Abstract
Objective
The goals were to identify the blood pressures of extremely low gestational age newborns that prompt intervention, to identify other infant characteristics associated with receipt of therapies intended to increase blood pressure, and to assess the interinstitutional variability in the use of these therapies.
Methods
The cohort included 1507 extremely low gestational age newborns born at 23 weeks to weeks of gestation, at 14 institutions, between March 2002 and August 2004; 1387 survived the first postnatal week. Blood pressures were measured as clinically indicated. Interventions were grouped as any treatment (ie, vasopressor and/or fluid boluses of >10 mL/kg) and vasopressor treatment, and logistic regression analyses were performed.
Results
At each gestational age, the lowest mean arterial pressures in treated and untreated infants tended to increase with advancing postnatal age. Infants who received any therapy tended to have lower mean arterial pressures than infants who did not, but uniform thresholds for treatment were not apparent. The proportion of infants receiving any treatment decreased with increasing gestational age from 93% at 23 weeks to 73% at 27 weeks. Treatment nearly always began during the first 24 hours of life. Lower gestational age, lower birth weight, male gender, and higher Score for Neonatal Acute Physiology–II values were associated with any treatment and vasopressor treatment. Institutions varied greatly in their tendency to offer any treatment and vasopressor treatment. Neither the lowest mean arterial pressure on the day of treatment nor other characteristics of the infants accounted for center differences in treatment.
Conclusions
Blood pressure in extremely premature infants not treated for hypotension increased directly with both increasing gestational age and postnatal age. The decision to provide treatment was associated more strongly with the center where care was provided than with infant attributes.
Keywords: premature infant, blood pressure, hypotension
Hypotension is one of the most common diagnoses assigned to extremely low gestational age newborns (ELGANs), and treatments to increase blood pressure are often used in this population.1 These treatments are administered under the assumption that increasing blood pressure increases tissue perfusion and that, below a threshold value, perfusion of vital organs is compromised, resulting in cell dysfunction and tissue injury.2 Among extremely premature infants, the range of blood pressures that maintain adequate perfusion and prevent injury is unknown.3 Blood pressure depends on systemic blood flow, cardiac output, and systemic vascular resistance, but most clinicians use blood pressure as a proxy for adequate tissue perfusion.4–7 To date, the range of “normal” blood pressures has been derived from the distribution of blood pressures in populations of infants who are not treated for hypotension. With this approach, reference ranges have been developed for gestational age and birth weight groups, forming the basis for recommendations for treatment of low blood pressure.8–16 In addition, there is a dearth of studies of commonly used therapies for hypotension with clinically relevant, long-term outcomes.17
The main problem with using a nontreated population approach to define infants who have abnormal blood pressures is that the treatment of hypotension depends on decisions made by clinicians without direct knowledge about the consequences of varying levels of blood pressure, systemic vascular resistance, or cardiac output. An additional problem is that previous studies designed to examine the incidence of hypotension enrolled relatively small numbers of infants at the lowest gestational ages, in whom morbidities potentially associated with hypotension are most likely to occur. In this study, we had 3 objectives: (1) to describe the distribution of the lowest blood pressure measurements for a large cohort of ELGANs, using the physician's decision to treat low blood pressure as an indicator of the clinician's concern regarding the likelihood of impaired perfusion; (2) to compare the lowest blood pressures of ELGANs who received treatment for low blood pressure with the lowest blood pressures of their peers who did not receive such treatment; and (3) to evaluate institutional differences in the use of treatments for low blood pressure.
Methods
All infants born between 23 weeks and weeks of gestation, between March 2002 and August 2004, at 1 of the 14 institutions were eligible for inclusion in the ELGAN Study, a study designed to identify the antecedents of brain damage in preterm infants. Eighty-three percent of eligible infants were enrolled. The enrollment rates according to center ranged from 67% to 93%. However, the consent processes were different at different sites. At some sites, recruitment occurred when a mother was admitted with threatening preterm delivery. At other sites, recruitment was not attempted until after delivery or admission to the intensive care nursery. Institutional review boards at each institution approved the ELGAN Study, and written informed consent was obtained before enrollment. All variables and outcomes were defined a priori, and research personnel were trained before study start. Historical, demographic, and clinical data were abstracted from maternal and infant clinical charts, and placentas were examined for evidence of inflammation.
