Abstract
Background
Antibody to hepatitis E virus (anti-HEV) is prevalent in Western countries, where clinical hepatitis E is rarely reported. The aim of this study was to determine the prevalence of anti-HEV among Danish blood donors and Danish farmers. In addition, we compared the prevalence among 2 sets of serum samples obtained from blood donors 20 years apart.
Methods
Samples from 291 Danish farmers and 169 blood donors that were collected in 1983 and samples from 461 blood donors that were collected in 2003 were tested for anti-HEV. Relevant information on HEV exposure was collected by self-administered questionnaire.
Results
Anti-HEV testing was performed on samples after 20 years of storage at −20°C. The prevalence of anti-HEV was 50.4% among farmers and 32.9% among donors in 1983 and 20.6% among donors in 2003 (P < .05). Presence of anti-HEV was significantly correlated with increasing age in all 3 groups (P < .05). Among donors who had serum samples obtained in 2003, age, contact with horses, and the presence of antibody to hepatitis A virus were associated with the presence of anti-HEV in multivariate analysis. Among farmers, only age was independently associated with the presence of anti-HEV.
Conclusion
Anti-HEV was highly prevalent among Danes but has decreased in prevalence over the past 50 years. Our study supports the hypothesis that HEV infection in Denmark may be an asymptomatic zoonotic infection.
Hepatitis E virus (HEV) is a single-stranded, nonenveloped virus with an RNA genome of ~7.5 kb. It is in the genus Hepevirus in the family Hepeviridae and can be further divided into 5 genotypes (including avian HEV) [1]. HEV has been the cause of waterborne outbreaks of hepatitis in Asia and Africa and is a major cause of sporadic hepatitis in these regions. Acute infection primarily affects young adults and is generally mild, except in women during late pregnancy, among whom 20% mortality has been reported. HEV infection usually does not become chronic, but chronic infections have recently been reported in transplant recipients [2]. Because of the development of an effective vaccine against hepatitis E, it has become increasingly important to understand the epidemiology of HEV infection [3].
Presence of antibody to HEV (anti-HEV) is more common than expected in areas where HEV infection is not endemic in western Europe and the United States, where cases of clinical hepatitis E are rarely reported [4–6]. Several findings suggest that HEV infection may be an asymptomatic zoonotic infection in industrialized countries [7–9].
Denmark is a country of low endemicity for viral hepatitis, including hepatitis E, which accounts for only 0–2 reported cases per year. In contrast, in a recent study, we found a high prevalence of anti-HEV among Danish prisoners and drug users (16.9%) [10]. The presence of anti-HEV was associated with the presence of antibody to hepatitis A virus (anti-HAV), but the study did not identify risk factors associated with injection drug use or imprisonment, and it was suggested that the high prevalence merely reflected the prevalence among the general population. The purpose of this study was to extend our previous study to serum samples obtained from Danish blood donors in 2003 and from Danish blood donors and farmers in 1983.
PATIENTS, MATERIALS, AND METHODS
Study Populations
Contemporary blood donors (serum samples obtained in 2003)
Consecutive volunteer blood donors at the blood bank of Odense University Hospital were recruited after written informed consent was obtained. They completed a questionnaire with demographic information, risk factors for viral hepatitis, hepatitis A vaccination status, ethnic origin, history of travel abroad, history of injection drug use, sexual orientation, water supply and sanitation, contact with animals (including pets), employment, and contact with farming.
Blood donors and farmers (serum samples obtained in 1983)
We included repository serum samples and questionnaires from blood donors and farmers participating in a study of farm-related allergy and respiratory illness that was performed in Aarhus during the period 1982–1983 [11]. The repository samples had been stored at −20°C since collection. The farmers were members of the Danish Farmers Association in 2 districts in the County of Aarhus. The group was selected by association officials to include farms with varying land area and livestock that used different farming methods. Among the farmers selected, 92% volunteered for the study. Serum samples from farmers were compared with those from consecutive voluntary blood donors from the blood bank at Aarhus University Hospital who were selected to match the age and sex distribution of the farmers. These donors were not screened for HIV infection and hepatitis C, because these tests were not performed at that time. Blood donors living on farms or with occupations related to agriculture were excluded from the initial study. This study did not contain information on risk factors for viral hepatitis.
