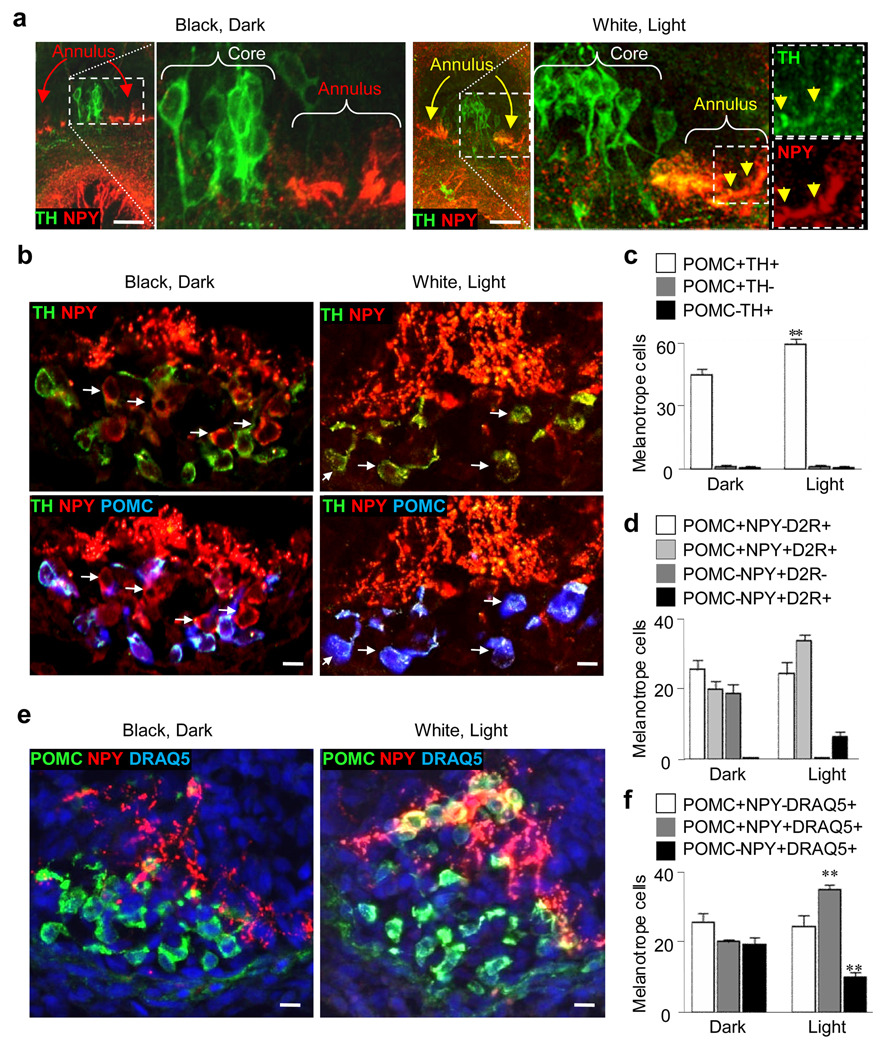
To identify the contribution of DA neurons to regulation of pigmentation we exposed stage 42 larvae for 30 min to 10 nM sulpiride, a dopamine D2 receptor antagonist, because coexpressed GABA and NPY can contribute to regulation of this behavior12,14. Sulpiride treatment blocks changes in skin pigmentation in response to altered illumination (Fig. 1c; Supplementary Fig. 2a,c), demonstrating the predominant role of DA neurons in this pathway in Xenopus larvae. The number of DA VSC neurons does not change in sulpiride-treated larvae versus controls raised at various illumination and background conditions (Supplementary Fig. 2b), excluding an indirect effect of sulpiride on the number of TH neurons of the VSC. Immunostaining for proopiomelanocortin (POMC) and NPY in combination with the nuclear marker DRAQ5 reveals that the number of POMC melanotrope cells is also unchanged (Supplementary Fig. 2d–e).
Because the modulatory actions of dopamine are complex21–23, we tested the blanching effect of a D2 receptor agonist, quinpirole hydrochloride, on dark-adapted larvae remaining in the dark. Blanching persists to the same extent after bilateral eye enucleation in the dark (Supplementary Fig. 3a–b), suggesting that D2 receptor activation in the retina is not involved in regulating adaptation to background. Other DA nuclei do not participate in this circuit. The D1 receptor agonist and antagonist, SKF 38393 and SCH23390, have the opposite effect on this behavior, darkening and lightening pigmentation, respectively (Supplementary Fig. 3c–d). The pineal gland, a light sensor controlling circadian oscillation of melatonin that initiates slow changes in pigmentation, is not involved in rapid background adaptation24. These results demonstrate participation of DA VSC neurons in the pathway controlling this camouflage behavior.
To understand their developmental origins we evaluated the time course of appearance of TH VSC neurons (Fig. 1d). They are first detected at 2 days of development (stage 37), and by 3 days (stage 42) they form a nucleus with a core that ranges from 10 to 30 neurons depending on the illumination conditions in which the larvae are raised. Lim1,2 and Pax6 transcription factors are useful markers for embryonic diencephalic DA neurons. VSC neurons are recognized by their expression of the Lim1,2 transcription factor and the absence of expression of Pax6 during the first 4 days of development25,26 (Fig. 2a,b control).