Data collected from the maternal charts included race, ethnicity, pregnancy complications, multifetal pregnancy, and duration of gestation. Gestational age was determined on the basis of (in order of decreasing preference) fetal ultrasound scans obtained before 14 weeks (63%), ultrasound scans obtained between 14 weeks and 18 weeks (17%), ultrasound scans obtained after 18 weeks with a consonant maternal report of the beginning of the last menstrual cycle (13%), and date of the last menstrual cycle only (7%). The presence of chorioamnionitis (defined on the basis of polymorphonuclear leukocytes in the chorion or chorioamnion) and funisitis (defined on the basis of polymorphonuclear leukocytes in the wall of a blood vessel in the umbilical cord or the chorionic plate) was determined by a pathologist who first engaged in training procedures to minimize inter-observer variability, was masked with respect to maternal history, and used the predefined operational definitions.
Neonatal blood pressure measurements are reported for postnatal day 0 through day 6. Because the first 24 hours of life represent a critical time period for ELGANs, day 0 began at the time of birth and continued through 24 hours of age. Day 1 began at the end of the first 24 hours of age and ended at midnight of that calendar day. For example, for an infant born at 1 pm on January 1, day 0 of life would be the next 24 hours, until 1 pm on January 2. Day 1 of life would start at 1 pm on January 2 and extend until midnight (11 hours). Therefore, day 1 varied in length and was always the “short day.” Days 2 through 6 began and ended at midnight. Mean blood pressure was measured either directly from an intraarterial catheter or from an automated blood pressure cuff. We did not record which oscillometric devices were used.
To identify blood pressures that prompted intervention, we compared the box plots of the lowest blood pressures on each day for infants who received any treatment for hypotension, including vasopressors (eg, dopamine, dobutamine, and epinephrine) or saline solution at >10 mL/kg per day, and infants who did not receive treatment.18 Saline therapy was recorded as a total amount received per day and included normal saline solution and Ringer's lactate solution; to avoid the cumulative amount of saline flushes, we considered a total amount of >10 mL/kg per day to be therapy for hypotension. Comparisons were made in 4 gestational age strata (ie, 23–24, 25, 26, and 27 weeks). Recognizing the possibility that some infants might have received saline therapy of >10 mL/kg per day because of abnormalities in skin perfusion, low urine output, or other indications that might or might not have been related directly to low blood pressure, we examined infants who received vasopressor treatment in analyses of risk factors for treatment and center variation. We did not include the administration of blood products as a treatment for hypotension because we could not distinguish this use from the treatment of a hematologic disorder (eg, red blood cell transfusion for the treatment of anemia). We did not include corticosteroids as a treatment for hypotension because all patients who received corticosteroids also received vasopressors, except for 10 patients. Those patients were treated at centers where randomized, controlled trials of corticosteroids were being conducted; the 10 patients were excluded because we had no way of knowing whether the patients received corticosteroids or placebo.
We examined candidate risk factors for any treatment and vasopressor treatment, including gender, gestational age, birth weight z score, maternal race, receipt of prenatal steroid treatment, chorioamnionitis, funisitis, pregnancy complications, multifetal pregnancy, Apgar score, and Score for Neonatal Acute Physiology–II (SNAP-II) score.19 Birth weight z score is the number of SDs (and direction) of the infant's birth weight from an assumed mean for gestational age.20 We considered these potential confounders when examining how centers differed in the use of any treatment and vasopressor treatment during the first 7 postnatal days.
We evaluated the associations between subject characteristics and nontreatment or treatment with saline boluses and/or vasopressor or between nontreatment and treatment with just vasopressor by using Fisher's exact test. Characteristics that distinguished the groups with a P value of ≤.3021 were included in logistic regression analyses that assessed the tendency of each of the 14 sites to treat at any time during the first 7 postnatal days.