The blood banks of Aarhus and Odense each covered a population of ~500,000 inhabitants, and the populations were comparable with regard to demographic characteristics. At both blood banks, the majority of blood donors were of urban origin.
Prisoners and drug users
For comparison, we included the results of a hepatitis E survey performed in 1996 with the same assay for 467 prisoners and drug users, as described elsewhere [10]. For comparison of the prevalence of anti-HEV, we included all 467 participants. For comparison of the prevalence of anti-HAV, we included only the 138 Danish prisoners who were not drug users, because these persons were comparable to blood donors (both foreign nationality and drug use were strongly associated with presence of anti-HAV in this population).
Laboratory Analysis
Anti-HEV was analyzed with use of an in-house assay that was developed at the National Institutes of Health, as described elsewhere [10, 12]. Briefly, a baculovirus vector containing a cDNA fragment corresponding to the major part of ORF2 of the Pakistani HEV strain SAR-55 was expressed in insect cells. A purified 55-kDa antigen representing a truncated form (aa112–607) of the 660aa capsid protein of HEV was coated on microtiter plates, and specific antibodies against this antigen were detected with horseradish-peroxidase–labeled antihuman IgG [13]. The assay was calibrated by a World Health Organization anti-HEV standard to a detection limit of 10 mU/mL. The assay was found to have a sensitivity of 96% and a specificity of 98% and to be superior to available commercial and in-house assays in a blinded evaluation of serum panels [14]. Positive results of this assay were previously confirmed by a blocking assay and by Western blot (unpublished data) [4].
Samples that were repeatedly reactive were classified as positive. Samples with anti-HEV levels within 10% of the cutoff level were retested. Because a higher background level was expected in the samples that had been stored since 1983, we retested all samples with anti-HEV levels within 30% of the cutoff level for this group. In addition, a subset of samples with low, medium, or high reactivity, including samples that had been stored since 1983 and samples that had discordant or borderline results, were retested with an antigen-specific blocking assay. Finally, 34 samples were tested against avian HEV capsid protein prepared in the same way as the human HEV capsid antigen. Avian HEV is sufficiently different from mammalian HEV to avoid significant cross-reaction in the ELISA, although the 2 antigens are structurally similar. This assay was included as a test for nonspecific binding of serum samples that could have occurred as a result of long-term storage.
Contemporary donors and prisoners were tested for the presence of anti-HAV with use of a commercial assay, as reported elsewhere [15]. In addition to being tested for the presence of anti-HAV, the contemporary donor samples were tested for the presence of antibody to HIV, hepatitis B surface antigen, and antibody to hepatitis C virus, and the results of all tests were negative. We compared the current age-specific prevalence of anti-HAV with the prevalence found in a study performed with use of samples from the same blood bank in 1980 [16].
Statistical Analysis
Data processing and statistical analysis were performed with use of EpiInfo (Centers for Disease Control and Prevention) and SPSS (SPSS). Nonparametric tests were used for univariate analysis; the χ2 test, Fisher’s exact test, the Mann-Whitney U test, and the Mantel-Haenszel stratified χ2 test were used as appropriate. Statistical significance was set at P < .05. The magnitude of the association between possible risk factors and seropositivity was expressed as ORs with 95% CIs in univariate analysis. To identify variables that were independently associated with presence of anti-HEV, we performed a multivariate logistic regression analysis. We included variables with P < .10 or with theoretical importance using stepwise backward elimination of insignificant variables according to the log rank statistic; interaction terms were not included in the model. The study was approved by the ethics committee for Vejle and Funen Counties (reference number VF 20020195).