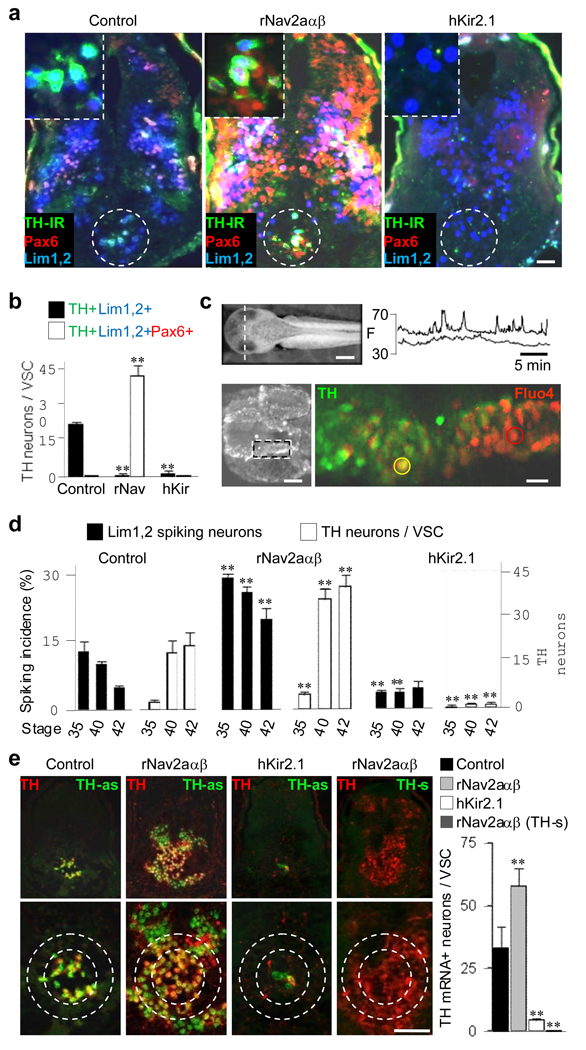
Figure 2.
Dopaminergic differentiation is activity-dependent. a, Molecular markers identify VSC neurons (dashed circles) in transverse sections in a control larva and in larvae following sodium or potassium channel overexpression (St 42). The VSC is shown at higher magnification in insets (top left). b, Altering spike activity drives proportional changes in the number of TH neurons. Quantification of data presented in (a). N≥6 larvae for each condition. **, significantly different from control, p<0.001. c, Neurons generate Ca spike activity in the developing brain: left, transverse section through the hypothalamus (dashed line) at stage 35 yields frontal view showing the Fluo-4AM-loaded tissue, below; confocal imaging, postfixation and staining are from the area in the inset. Right, digitized fluorescence (F) of representative cells circled in the panel below; traces are Ca spike activity recorded from dorsal (top trace, red circle) TH- and ventral (bottom trace, yellow circle) TH+ cells. N=3 larvae.d, Across developmental stages the number of TH neurons is inversely proportional to the incidence of spiking in Lim1,2 neurons in controls and following alterations of spike activity. **, significantly different from control, p<0.001. e, TH mRNA expression is altered by ion channel overexpression. VSC double labeling with TH antibodies and TH-antisense probe (TH-as) in transverse brain sections (top row) and enlargements of the VSC (dashed circles, bottom row) in larvae injected with cascade blue (left panel, control), overexpressing sodium channels (second panel), or potassium channels (third panel). Antisense probe binding in sodium channel-overexpressing larvae (probe-s, fourth panel). Quantification of the results of channel overexpression is shown at right. **, significantly different from control, p<0.001. a–e, Larvae were raised on a 12/12 day/night cycle on a gray background. N≥6 larvae for each condition. Scale bars: a, 50 µm; c, 500 µm (left), 30 µm (right); e, 100 µm.
Activity-dependence of dopaminergic differentiation
To discover whether the temporal and spatial parameters of activity have a role in selective neuronal recruitment for neurotransmitter respecification, we compared the effect of global changes of activity throughout the CNS at early developmental stages with the result of changes in circuit-specific activation later after synapses are formed. To test the general activity-dependence of dopamine expression, we investigated the effect of ion channel overexpression on the number of DA neurons. We first confocally imaged intracellular calcium with Fluo-4AM to determine whether neurons in the brain generate spontaneous calcium spikes. Neurons in the developing hypothalamus exhibit calcium spikes during stages 35–42 that are similar to those in the posterior neural tube3; fixing and immunostaining these preparations identifies inactive neurons that express TH (Fig. 2c; Supplementary Fig. 4 and Supplementary Fig. 5). These results suggest that, as in the spinal cord, calcium spikes precede the appearance of the transmitter, and raise the possibility that here too calcium spike activity regulates transmitter expression.
To test this hypothesis we overexpressed inward rectifier potassium channels (hKir2.1) and voltage-gated sodium channels (rNav2aαβ) in separate experiments to suppress or enhance calcium spike generation, injecting transcripts into both blastomeres at the two cell stage3. Increasing activity increases the number of TH neurons in and around the VSC, while suppressing activity decreases the number of TH neurons (Fig. 2a,b). Similar changes are observed for the dorsolateral suprachiasmatic nucleus (data not shown). Changes in the incidence of calcium spiking (% neurons spiking/hr) during development are inversely correlated with changes in TH expression, establishing a quantitative connection between the two (Fig. 2d). Increasing activity also induces expression of Pax6, but not Lim1,2 (data not shown), and newly TH neurons are recruited from the large Pax6 and stable Lim1,2 pool of cells (Fig. 2a,b). Increasing calcium spike generation leads to widespread expression of TH mRNA in cells not normally expressing it, assessed by in situ hybridization; decreasing calcium spike generation leads to a decrease in the number of cells expressing these transcripts (Fig. 2e). Thus the changes in DA phenotype are regulated transcriptionally. These findings suggest that spontaneous calcium spike activity controls the number of VSC DA neurons in order to homeostatically regulate circuit function, because these neurons inhibit melanotrope cells14, while global ion channel misexpression has widespread effects that seem less specific.