Results
A total of 1507 infants were enrolled in the ELGAN Study; 1387 infants survived the first postnatal week and contributed data to this analysis. There were 119 total deaths. Of the 76 infants who died in the first 48 hours of life, we were unable to collect information for 46 (61%). Of the 43 infants who died between 48 hours and day 6 of life, we were unable to collect information for 18 (42%). In addition, some centers collected data on all infants delivered between 23 and 27 weeks, whereas others collected data only on infants admitted to the intensive care nursery. Moreover, because we were examining factors that might be related to treatment during the first postnatal week, each infant needed to be eligible for treatment during the entire week. Therefore, we elected not to include infants who died during the first week in this analysis. A description of the study population revealed that 45% of the patients were born between 23 and 25 weeks of gestation and 55% were born between 26 and 27 weeks of gestation (Table 1). Approximately 20% of the birth weight z scores were >1 SD below the external mean.20 More than 30% of the infants were the products of multifetal pregnancies. Almost 90% were exposed to ≥1 prenatal steroid dose. Nearly 50% of placentas had evidence of chorioamnionitis, and 42% had funisitis.
TABLE 1. Characteristics and Antecedents Associated With Treatment for Hypotension.
| Characteristic | No. of Infants (Maximal N = 1387)a | Proportion of Infants, % | ||
|---|---|---|---|---|
| No Treatment (Maximal n = 249) | Any Treatment (Maximal n = 1138)b | Vasopressor Treatment (Maximal n = 470)c | ||
| Gestational age, wk | P = .001 | P≤.0005 | ||
| 23 | 85 | 7 | 93 | 52 |
| 24 | 246 | 10 | 90 | 47 |
| 25 | 289 | 16 | 84 | 34 |
| 26 | 338 | 18 | 82 | 32 |
| 27 | 429 | 27 | 73 | 25 |
| Birth weight, g | P≤.0005 | P≤.0005 | ||
| ≤750 | 564 | 13 | 87 | 43 |
| 751–1000 | 570 | 16 | 84 | 30 |
| 1001–1250 | 230 | 34 | 66 | 20 |
| >1250 | 23 | 35 | 65 | 48 |
| Birth weight z score | P = .07 | P = .03 | ||
| Less than −2 | 92 | 20 | 80 | 38 |
| −2 to less than −1 | 192 | 16 | 84 | 35 |
| −1 to 0 | 508 | 13 | 87 | 38 |
| ≥0 | 595 | 23 | 77 | 30 |
| Gender | P = .03 | P = .007 | ||
| Male | 737 | 16 | 84 | 37 |
| Female | 650 | 20 | 80 | 31 |
| Race | P = .94 | P = .75 | ||
| White | 774 | 17 | 83 | 34 |
| Black | 397 | 20 | 80 | 36 |
| Asian | 26 | 19 | 81 | 31 |
| Native American | 14 | 14 | 86 | 50 |
| Mixed | 51 | 16 | 84 | 31 |
| Other | 104 | 14 | 86 | 23 |
| Ethnicity | P = .29 | P >.99 | ||
| Hispanic | 166 | 13 | 87 | 28 |
| Non-Hispanic | 1213 | 19 | 81 | 35 |
| Fetal number | P = .11 | P = .02 | ||
| Multifetal pregnancy | 442 | 16 | 84 | 38 |
| Singleton pregnancy | 945 | 19 | 81 | 32 |
| Prenatal steroid treatment | P = .82 | P >.99 | ||
| Any | 1232 | 18 | 82 | 34 |
| None | 147 | 18 | 82 | 36 |
| SNAP-II score | P≤.0005 | P≤.0005 | ||
| <20 | 694 | 23 | 77 | 23 |
| 20–29 | 341 | 14 | 86 | 35 |
| ≥30 | 330 | 11 | 89 | 56 |
| Initiator of delivery | P = .38 | P = .34 | ||
| Preterm labor | 614 | 17 | 83 | 35 |
| pPROM | 297 | 19 | 81 | 31 |
| PIH | 187 | 19 | 81 | 29 |
| Abruption | 144 | 18 | 82 | 33 |
| Cervical insufficiency | 86 | 17 | 83 | 40 |
| Fetal indication | 59 | 24 | 76 | 42 |
| Mode of delivery | P = .87 | P = .87 | ||
| Cesarean | 896 | 18 | 82 | 34 |
| Vaginal | 490 | 18 | 82 | 34 |
| Chorioamnionitis | P = .77 | P = .87 | ||
| Any | 591 | 18 | 82 | 32 |
| None | 611 | 19 | 81 | 34 |
| Funisitis | P = .94 | P = .78 | ||
| Any | 508 | 19 | 81 | 31 |
| None | 675 | 19 | 81 | 33 |
For each characteristic, the comparison group used to obtain P values was the no-treatment group. Associations were evaluated by using Fisher's exact test (2-sided). pPROM indicates preterm prelabor rupture of membranes; PIH, pregnancy-induced hypertension.