RESULTS
Anti-HEV assay validation
To analyze for nonspecific reactivity of stored serum samples, we performed a confirmatory blocking assay for anti-HEV on a subset of old samples (from 6 donors and 30 farmers), including 10 samples that were positive for anti-HEV, 15 that were borderline positive, and 11 that were negative. The initial results for 34 of the 36 samples were confirmed by specific blocking, whereas results for 2 borderline-positive samples were not confirmed by blocking (data not shown). Of the 36 samples, 34 were tested for reactivity with avian HEV antigen, and only 2 were reactive with the avian antigen (2 samples were not tested, including 1 sample that was borderline positive and 1 that was negative, because the amount of the samples was insufficient for analysis). Both reactive samples were specific for human HEV antigen in the blocking assay. In summary, the status of these serum samples, with the exception of 2 borderline positive samples, was confirmed as originally classified, and a low level of cross-reactivity with avian anti-HEV was also confirmed [17].
Contemporary donors
Of 488 consecutive blood donors, 461 (94.5%) completed the questionnaire and had a blood sample obtained. Five samples for which results were inconclusive (repeated gray zone) were excluded. Among the remaining 456 samples, 94 (20.6%) were anti-HEV positive (table 1).
Table 1.
Prevalence of antibody to hepatitis E virus (anti-HEV), by age.
| Farmers (1983) | Blood donors | |||||
|---|---|---|---|---|---|---|
| 1983 | 2003 | |||||
| Age, years | No. of persons who were anti-HEV positive/no. of persons tested | Percentage of persons who were anti-HEV positive (95% CI) | No. of persons who were anti-HEV positive/no. of persons tested | Percentage of persons who were anti-HEV positive (95% CI) | No. of persons who were anti-HEV positive/no. of persons tested | Percentage of persons who were anti-HEV positive (95% CI) |
| <25 | 1/11 | 9.1 (0.2–42) | 1/11 | 9.1 (0.2–42) | 0/28 | 0.0 (0–12) |
| 25–29 | 6/18 | 33.3 (13–59) | 5/22 | 19.0 (8–45) | 1/37 | 2.7 (0.1–14) |
| 30–34 | 6/21 | 28.6 (11–52) | 8/25 | 32.0 (15–54) | 3/36 | 8.3 (2–22) |
| 35–39 | 12/28 | 42.9 (24–63) | 7/21 | 28.6 (15–57) | 9/67 | 13.4 (6–24) |
| 40–44 | 16/34 | 47.1 (30–65) | 9/25 | 36.0 (18–57) | 9/69 | 13.0 (6–23) |
| 45–49 | 28/51 | 54.9 (40–69) | 10/22 | 42.9 (24–68) | 17/72 | 23.6 (14–35) |
| 50–54 | 21/44 | 47.7 (33–63) | 2/14 | 14.3 (2–43) | 15/62 | 24.2 (14–37) |
| 55–59 | 21/32 | 65.6 (47–81) | 8/16 | 50.0 (25–75) | 30/58 | 51.7 (38–65) |
| >59 | 33/47 | 70.2 (55–83) | 5/11 | 45.5 (17–77) | 10/27 | 37.0 (19–58) |
| All | 144/283 | 50.3 (45–57) | 55/167 | 31.5 (26–41) | 94/456 | 20.6 (17–25) |
In univariate analysis, older age (P < .001), male sex (P = .03), having children (P = .007), contact with horses (P = .02), and being anti-HAV positive (P = .005) were associated with the presence of anti-HEV. No association was found between presence of anti-HEV and traveling, water supply and sewage system, contact with children, contact with pet animals, farm exposure (only 4 donors were working as farmers, and only 2 had ever worked with pigs), or ABO and RhD blood types.
We examined the geographical variation by county of residence for the donors and the distribution of pig farms. One-third of donors lived in rural areas, and the median pig density in the area of residence was 179 pigs per km2 (range, 19–669 pigs per km2). For comparison, the mean pig densities in Denmark and Funen were 270 pigs per km2 and 315 pigs per km2, respectively. There was no geographical clustering of anti-HEV and no relationship to pig density in the area of residence.