Light-dependence of dopamine expression
To determine whether newly DA neurons can be selectively recruited in specific circuits we investigated the effect of activity of the retinohypothalamic projection on neurotransmitter expression of VSC neurons that already appear to project to appropriate targets. Remarkably, raising animals in different levels of illumination and background changes the number of TH VSC neurons (Fig. 3a), and light on white background is most effective in inducing an increase. Exposure of dark-raised animals to this illumination protocol for as little as 2 hr leads to appearance of TH neurons in the annular region surrounding the core (Fig. 3b). Immunostaining neurons with antibodies to dopamine (DA), the dopamine transporter (DAT) and vesicular monoamine transporter (VMAT2), we find that they are coregulated with TH: all four markers are undetected in annular neurons of larvae kept in the dark and expressed in the NPY annular neurons of larvae that are exposed to 2 hr light (Supplementary Fig. 6 – Supplementary Fig. 8). During the respecification process these annular DA neurons are immunoreactive for NPY and for Lim1,2 but not Pax6 (Fig. 3b–e; Supplementary Fig. 9). Thus the more restricted physiological stimulus, in contrast to ubiquitous ion channel overexpression, recruits newly DA neurons from a restricted population. The absence of Pax6 induction may be due to the difference in the extent of altered activity. Increasing light exposure leads to the appearance of TH mRNA in annular NPY neurons. This transmitter respecification is reversible; decreasing light exposure reduces the extent of expression of TH mRNA in neurons in both the annulus and the core of the VSC (Fig. 3f), and TH protein is no longer detected in 90% of TH annular neurons when larvae are kept in the dark for >2 hr (Supplementary Fig. 10).
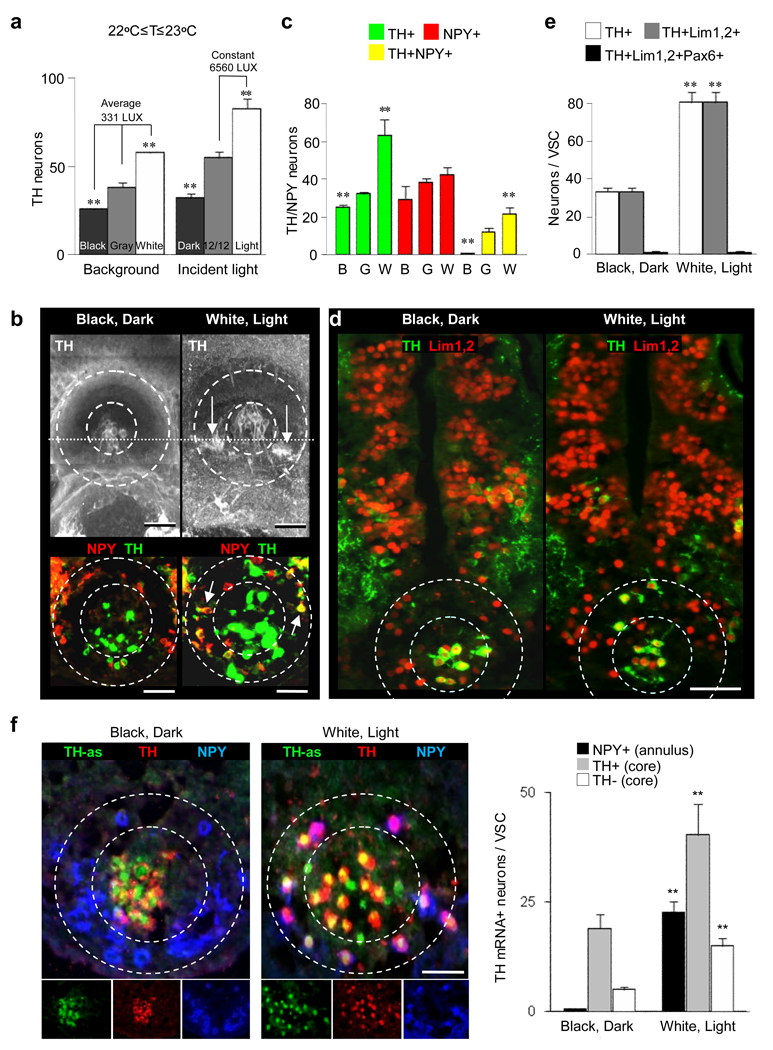
Figure 3.
Illumination changes the numbers of dopaminergic neurons selectively in the VSC. a, Raising animals in different levels of light changes the number of TH neurons in the VSC. and A constant level of incident light was tested with different backgrounds and different levels of incident light were tested on a gray background. **, indicates significantly different from intermediate illumination, p<0.001. N≥9 St 42 larvae for each condition. b, TH viewed in wholemounts from above (top) and in transverse sections (bottom) shows the core (dashed inner circle) and the annular DA neurons (arrows, between dashed circles) in 2 hr black- and white-adapted St 42 larvae. Dotted line in wholemounts indicates section orientation. c, Two hr exposure of larvae to different background illumination (B, black; G, gray; W, white) changes the number of TH/NPY neurons in the VSC (core + annulus). ** indicates significantly different from gray background, p<0.001. d, Core DA neurons and newly recruited annular DA neurons from 2 hr white-adapted larvae are both from the Lim1,2 pool; sections as in (b). e, Exposure to different background illumination for 2 hr changes the number of Lim1,2 neurons expressing TH. ** indicates significantly different from dark rearing, p<0.001. f, TH mRNA expression is altered by light or dark adaptation. Triple labeling of transverse VSC sections (dashed circles) with TH, NPY and TH-as probe in 2 hr dark-, black-adapted and 2 hr light-, white-adapted larvae. Merged images (top row) and separate channels (bottom row) are shown. Quantification of the results of differential illumination is presented at right. **, value following light adaptation is significantly different from value following dark adaptation, p<0.001. b–f, St 42 larvae raised in the dark. c,e,f, N≥6 larvae for each condition. Scale bars: b, 100 µm (top), 60 µm (bottom); d, 100 µm; f, 60 µm.