Column characteristics do not add to 1387 because of missing data.
Any treatment represents administration of vasopressor and/or normal saline solution bolus of >10 mL/kg.
Vasopressor treatment represents dopamine, dobutamine, and/or epinephrine treatment.
Among infants treated for hypotension during the first postnatal week, treatment began in the first 24 hours of life for 90%, 89%, 91%, and 89% of infants born at 23 to 24 weeks, 25 weeks, 26 weeks, and 27 weeks of gestation, respectively. Among all infants, the proportion of infants who received treatment on subsequent days during the first week of life declined in all gestational age groups, but the likelihood of treatment was always higher for the least-mature infants (Table 2). On day 6 of life, 69% of infants at 23 to 24 weeks of gestation received treatment, compared with 26% at 27 weeks.
TABLE 2. Gestational and Postnatal Age-Specific Proportions of Infants Who Survived the First Postnatal Week and Received Any Treatment for Hypotension.
| Gestational Age, wk | Postnatal Age, d | |||||
|---|---|---|---|---|---|---|
| 0a | 2 | 3 | 4 | 5 | 6 | |
| 23–24 | 83 | 80 | 78 | 75 | 72 | 69 |
| 25 | 77 | 69 | 63 | 57 | 54 | 49 |
| 26 | 74 | 65 | 59 | 51 | 44 | 37 |
| 27 | 63 | 57 | 50 | 42 | 30 | 24 |
Day 0 of life is the first 24 hours after birth; day 1 of life is the time from the end of day 0 until midnight of that day (omitted because of variable periods of time); and days 2 to 6 are 24-hour periods beginning at midnight at the end of day 1.
Among infants who survived the first postnatal week, treated infants tended to have lower blood pressures on each day (Fig 1). This difference was present on the first day of life, reflecting differences before initiation of treatment. This difference persisted throughout the first postnatal week, reflecting blood pressure values during treatment and after cessation of treatment. Although the differences in median values were not large, the treated infants tended to exhibit longer plot whiskers (defined in the legend to Fig 1), compared with the untreated infants. This indicates greater variability of the blood pressures in the lowest quartile. With infants not treated for hypotension as a reference group, infants who received treatment were more likely to be less mature, to have a lower birth weight z score, to have a higher SNAP-II score (all P≤.0005), and to be male (any treatment, P = .03; drug treatment, P = .007) (Table 1).
FIGURE 1.
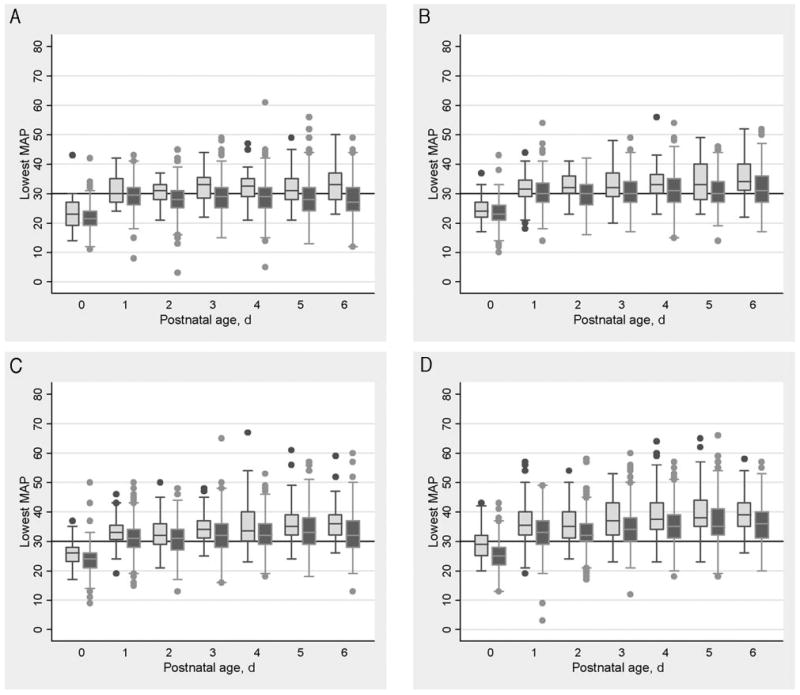
Lowest MAP values on postnatal days 0 to 6 for infants who survived the first postnatal week, according to gestational age. Dark shaded boxes indicate data for infants who received any treatment for low blood pressure at any time during the first week of life; light shaded boxes indicate data for infants not treated for low blood pressure. Each box represents the 25th to 75th percentiles, the center line represents the median, and the whiskers mark the real data point that is closest to 1.5 times the interquartile range. The dots indicate outliers. A, Infants born at 23 and 24 weeks (n = 331); B, infants born at 25 weeks (n = 289); C, infants born at 26 weeks (n = 338); D, infants born at 27 weeks (n = 429).