Most correlations were confounded by age; older donors were predominantly male, were more likely to have children and to be anti-HAV positive, and were less likely to have a history of travel. In a backwards stepwise logistic regression analysis that included only variables of univariate significance (age, sex, having children, presence of anti-HAV, and contact with horses), only age (OR, 6.8; 95% CI, 2.8–16.5; P < .001) and contact with horses (OR, 2.2; 95% CI, 1.1–4.6; P = .035) were associated with presence of anti-HEV. However, this analysis was restricted to only 311 of 456 donors (excluding 87 donors who had received HAV vaccination and 58 for whom there was a lack of information on contact with horses). Substituting contact with horses for any contact with farms allowed inclusion of 357 donors in the model, and in this analysis, both age (OR, 4.5; 95% CI, 1.9–10.5; P < .001) and the presence of anti-HAV (OR, 2.8; 95% CI, 1.1–7.0; P = .039) were associated with presence of anti-HEV.
Donors from 1983
One hundred sixty-nine (79.7%) of the 212 original donors from 1983 had a case form available and serum samples available for testing. We excluded 2 samples for which the test results were inconclusive. Among the remaining samples, the prevalence of anti-HEV was 32.9% (55 of 167 samples). Prevalence of anti-HEV increased significantly with age (P = .04), and antibodies were acquired earlier in life in the donors from 1983 than in the donors from 2003 (table 1). No difference in sex was found. The 1983 donors were younger and had a more equal sex distribution, compared with the 2003 donors, because the 1983 group was selected by age and sex to match the age and sex of the farmers.
Farmers
Of the 291 farmers with complete data, 5 were excluded because of inconclusive test results. Of the remaining 286 samples, 144 (50.3%) were anti-HEV positive. There was a strong correlation between age and presence of anti-HEV (table 1). Among farm exposures, only pig farming was significantly associated with presence of anti-HEV (OR, 1.9; 95% CI, 1.02–3.5; P = .046), but the association between working as a full-time farmer and presence of anti-HEV nearly reached statistical significance (OR, 2.8; P = .055). In a logistic regression analysis of age, sex, pig farming, and full–time farming, only age was independently associated with presence of anti-HEV (OR, 2.7; 95% CI, 1.5–4.8; P = .001).
When stratified by age, the prevalence of anti-HEV was significantly different between each group and the other 2 groups (table 1). To determine whether the increasing prevalence of anti-HEV with age was the result of constant exposure to HEV over time, resulting in a progressively higher prevalence of anti-HEV that paralleled age, or was the result of a higher frequency of exposure to HEV infection in the past, manifested as a higher prevalence of anti-HEV related to year of birth rather than age (i.e., the “cohort effect”), we repeated the analysis after substituting each individual’s age with his or her year of birth. A strong cohort effect was demonstrated among the blood donors, regardless of age (figure 1). In addition, we demonstrated that, within birth cohorts, the prevalence of anti-HEV was significantly higher among farmers who had blood samples obtained in 1983 than among donors in the same year (OR, 1.8; P < .001, by stratified Mantel-Haenzel test). Among the old and new donors, there was no difference in the prevalence of anti-HEV by year of birth. Based on these data, the incidence of HEV transmission has been decreasing since World War II and is currently at a low level. This finding is similar to what has been reported previously for transmission of HAV infection; for the comparison in Denmark, we included data on the prevalence of anti-HAV over time by year of birth among the blood donor population in Odense and among non–drug-using Danish prisoners who had blood samples obtained in 1996 (figure 2) [15, 16]. There has been a sharp decrease in the rate of transmission of HAV infection in a pattern similar to (but not identical to) that of the decrease in the rate of transmission of HEV infection. However, the rates of transmission of both HEV and HAV infection reached very low levels in the 1970s.
Figure 1.