Because the NPY annular neurons in the VSC of dark-adapted larvae are reported to share similar functional properties with the DA core neurons, both innervating the melanotrope cells13 and inhibiting MSH production for long periods14, their light-dependent acquisition of the TH phenotype produces a new set of DA neurons that could regulate pigmentation. There is no change in the total number of DAPI or Lim1,2 stained nuclei, implying that neither cell proliferation nor migration account for the observed change in the total number of DA neurons (Supplementary Fig. 11a,b). BrdU labeling and TUNEL assays do not reveal apoptosis or cell birth in these cells (Supplementary Fig. 11c,d). However, the number of POMC melanotrope cells also increases following light exposure and decreases in the dark, associated with changes in intensity of POMC staining, while the total number of DRAQ5-positive melanotrope cells remain constant. Similar light-dependent accumulation of POMC in melanotrope cells occurs in white background-adapted adult frogs27.
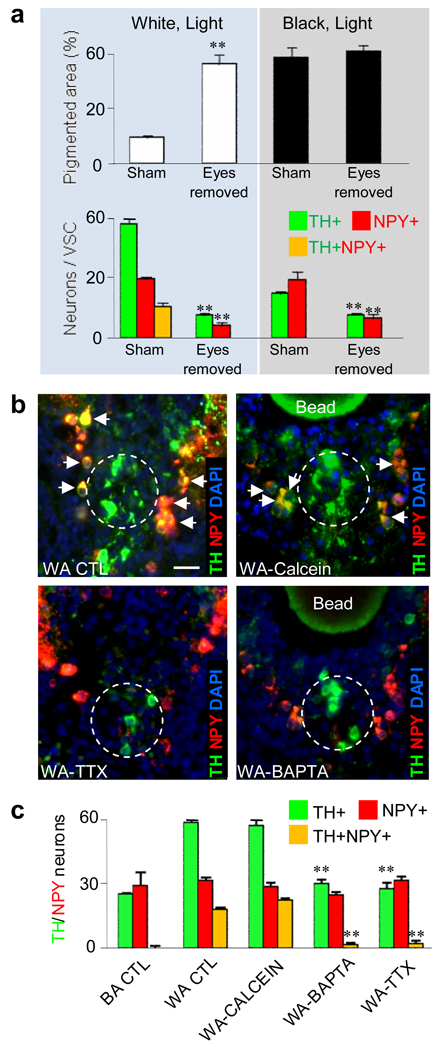
Suppressing activity eliminates dopamine expression elicited by light. Binocular eye enucleation that eliminates retinal input abolishes both white background adaptation and light-induced changes in the number of TH neurons (Fig. 4a). We then used a pharmacological approach to test the involvement of electrical activity and calcium signaling. We first implanted 80 µm agarose beads loaded with tracer in the roof of the hypothalamus above the VSC to evaluate the time course of drug delivery. Twenty-four hr after implantation, the spread of calcein-AM from the bead is localized within a radius of ∼100 µm that includes the VSC (Supplementary Fig. 12). Larvae implanted with beads loaded with the sodium channel blocker tetrodotoxin or with the calcium buffer BAPTA-AM (similar in molecular weight and diffusion profile to calcein-AM) show no increase in numbers of either TH neurons or NPY/TH annular neurons in the VSC following 2 hr illumination in white light after 24 hr in the dark (Fig. 4b,c).
Figure 4.
Blocking physiological activity blocks illumination-dependent changes in numbers of TH VSC neurons. a, Binocular eye enucleation abolishes white adaptation of skin pigmentation and background illumination-dependent changes in numbers of DA neurons. **, significantly different relative to sham operation, p<0.001. b, Implanted beads delivering activity blockers prevent appearance of TH/NPY neurons in larvae exposed to 2 hr light relative to wild type or diffusion marker controls. Transverse sections through the VSC (dashed circles) show NPY/TH colocalization (yellow, arrows) in white-adapted wild type (WA WT) and calcein bead-implanted larvae that is absent in TTX and BAPTA-bead implanted larvae. Scale bar: 40 µm. c, TH and NPY identify VSC neurons in WA WT and BA WT (black-adapted wild type) controls and following suppression of activity. **, significantly different relative to calcein-bead implanted larvae, p<0.001. a,c, N≥6 St 42 larvae raised in the dark for each condition.
Functional significance of illumination-induced dopaminergic neurons
To assess the behavioral potential of newly DA neurons we determined whether they project to appropriate targets. Following adaptation in the light for a minimum of 2 hr, annular neurons, identified by Lim1,2 and NPY expression, co-express TH in their somata and axonal projections to the melanotrope cells (Fig. 5a; Supplementary Fig. 13). Localization of TH/NPY immunoreactivity adjacent to melanotrope cells, identified by staining for POMC28, shows that NPY terminals are present in dark-adapted larvae and become TH in white-adapted larvae (Fig. 5b). Interestingly, POMC is not detected in a subpopulation of melanotrope cells that receive only NPY terminals, but appears following white adaptation when closely apposed nerve terminals become both NPY and TH (Fig. 5b,c). Moreover, D2 receptors are not observed at NPY terminals in dark-adapted larvae but are evident at TH/NPY terminals of white-adapted larvae (Fig. 5d and Supplementary Fig. 14). These results suggest that the inhibitory output from the increased number of TH-expressing neurons in the VSC stimulates POMC storage in a larger number of melanotrope cells (Fig. 5e,f) and in turn reduces MSH release, which may account for accumulation of POMC in the melanotrope cells of white background-adapted adult frogs27.