The centers differed considerably in the proportions of infants treated (Table 3). For example, at the center that treated the smallest proportions of infants, 29% received any treatment and 6% received drug treatment. In the center providing treatment to the greatest proportions of infants, 98% received any treatment and 64% received vasopressor treatment. With the center treating the smallest proportions as the reference group, we used logistic regression analyses to evaluate each center's tendency to treat. Adjustment for maternal and neonatal risk factors showed little effect on the predicted proportions of infants treated for hypotension. The lowest mean arterial pressure (MAP) on the day of treatment varied widely but did not correlate with the likelihood of treatment according to center; centers with lower MAP values on the first day of treatment did not have more infants treated, and centers with higher MAP values on the first day of treatment did not have fewer infants treated.
TABLE 3. Center Variation in Treatments for Hypotension.
| Center | Proportion Treated, % | Lowest MAP on First Day,a mm Hg | OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Any treatmentb | ||||
| A | 29 | 28 | 1 | 1c |
| B | 46 | 27 | 2 (1–4) | 3 (1–6) |
| C | 61 | 20 | 4 (2–7) | 5 (2–10) |
| D | 69 | 24 | 5 (3–9) | 9 (5–18) |
| E | 80 | 25 | 9 (5–20) | 33 (14–80) |
| F | 85 | 24 | 13 (6–27) | 25 (11–56) |
| G | 91 | 23 | 24 (11–50) | 44 (19–102) |
| H | 92 | 23 | 26 (13–52) | 54 (25–118) |
| I | 93 | 23 | 32 (7–145) | 84 (17–404) |
| J | 93 | 25 | 34 (15–78) | 80 (32–203) |
| K | 94 | 22 | 37 (16–82) | 58 (24–140) |
| L | 94 | 23 | 39 (14–106) | 92 (31–275) |
| M | 96 | 26 | 65 (19–225) | 105 (29–385) |
| N | 98 | 23 | 116 (27–504) | 299 (65–1383) |
| Vasopressor treatmentd | ||||
| A | 6 | 19 | 1 | 1c |
| N | 12 | 20 | 2 (1–6) | 3 (1–9) |
| F | 15 | 21 | 3 (1–7) | 3 (1–10) |
| M | 18 | 25 | 3 (1–9) | 4 (2–12) |
| D | 20 | 22 | 4 (1–10) | 5 (2–14) |
| B | 27 | 37 | 6 (2–15) | 8 (3–22) |
| H | 32 | 21 | 7 (3–17) | 12 (5–30) |
| K | 38 | 21 | 9 (4–22) | 11 (4–27) |
| C | 44 | 19 | 12 (4–30) | 19 (7–52) |
| J | 46 | 23 | 13 (5–31) | 25 (10–65) |
| I | 48 | 25 | 14 (5–42) | 34 (11–107) |
| E | 52 | 24 | 16 (6–42) | 48 (17–132) |
| G | 60 | 23 | 22 (9–54) | 35 (14–91) |
| L | 64 | 24 | 26 (10–67) | 61 (23–165) |
Center A had the lowest rate of treatment and was used as the reference. OR indicates odds ratio; CI, confidence interval.
Values are median of the lowest MAP value on the first day of treatment.
Any treatment represents administration of vasopressor and/or normal saline solution bolus of >10 mL/kg.
Adjusted for gestational age, birth weight z score, gender, Hispanic ethnicity, fetal number, and SNAP-II score.