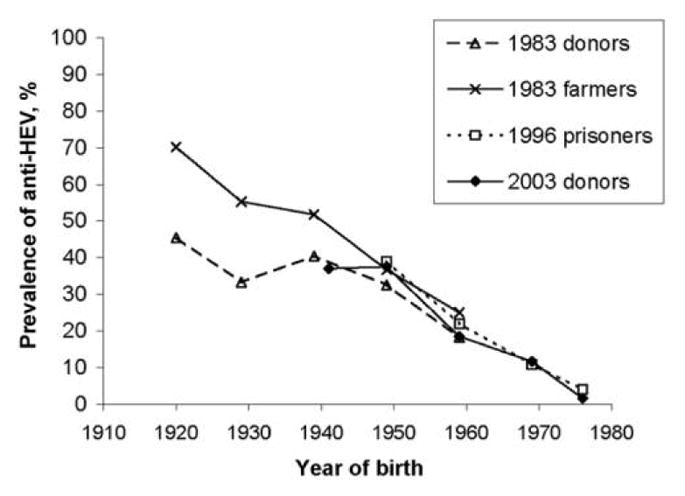
Prevalence of antibody to hepatitis E virus (anti-HEV) among blood donors who had serum samples obtained in 2003, compared with prevalence among donors who had samples obtained in 1983, farmers who had samples obtained in 1983, and prisoners who had samples obtained in 1996, by year of birth.
Figure 2.
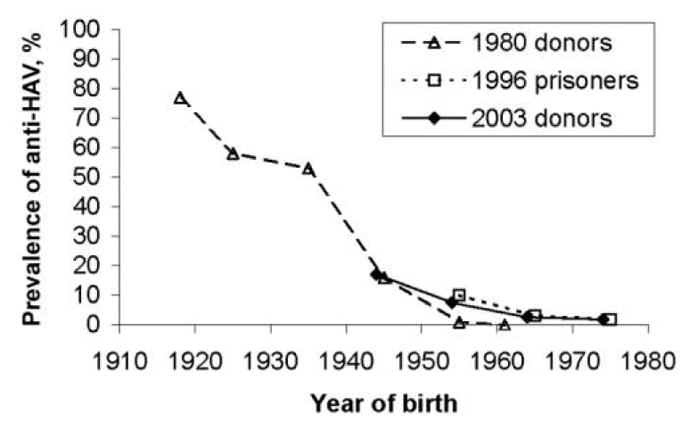
Prevalence of antibody to hepatitis A virus (anti-HAV) among blood donors who had serum samples obtained in 2003, compared with prevalence among donors who had samples obtained in 1980 and prisoners who had samples obtained in 1996, by year of birth. Prisoners included only Danes who were not drug users (n = 138).
DISCUSSION
This study confirms a high prevalence of anti-HEV in a country where clinical hepatitis E is not endemic. Our findings are consistent with previous studies that have found a higher prevalence of anti-HEV with use of the ELISA that we used than with commercial assays [14, 18]. This may be because the National Institutes of Health assay uses a recombinant ORF2-derived capture antigen with a higher sensitivity and with a high level of detection of antibody to both HEV genotype 1 and HEV genotype 3 [12]. This assay may also detect remote infections better than commercial assays that were developed for diagnosis of acute HEV infection [14]. The high prevalence of anti-HEV that was found in industrialized countries has been suggested to reflect false-positive or nonspecific serum reactions [6]. The strong birth cohort effect in our surveys, with uniform decrease in prevalence among different populations tested at different times, is unlikely to have been the result of a non-specific reaction; a similar decrease in prevalence was found in our previous study involving Danish prisoners [10]. Furthermore, we found very little reactivity of stored serum samples with avian HEV capsid antigen, a marker that we used to test for nonspecificity.
The prevalence of anti-HEV increased with age in all populations studied; furthermore, this is, to our knowledge, the first time that a cohort effect on prevalence of anti-HEV has been demonstrated. If the increasing prevalence of anti-HEV by age was the result of ongoing low-level exposure, we would expect to find the same prevalence among persons of the same age, regardless of their year of birth. If, on the other hand, a cohort effect (e.g., higher levels of HEV exposure in the past) was the explanation, we would expect higher prevalence among persons born in the past and unchanged prevalence among each birth cohort, regardless of year of testing, as was found in our study (figure 1). The results of the comparison of birth cohorts suggest that the rate of exposure has decreased significantly in the recent past, especially among persons (both donors and farmers) born after World War II. How long anti-HEV is maintained in the absence of new exposure is not well documented, but long-term persistence was found in studies of hepatitis B virus markers, in which antibodies were demonstrated 40 years after exposure [19].