Figure 5.
NPY neurons projecting to melanotrope cells express TH following illumination of larvae. a, Left, TH is absent in NPY annular neurons (red arrows) in transverse sections of larvae dark-adapted for 2 hr. Right, TH is present in somata (yellow arrows) and axons (yellow arrowheads) of NPY annular neurons in 2 hr white- and light-adapted larvae; color separation of green TH and red NPY (insets far right) facilitates visualization of double labeling of axons. b, Left, TH nerve terminals invest POMC-stained melanotrope cells in larvae dark-adapted for 2 hr, and terminals that are NPY and not TH project to melanotrope cells in which POMC is not detected (arrows). Right, TH/NPY terminals project to POMC melanotrope cells (arrows) in 2 hr white-adapted larvae. c, Quantification of the changes illustrated in (b). **, significantly different from value from dark-adapted larvae, p<0.001. d, D2 receptors are expressed in melanotrope cells of larvae light-adapted for 2 hr that newly express or may be acquiring POMC (light gray and black bars). e, DRAQ5-labeled nuclei identify 2 hr dark/light-dependent changes in melanotrope POMC expression f, Quantification of the changes illustrated in (e). **, significantly different from dark-adapted values, p<0.001. a–f, larvae raised in the dark. c,d,f, N≥6 stage 42 larvae for each condition. Scale bars: a, 100 µm; b, e, 40 µm.
To determine whether the larger population of DA neurons enhances light sensitivity of changes in pigmentation, we compared white-adaptation of larvae raised in the dark or in the light. These larvae were black background-adapted for 30 min to achieve the same starting level of pigmentation before being tested. Larvae raised in the light, which have more core TH neurons as well as TH/NPY annular neurons, white-adapt more rapidly and to a greater extent than larvae raised in the dark, which have only core TH neurons. (Supplementary Fig. 15). Prolonged white adaptation causes increases in the number of both core and annular TH neurons, with a slower time course than the initial changes in pigmentation (Supplementary Fig. 16). These results suggest that transmitter respecification provides a behavioral advantage. In contrast, dark adaptation of larvae with DA annular and core neurons placed on a dark background for 30 min occurs at the same rate as in larvae with only DA core neurons (Supplementary Fig. 17). This result is consistent with dark adaptation in adult frogs, which occurs in the absence of TH VSC inhibition of the MC and via a different circuit involving the activity of serotonergic raphe neurons14,18. These dynamics contribute to plasticity at the neuroendocrine-melanotrope interface29,30.
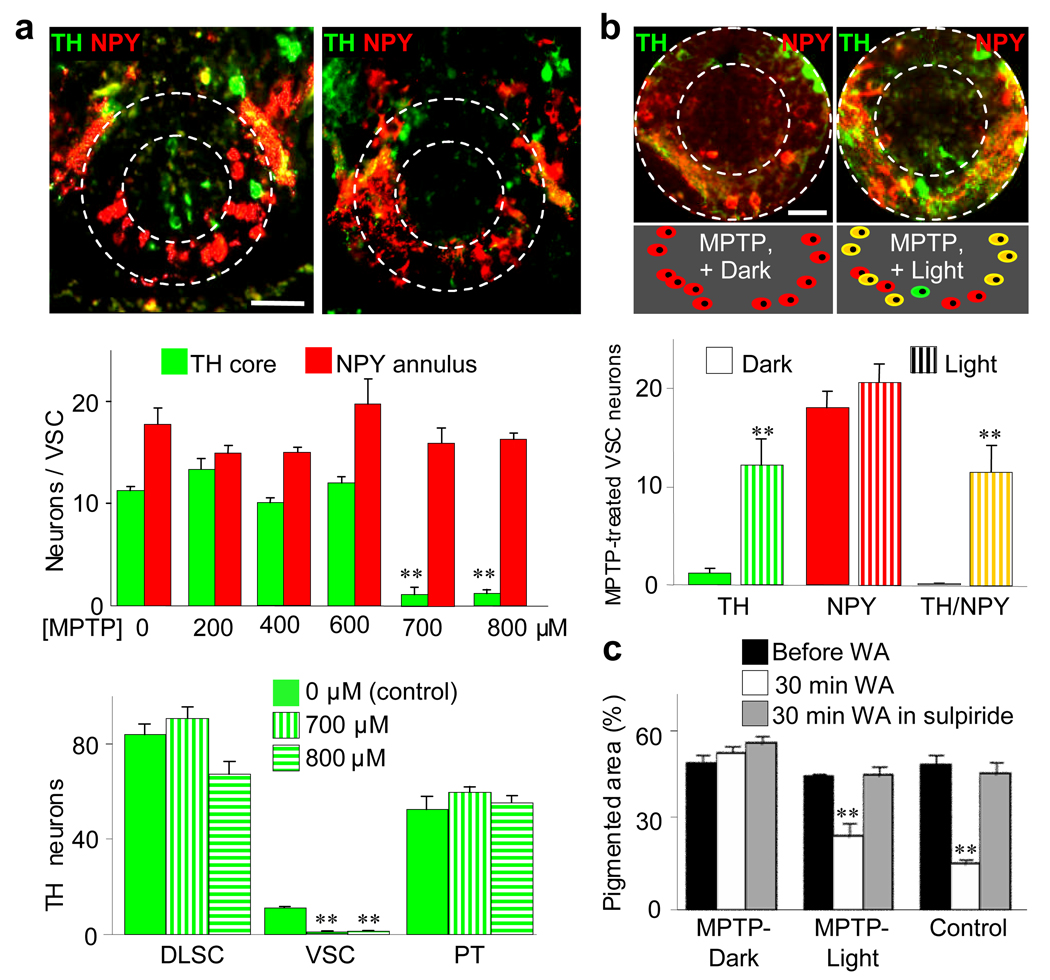
To investigate the independent function of these newly DA neurons we eliminated the core DA neurons. Testing a range of concentrations of the dopaminergic toxin, MPTP31,32, we found that 700 µM MPTP for 8 hr selectively kills the core VSC neurons while sparing other DA nuclei (Fig. 6a). Since the number of TH neurons in the VSC remains constant for the first 40 min of light adaptation (Supplementary Fig. 16), we evaluated dark-raised, MPTP-treated larvae after 30 min exposure to light, and found that this treatment abolishes changes in pigmentation (Fig. 6b,c, left).