Vasopressor treatment represents dopamine, dobutamine, and/or epinephrine treatment.
Adjusted for gestational age, birth weight z score, gender, fetal number, and SNAP-II score.
Discussion
In a large cohort of ELGANs, we describe the distributions of the lowest blood pressures in the first week of life among the relatively small proportion of infants who did not receive treatment for hypotension and separately among infants who received any treatment. As reported previously, the lowest blood pressure increased with both increasing gestational age and postnatal age.8–16,22 More than 80% of infants received some form of treatment for hypotension. Lower gestational age, lower birth weight, male gender, and higher scores of illness severity (ie, SNAP-II scores) were associated with treatment. After adjustment for these factors, no other clinical factors influenced the likelihood of being treated. Specifically, chorioamnionitis, funisitis, and placental abruption did not increase the likelihood of treatment. This finding is similar to that of Yanowitz et al,23 who demonstrated that blood pressure in the first week of life was lower among very low birth weight infants with fetal vessel inflammation only when the gestational age was >29 weeks. These results are also in general agreement with other reports, with the exception that we did not identify pregnancy-induced hypertension as a factor associated with higher blood pressure.12 It is possible that the current study permitted a more-precise examination of the influence of other confounders, compared with the study by Hegyi et al.12 For that study, the primary inclusion criterion was based on birth weight, so that factors associated with fetal growth restriction (such as pregnancy-induced hypertension) would be expected to be associated with greater gestational age. In contrast, our study cohort included infants according to gestational age instead of birth weight, minimizing the potential bias of the birth weight-defined cohort, namely, including a greater proportion of more-mature, growth-restricted infants. This difference in study design might have contributed to the observed variation in results among these studies.24
Hypotension in the first postnatal week is a phenomenon that is identified and treated early, predominantly during the first 24 hours. The percentage of infants receiving treatment according to day of life is inversely proportional to gestational age, which suggests that a pathologic state is more likely to persist in the least-mature infants. A unique finding in this study is that blood pressures among treated infants remained lower than those among nontreated infants both during and after completion of treatment. There are several possible explanations for this observation. The goal of clinicians might be not to achieve MAPs comparable to those of untreated infants but to improve perfusion, urinary output, or some other clinical indication of adequate blood pressure for a particular infant. Therapies to increase blood pressure might be less effective than desired in this population, or the clinicians' target blood pressures might be the lowest values deemed acceptable at a given gestational age. For example, in some nurseries, the target MAP might be 24 mm Hg for a 24-week infant. We would not expect the treated infants to have similar blood pressures unless clinicians targeted the mean seen among untreated infants.
As reported previously, we observed a marked difference among centers in the tendency to use treatments for hypotension.22 This variability is explained neither by variation in infant attributes nor by center differences in blood pressure distributions. Other possible explanations for intercenter variation include differences in care practices that can influence blood pressure secondarily, such as use of positive airway pressure, variations in the perception of when therapy is needed, and variations in the perception of the efficacy of blood pressure therapy. This finding of such large intercenter variation in treatment demonstrates that previous studies of untreated infants whose data were used to determine “normal” blood pressures might reflect physicians' preferences rather than wellness.
The ranges of blood pressures observed in infants who were not treated for hypotension seemed to convey information about treatment practices and infant attributes, such as gestational and postnatal age, but do not necessarily identify a range of blood pressures over which treatment is not beneficial. The small differences in blood pressure between treated and untreated infants raise the possibility of misclassification. For example, infants who had normal tissue perfusion might have been treated. Recent data suggest that infants autoregulate blood flow to vital organs to a greater extent than previously assumed25; therefore, blood pressure alone may be a poor predictor of need for treatment. Similarly, some infants whose blood pressures were considered normal and were not treated might have suffered from insufficient end-organ perfusion.
There are several potential limitations to this study. We report blood pressures recorded in the bedside chart, and we cannot be certain that these reflect accurately the true variability of pressures. Furthermore, some blood pressures were measured with central or peripheral arterial line transducers, whereas others were obtained from cuff blood pressure measurements. We included among blood pressure therapies most (drugs and saline fluid boluses) but not all potential forms of blood pressure treatment. For example, we did not consider transfusion of blood products as a blood pressure treatment. In addition, we report only MAP values because recording of systolic and diastolic blood pressure values was not a component of the ELGAN Study. Because of the design of the data collection of the ELGAN study, we could not distinguish between dopamine, dobutamine, and epinephrine as part of vasopressor therapy.