Few studies have monitored the prevalence of anti-HEV over time. In Japan, no statistically significant difference was detected in overall prevalence between 1974 and 1994; the prevalence increased with age, and there was a significantly higher prevalence in 1974 only among male persons (among whom the prevalence was highest) [20]. In India, the age-specific prevalence of anti-HEV and anti-HAV did not change between 1982 and 1992, suggesting that both HEV and HAV infection were endemic and transmission was ongoing [21]. In contrast, the prevalence of anti-HAV significantly decreased during the same period, both in our study and in several others [15, 20, 22].
Because both HAV and HEV infection are transmitted through the fecal-oral route, improvement in sanitation and general living conditions could be an explanation for the decreasing rate of transmission of both viruses over time. Indeed, the historical record is consistent with a high incidence of both HAV and HEV infection in industrialized countries before the 20th century [1].
A main unanswered question remains: how did 20% of the Danish population became infected? Our results suggest a reservoir of HEV in Denmark, and multiple case reports have confirmed transmission of HEV infection in industrialized countries that are not located near regions of endemicity [23–30]. HEV has been demonstrated in domestic pigs worldwide, and prevalence of anti-HEV was shown to be higher among pig handlers and veterinarians working with swine than among matched control subjects [7, 31–33]. In Sweden, the prevalence of anti-HEV was higher among pig farmers than among others and increased with age, but the differences were not statistically significant [6]. The higher prevalence among farmers in our study is in agreement with findings of most other studies, but this does not explain the high prevalence among donors; only one-half of our donors reported contact with farms, and farm exposure was not associated with presence of anti-HEV. HEV RNA has been demonstrated in pig urine and stool samples, and we speculated that the heavy use of manure as fertilizer in Danish agriculture could lead to low-level exposure to HEV in the general rural population. Several studies have found significant geographical variation in the prevalence of anti-HEV, but we could not confirm this finding [6, 33].
Recently, in 2 independent surveys of raw pig liver purchased in Dutch and US butcher shops, HEV RNA was detected in 6% and 11% of specimens, respectively, and transmission of virus from HEV-positive livers to pigs was demonstrated [34, 35]. Although raw pig liver is rarely consumed in Western countries, handling and preparing liver may pose a risk for transmission. This factor, in addition to the documented transmission of HEV to humans through eating raw deer meat and boar liver in Japan, suggests a transmission route for human infection that could partly explain the high prevalence of anti-HEV in developed countries [36–38].
The association between the presence of anti-HEV and contact with horses is in agreement with a recent Egyptian study that showed a 13% prevalence of anti-HEV in serum samples from work horses [39]. However, contact with horses was infrequently reported by Danish blood donors.
In conclusion, the prevalence of anti-HEV was significant but decreasing in Denmark. The prevalence of anti-HEV was higher among farmers than among blood donors, and this supports the hypothesis that HEV infection is a zoonotic infection transmitted from domestic animals. However, direct contact with farms is probably not the primary route of infection in the Danish population.
Acknowledgments
We thank Keiko Tomioka, for excellent technical assistance in performing testing for antibody to hepatitis E virus, and Thomas Strickland, for thoughtful review of the manuscript.
Financial support. Director of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Potential conflict of interest. All authors: no conflicts.