Figure 6.
Newly DA neurons regulate pigmentation. a, MPTP selectively eliminates the core VSC neurons. Top, TH/NPY in transverse sections before (left) and after (right) 8 hr incubation of larvae in 700 µM MPTP in the dark. Middle, the effect of different MPTP concentrations on the number of TH/NPY VSC neurons. Bottom, 700 µM MPTP eliminates VSC but not dorsolateral suprachiasmatic nucleus (DLSC) and posterior tuberculum (PT) DA nuclei. **, significantly different from 0 µM MPTP, p<0.001. b, Top, following MPTP treatment TH is largely absent from NPY annular neurons after dark-adaptation (left), and abundant following 2 hr light-adaptation (right). Middle, scheme illustrating transmitter expression in annular neurons in these conditions. Bottom, quantification of induction of TH; **, significantly different from values from dark adapted larvae, p<0.001. a,b, N>6 St 42 larvae raised in the dark. Scale bars: 50 µm. c, Illumination-dependent changes in skin pigmentation are rescued when MPTP treatment is followed by 2 hr exposure to light. Pigmentation of three groups of larvae raised in the dark: 1) MPTP-treated in the dark and kept in the dark; 2) MPTP-treated in the dark followed by 2 hr exposure to light; or 3) MPTP-untreated followed by 2 hr exposure to light (control). Pigmentation was measured before (black bars) or after (white bars) 30 min of white adaptation (WA), or after WA in the presence of sulpiride (gray bars). 3 left bars: MPTP followed by 2 hr incubation in the dark abolishes the change in pigmentation in response to 30 min white background adaptation. 3 center bars: MPTP followed by 2 hr incubation in the light recovers most of the change in pigmentation in response to a 30 min exposure to white background, relative to control (3 right bars). Sulpiride (10 nM) during 30 min exposure to white background blocks the reduction in pigmented area (gray bars, center and right groups). **, significantly different from value prior to white adaptation, p<0.001. N≥10 St 42 larvae.
To determine the behavioral role of newly DA neurons we exposed animals previously treated with MPTP and lacking VSC core neurons to a 2 hr period of illumination in the absence of MPTP and the presence of deprenyl, a MAO inhibitor that blocks the production of MPP+, the active form of MPTP. This protocol again leads to induction of TH/NPY annular neurons in the VSC (Fig. 6b), consistent with recruitment from the NPY population. Strikingly, these neurons alone drive changes in pigmentation in response to light. A high level of ambient illumination leads to aggregation of melanin granules in melanocytes and a low level of illumination leads to their dispersion, as in controls (Fig. 6c). These changes in pigmentation are blocked by exposure to sulpiride, identifying the contribution of DA neurons.
Discussion
Our results indicate that physiological levels of environmental illumination dynamically regulate the number of DA VSC neurons innervating hypothalamic melanotrope cells that control pigmentation. Appropriate postsynaptic receptors are regulated in parallel, as observed during transmitter respecification in the peripheral nervous system8,9. The recruitment of additional DA neurons is activity- and calcium-dependent and derived from a subpopulation of neurons that display a characteristic molecular signature and already project to a relevant target. When the retinohypothalamic projection is selectively activated, the DA synthesis and transport machinery is acquired by annular NPY neurons that surround the TH core of the VSC nucleus. As previously observed in GABA/TH-expressing neurons of tottering mutant mice11, respecification of the TH phenotype occurs without influencing expression of the default original transmitter (NPY) in the annular neurons, and seems to follow a homeostatic rule. The number of inhibitory dopaminergic neurons increases following enhanced circuit activation due to higher light exposure, increasing inhibitory input to melanotrope cells and decreasing pigmentation. Conversely, dark-adapted animals respond to lower light-induced activation of this circuit by reducing the number of DA neurons; decreased inhibitory input to the melanotrope cells then boosts pigmentation. This form of plasticity evoked by sensory stimulation parallels use-dependent changes in neuropeptide expression in the hypothalamus33–35 and activity-dependent alterations in cortical receptive fields in response to alteration of sensory experience36.
We propose a model in which the neurons innervating a class of target neurons express different transmitters but belong to a constellation of cells whose transmitter phenotype can be expanded (by co-expression) or reduced (by elimination), depending on the physiological requirements of the regulated behavior. Global changes in activity caused by ion channel overexpression throughout the brain drive ectopic TH expression, but this includes neurons that do not express NPY- and lim1,2 and do not innervate melanotrope cells. These findings suggest a general role of activity in homeostatic specification of monoamines expressed by CNS neurons. Importantly, our results demonstrate that ectopic neurotransmitter expression can be functionally significant when activity is manipulated by activation of the neural circuit controlling a specific behavior.