Conclusions
Our results support previously reported relationships between gestational age, advancing postnatal age, and blood pressure. In addition, we identified associations between lower blood pressure and both male gender and illness severity. Although most infants at the earliest gestational ages received some treatment, there was marked variability among centers. Our observation that this variation was not explained by differences in infant attributes or distribution of MAPs at the different centers indicates a lack of uniformity with respect to the definition of hypotension requiring treatment. These findings call into question the validity of the definitions of both normotension and hypotension derived from most previous studies, because both definitions hinge on whether the infant received treatment to increase blood pressure. Current treatments for hypotension do not seem to produce uniformly blood pressures that are comparable to those observed in an untreated population.
Wanting Babies Like Themselves, Some Parents Choose Genetic Defects —
“Wanting to have children who follow in one's footsteps is an understandable desire. But a coming article in the journal Fertility and Sterility offers a fascinating glimpse into how far some parents may go to ensure that their children stay in their world—by intentionally choosing malfunctioning genes that produce disabilities like deafness or dwarfism. The article reviews the use of pre-implantation genetic diagnosis, or PGD, a process in which embryos are created in a test tube and their DNA is analyzed before being transferred to a woman's uterus. In this manner, embryos destined to have, for example, cystic fibrosis or Huntington's disease can be excluded, and only healthy embryos implanted. Yet Susannah A. Baruch and colleagues at the Genetics and Public Policy Center at Johns Hopkins University recently surveyed 190 American PGD clinics, and found that 3 percent reported having intentionally used PGD ‘to select an embryo for the presence of a disability.’ In other words, some parents had the painful and expensive fertility procedure for the express purpose of having children with a defective gene. It turns out that some mothers and fathers don't view certain genetic conditions as disabilities but as a way to enter into a rich, shared culture.”
Sanghavi DM. New York Times. December 5, 2006
Noted by Roger Soll, MD
Acknowledgments
This study was supported by National Institute of Neurological Disorders and Stroke grant 5U01NS040069.
We acknowledge the site principal investigators for the ELGAN Study: Bhavesh L. Shah, Baystate Medical Center (Springfield, MA); Camilia Martin, Beth Israel Deaconess Medical Center (Boston, MA); Linda Van Marter, Brigham and Women's Hospital (Boston, MA); Karl Kuban, Boston Medical Center (Boston, MA); Robert Insoft, Massachusetts General Hospital (Boston, MA); Francis Bednarek, University of Massachusetts Memorial Health Center, Worcester (Boston, MA); Cynthia Cole and John Fiascone, New England Medical Center (Boston, MA); Richard Ehrenkranz, Yale-New Haven Hospital (New Haven, CT); T. Michael O'Shea, Wake Forest University/Baptist Medical Center (Winston-Salem, NC); Stephen C. Engelke, University Health Systems of Eastern Carolina (Greenville, NC); Carl Bose, University of North Carolina at Chapel Hill (Chapel Hill, NC); Mariel Poortenga, DeVos Children's Hospital (Grand Rapids, MI); Padmani Karna, Sparrow Hospital (Lansing, MI); Michael D. Schreiber, University of Chicago Hospital (Chicago, IL); Daniel Batton, William Beaumont Hospital (Royal Oak, MI).