Bright light therapy has been used for more than 20 years to treat seasonal affective disorder (SAD), a form of major depression characterized by recurrent seasonal episodes37. Typically a fall/winter onset is followed by full remission of symptoms or switch into mania during spring/summer. Exposure to bright artificial light, termed phototherapy, a treatment currently recommended in clinical guidelines for SAD38, is as effective as antidepressant medications. Dysfunction of dopaminergic signaling appears to be an important element of SAD, because depressed symptoms are induced in SAD patients during summer remission by pharmacologically inhibiting TH39. Although it has been shown in primates as in amphibians that retinal ganglion cells project monosynaptically to the suprachiasmatic nucleus and DA neurons in the hypothalamus16, the mechanism through which light therapy exerts its effect is not known. Our findings link light exposure to neurotransmitter respecification and neuroendocrine function.
Signaling molecules potentially implicated in this form of plasticity include AMPA/KA glutamate receptors involved in the light-dependent excitatory response of DA suprachiasmatic neurons40 and BDNF, FGF and microRNAs that have roles in establishing the proper number of DA neurons41–44. The present study demonstrates that physiological stimuli can respecify neurotransmitter expression and functional output of selective neuronal networks by harnessing circuit activity: the nervous system then identifies the right molecules to express, and delivers them at the right time, to the right place and in the right dose. This approach could guide future research to discover novel brain stimulation methods tuned to activate selected neuronal circuits, aimed at preventing or slowing the progression of cognitive and neurodegenerative disorders prior to discovery of the activity-dependent molecular mechanisms involved.
Methods Summary
Illumination experiments
We determined the optimal condition for altering skin pigmentation and numbers of TH neurons by raising larvae under two illumination conditions. In the first, larvae were placed on black, gray or white backgrounds (corresponding to N 2.25, N 7.25, or N 9.5 on the Munsell neutral value scale of a monochromatic color wheel) with constant illumination (331 lux). In the second, larvae on a white, gray or black background were exposed to constant illumination (6,560 lux, measured with a light meter (Sper Scientific) – equivalent to shady illumination on a sunny day), or to constant dark or to 12 hr light/12 hr dark. Light on white background vs dark proved most effective. Subsequently, when testing the effect of light on pigmentation or the numbers of TH neurons, animals were usually raised in the dark (stages 35–42) and exposed (typically for 2 hr at stage 42) to these optimal conditions. For some tests of the functional significance of newly TH neurons, animals were raised under different light/background conditions and a conditioning step of 30 min light/white background exposure was imposed to achieve similar levels of skin pigmentation before comparing light or dark adaptation between these groups.
Neuropharmacology
In vivo drug application was achieved with agarose beads3, implanted 100–200 µm into the brain of stage 41 larvae. For selective ablation of VSC dopamine neurons, MPTP was bath-applied in the dark to larvae for 8 hr at stage 41. 100 µM deprenyl (Sigma) was bath applied to inhibit MAO.
Immunocytochemistry
The number of neurons in the VSC was quantified by counting the cells within the core and surrounding annular region in sections of the diencephalon. Details are described in Methods on-line.
Calcium imaging
Procedures are described on-line.
In situ hybridization
Tyrosine hydroxylase mRNA was detected by locked nucleic acid-based in situ hybridization45 optimized for cryostat sections with the tyramide-fluorescein amplification system (Perkin Elmer).
Statistics
Data were collected from 6–9 X. laevis larvae from 3 or more clutches at stages 35, 37, 42, and 46 and are presented as mean±SEM. Significance was assessed with Student’s t-test.
Supplementary Material
Acknowledgements
We thank Darwin Berg, Laura Borodinsky, and Richard Levine for critical comments on the manuscript and I-Teh Hsieh and Daniela Boassa for technical support. This work was supported by a grant to N.C.S. from the National Institutes of Health.
Footnotes
Competing interest statement The authors declare that they have no competing interests.
References
- 1.Walicke PA, Patterson PH. On the role of Ca2+ in the transmitter choice made by cultured sympathetic neurons. Journal of Neuroscience. 1981;1:343–350. doi: 10.1523/JNEUROSCI.01-04-00343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosenitsch TA, Katz DM. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Molecular and Cellular Neuroscience. 2002;20:447–457. doi: 10.1006/mcne.2002.1135. [DOI] [PubMed] [Google Scholar]
- 3.Borodinsky LN, et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. Journal of Neuroscience. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano SM, Chang CK, Shatz CJ. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. Journal of Neuroscience. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd FL, Isaac JTR. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli G, et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CF, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 11.Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine-hydroxylase in embryologically distinct Purkinje-cells. Neuron. 1991;6:123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- 12.Ubink R, Tuinhof R, Roubos EW. Identification of suprachiasmatic melanotrope-inhibiting neurons in Xenopus laevis: A confocal laser-scanning microscopy study. Journal of Comparative Neurology. 1998;397:60–68. [PubMed] [Google Scholar]
- 13.Tuinhof R, et al. Involvement of retinohypothalamic input, suprachiasmatic nucleus, magnocellular nucleus and locus-coeruleus in control of melanotrope cells of Xenopus-laevis-a retrograde and anterograde tracing study. Neuroscience. 1994;61:411–420. doi: 10.1016/0306-4522(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 14.Kramer BMR, et al. Dynamics and plasticity of peptidergic control centres in the retino-brain-pituitary system of Xenopus laevis. Microscopy Research and Technique. 2001;54:188–199. doi: 10.1002/jemt.1132. [DOI] [PubMed] [Google Scholar]