Abbreviations
- ELGAN
extremely low gestational age newborn
- MAP
mean arterial pressure
- SNAP-II
Score for Neonatal Acute Physiology–II
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Osborn DA. Diagnosis and treatment of preterm transitional circulatory compromise. Early Hum Dev. 2005;81:413–422. doi: 10.1016/j.earlhumdev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Martens SE, Rijken M, Stoelhorst GM, et al. Is hypotension a major risk factor for neurological morbidity at term age in very preterm infants? Early Hum Dev. 2003;75:79–89. doi: 10.1016/j.earlhumdev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Kluckow M, Evans N. Low systemic blood flow in the preterm infant. Semin Neonatol. 2001;6:75–84. doi: 10.1053/siny.2000.0035. [DOI] [PubMed] [Google Scholar]
- 4.Pladys P, Wodey E, Beuchee A, Branger B, Betremieux P. Left ventricle output and mean arterial blood pressure in preterm infants during the 1st day of life. Eur J Pediatr. 1999;158:817–824. doi: 10.1007/s004310051213. [DOI] [PubMed] [Google Scholar]
- 5.Lopez SL, Leighton JO, Walther FJ. Supranormal cardiac output in the dopamine- and dobutamine-dependent preterm infant. Pediatr Cardiol. 1997;18:292–296. doi: 10.1007/s002469900177. [DOI] [PubMed] [Google Scholar]
- 6.Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr. 1996;129:506–512. doi: 10.1016/s0022-3476(96)70114-2. [DOI] [PubMed] [Google Scholar]
- 7.Wardle SP, Yoxall CW, Weindling AM. Peripheral oxygenation in hypotensive preterm babies. Pediatr Res. 1999;45:343–349. doi: 10.1203/00006450-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kitterman JA, Phibbs RH, Tooley WH. Aortic blood pressure in normal newborn infants during the first 12 hours of life. Pediatrics. 1969;44:959–968. [PubMed] [Google Scholar]
- 9.Versmold HT, Kitterman JA, Phibbs RH, Gregory GA, Tooley WH. Aortic blood pressure during the first 12 hours of life in infants with birth weight 610 to 4220 grams. Pediatrics. 1981;67:607–613. [PubMed] [Google Scholar]
- 10.Shortland DB, Evans DH, Levene MI. Blood pressure measurements in very low birth weight infants over the first week of life. J Perinat Med. 1988;16:93–97. doi: 10.1515/jpme.1988.16.2.93. [DOI] [PubMed] [Google Scholar]
- 11.Spinazzola RM, Harper RG, de Soler M, Lesser M. Blood pressure values in 500- to 750-gram birthweight infants in the first week of life. J Perinatol. 1991;11:147–151. [PubMed] [Google Scholar]
- 12.Hegyi T, Carbone MT, Anwar M, et al. Blood pressure ranges in premature infants, part I: the first hours of life. J Pediatr. 1994;124:627–633. doi: 10.1016/s0022-3476(05)83146-4. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi T, Anwar M, Carbone MT, et al. Blood pressure ranges in premature infants, part II: the first week of life. Pediatrics. 1996;97:336–342. [PubMed] [Google Scholar]
- 14.Lee J, Rajadurai VS, Tan KW. Blood pressure standards for very low birthweight infants during the first day of life. Arch Dis Child Fetal Neonatal Ed. 1999;81:F168–F170. doi: 10.1136/fn.81.3.f168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuntnarumit P, Yang W, Bada-Ellzey HS. Blood pressure measurements in the newborn. Clin Perinatol. 1999;26:981–996. [PubMed] [Google Scholar]
- 16.Cordero L, Timan CJ, Waters HH, Sachs LA. Mean arterial pressures during the first 24 hours of life in ≤600-gram birth weight infants. J Perinatol. 2002;22:348–353. doi: 10.1038/sj.jp.7210736. [DOI] [PubMed] [Google Scholar]
- 17.Evans N. Which inotrope for which baby? Arch Dis Child Fetal Neonatal Ed. 2006;91:F213–F220. doi: 10.1136/adc.2005.071829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tukey JW. Some thoughts on clinical trials, especially problems of multiplicity. Science. 1977;198:679–684. doi: 10.1126/science.333584. [DOI] [PubMed] [Google Scholar]
- 19.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 20.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 21.Dales LG, Ury HK. An improper use of statistical significance testing in studying covariables. Int J Epidemiol. 1978;7:373–375. doi: 10.1093/ije/7.4.373. [DOI] [PubMed] [Google Scholar]
- 22.Al-Aweel I, Pursley DM, Rubin LP, Shah B, Weisberger S, Richardson DK. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol. 2001;21:272–278. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- 23.Yanowitz TD, Baker RW, Roberts JM, Brozanski BS. Low blood pressure among very-low-birth-weight infants with fetal vessel inflammation. J Perinatol. 2004;24:299–304. doi: 10.1038/sj.jp.7211091. [DOI] [PubMed] [Google Scholar]
- 24.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134:604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 25.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]