- 15.Kolk SM, Berghs C, Vaudry H, Verhage M, Roubos EW. Physiological control of Xunc18 expression in neuroendocrine melanotrope cells of Xenopus laevis. Endocrinology. 2001;142:1950–1957. doi: 10.1210/endo.142.5.8131. [DOI] [PubMed] [Google Scholar]
- 16.Abizaid A, Horvath B, Keefe DL, Leranth C, Horvath TL. Direct visual and circadian pathways target neuroendocrine cells in primates. European Journal of Neuroscience. 2004;20:2767–2776. doi: 10.1111/j.1460-9568.2004.03737.x. [DOI] [PubMed] [Google Scholar]
- 17.Logan DW, Burn SF, Jackson IJ. Regulation of pigmentation in zebrafish melanophores. Pigment Cell Research. 2006;19:206–213. doi: 10.1111/j.1600-0749.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 18.Roubos EW, Scheenen W, Jenks BG. Trends in Comparative Endocrinology and Neurobiology. 2005:172–183. [Google Scholar]
- 19.Nordland JJ, et al. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press; 2006. [Google Scholar]
- 20.Tonosaki Y, Nishiyama K, Honda T, Ozaki N, Sugiura Y. D-2-Like Dopamine-receptor mediates dopaminergic or gamma-aminobutyric acidergic inhibition of melanotropin-releasing hormone release from the pars intermedia in frogs (Rana-nigromaculata) Endocrinology. 1995;136:5260–5265. doi: 10.1210/endo.136.12.7588269. [DOI] [PubMed] [Google Scholar]
- 21.Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. Journal of Neurophysiology. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Harsanyi K, Mangel SC. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. Journal of Neurophysiology. 1997;78:439–449. doi: 10.1152/jn.1997.78.1.439. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Dowling JE. Effects of dopamine depletion on visual sensitivity of zebrafish. Journal of Neuroscience. 2000;20:1893–1903. doi: 10.1523/JNEUROSCI.20-05-01893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green CB, Liang MY, Steenhard BM, Besharse JC. Ontogeny of circadian and light regulation of melatonin release in Xenopus laevis embryos. Developmental Brain Research. 1999;117:109–116. doi: 10.1016/s0165-3806(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 25.Mastick GS, Andrews GL. Pax6 regulates the identity of embryonic diencephalic neurons. Molecular and Cellular Neuroscience. 2001;17:190–207. doi: 10.1006/mcne.2000.0924. [DOI] [PubMed] [Google Scholar]
- 26.Wullimann MF, Rink E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Developmental Brain Research. 2001;131:173–191. doi: 10.1016/s0165-3806(01)00270-x. [DOI] [PubMed] [Google Scholar]
- 27.Vazquez-Martinez R, et al. Melanotrope cell plasticity: A key mechanism for the physiological adaptation to background color changes. Endocrinology. 2001;142:3060–3067. doi: 10.1210/endo.142.7.8266. [DOI] [PubMed] [Google Scholar]
- 28.Berghs C, Tanaka S, VanStrien FJC, Kurabuchi S, Roubos EW. The secretory granule and pro-opiomelanocortin processing in Xenopus melanotrope cells during background adaptation. Journal of Histochemistry & Cytochemistry. 1997;45:1673–1682. doi: 10.1177/002215549704501211. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HLM, Breukels V, Jenks BG, Roubos EW, Scheenen WJ. Calcium channel kinetics of melanotrope cells in Xenopus laevis depend on environmental stimulation. Gen Comp Endocrinol. 2008;156:104–112. doi: 10.1016/j.ygcen.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Jenks BG, Kidane AH, Scheenen W, Roubos EW. Plasticity in the melanotrope neuroendocrine interface of Xenopus laevis. Neuroendocrinology. 2007;85:177–185. doi: 10.1159/000101434. [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Korzh V, Strahle U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. European Journal of Neuroscience. 2005;21:1758–1762. doi: 10.1111/j.1460-9568.2005.03988.x. [DOI] [PubMed] [Google Scholar]
- 32.McKinley ET, et al. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Molecular Brain Research. 2005;141:128–137. doi: 10.1016/j.molbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Olive S, Rougon G, Pierre K, Theodosis DT. Expression of a glycosyl phosphatidylinositol-anchored adhesion molecule, the glycoprotein F3, in the adult rat hypothalamoneurohypophyseal system. Brain Research. 1995;689:271–280. doi: 10.1016/0006-8993(95)00555-5. [DOI] [PubMed] [Google Scholar]
- 34.El Majdoubi M, Poulain DA, Theodosis DT. Activity-dependent morphological synaptic plasticity in an adult neurosecretory system: magnocellular oxytocin neurons of the hypothalamus. Biochemistry and Cell Biology-Biochimie et Biologie Cellulaire. 2000;78:317–327. [PubMed] [Google Scholar]
- 35.Mueller NK, Di S, Paden CM, Herman JP. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology. 2005;146:348–354. doi: 10.1210/en.2004-0539. [DOI] [PubMed] [Google Scholar]
- 36.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 37.Lam RW, Levitan RD. Pathophysiology of seasonal affective disorder: a review. Journal of Psychiatry & Neuroscience. 2000;25:469–480. [PMC free article] [PubMed] [Google Scholar]
- 38.Lam RW, Levitt AJ. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Vancouver BC: Clinical and Academic Publishing; 1999. [Google Scholar]
- 39.Lam RW, Tam EM, Grewal A, Yatham LN. Effects of alpha-methyl-para-tyrosine-induced catecholamine depletion in patients with seasonal affective disorder in summer remission. Neuropsychopharmacology. 2001;25:S97–S101. doi: 10.1016/S0893-133X(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 40.Michel S, Itri J, Colwell CS. Excitatory mechanisms in the suprachiasmatic nucleus: The role of AMPA/KA glutamate receptors. Journal of Neurophysiology. 2002;88:817–828. doi: 10.1152/jn.2002.88.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. Journal of Neuroscience. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFarlane S, McNeill L, Holt CE. FGF signaling and target recognition in the developing Xenopus visual system. Neuron. 1995;15:1017–1028. doi: 10.1016/0896-6273(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nature Reviews Neuroscience. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- 45.Obernosterer G, Martinez J, Alenius M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nature Protocols. 2007;2:1508–1514. doi: 10.1038/nprot.2007.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.