References
- 1.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–7. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DL, Yarbough PO, Vlahov D, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244–7. doi: 10.1128/jcm.35.5.1244-1247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mast EE, Kuramoto IK, Favorov MO, et al. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J Infect Dis. 1997;176:34–40. doi: 10.1086/514037. [DOI] [PubMed] [Google Scholar]
- 6.Olsen B, Axelsson-Olsson D, Thelin A, Weiland O. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis. 2006;38:55–8. doi: 10.1080/00365540500321470. [DOI] [PubMed] [Google Scholar]
- 7.Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860–5. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng XJ, Halbur PG, Shapiro MS, et al. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–21. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teo CG. Hepatitis E indigenous to economically developed countries: to what extent a zoonosis? Curr Opin Infect Dis. 2006;19:460–6. doi: 10.1097/01.qco.0000244052.61629.49. [DOI] [PubMed] [Google Scholar]
- 10.Christensen PB, Engle RE, Jacobsen SE, Krarup HB, Georgsen J, Purcell RH. High prevalence of hepatitis E antibodies among Danish prisoners and drug users. J Med Virol. 2002;66:49–55. doi: 10.1002/jmv.2110. [DOI] [PubMed] [Google Scholar]
- 11.Hjort C, Andersen P, Schonheyder H, Stenderup A. Occurrence of Aspergillus umbrosus antibodies in Danish farmers. Allergy. 1986;41:104–9. doi: 10.1111/j.1398-9995.1986.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 12.Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–80. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsarev SA, Tsareva TS, Emerson SU, et al. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J Infect Dis. 1993;168:369–78. doi: 10.1093/infdis/168.2.369. [DOI] [PubMed] [Google Scholar]
- 14.Mast EE, Alter MJ, Holland PV, Purcell RH. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27:857–61. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 15.Christensen PB, Homburg KM, Sorensen LT, Georgsen J. Hepatitis A infection and vaccination among Danish blood donors. Scand J Infect Dis. 2005;37:127–30. [PubMed] [Google Scholar]
- 16.Tonnesen E, Sorensen PG, Diederichsen H, Martinussen K. Antibodies to hepatitis A virus in blood donors. Ugeskr Laeger. 1981;143:3301–3. [PubMed] [Google Scholar]
- 17.Guo H, Zhou EM, Sun ZF, Meng XJ, Halbur PG. Identification of B-cell epitopes in the capsid protein of avian hepatitis E virus (avian HEV) that are common to human and swine HEVs or unique to avian HEV. J Gen Virol. 2006;87:217–23. doi: 10.1099/vir.0.81393-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghabrah TM, Tsarev S, Yarbough PO, Emerson SU, Strickland GT, Purcell RH. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134–7. [PubMed] [Google Scholar]
- 19.Seeff LB, Beebe GW, Hoofnagle JH, et al. A serologic follow-up of the 1942 epidemic of post-vaccination hepatitis in the United States Army. N Engl J Med. 1987;316:965–70. doi: 10.1056/NEJM198704163161601. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka E, Matsumoto A, Takeda N, et al. Age-specific antibody to hepatitis E virus has remained constant during the past 20 years in Japan. J Viral Hepat. 2005;12:439–42. doi: 10.1111/j.1365-2893.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 21.Arankalle VA, Tsarev SA, Chadha MS, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–50. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 22.Bottiger M, Christenson B, Grillner L. Hepatitis A immunity in the Swedish population: a study of the prevalence of markers in the Swedish population. Scand J Infect Dis. 1997;29:99–102. doi: 10.3109/00365549709035867. [DOI] [PubMed] [Google Scholar]
- 23.Borgen K, Herremans T, Duizer E, et al. Non–travel-related hepatitis E virus genotype 3 infections in The Netherlands: a case series 2004–2006. BMC Infect Dis. 2008;8:61. doi: 10.1186/1471-2334-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansuy JM, Peron JM, Abravanel F, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419–24. doi: 10.1002/jmv.20206. [DOI] [PubMed] [Google Scholar]
- 25.Ijaz S, Arnold E, Banks M, et al. Non–travel-associated hepatitis E in England and wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;192:1166–72. doi: 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 26.Erker JC, Desai SM, Schlauder GG, Dawson GJ, Mushahwar IK. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J Gen Virol. 1999;80:681–90. doi: 10.1099/0022-1317-80-3-681. [DOI] [PubMed] [Google Scholar]
- 27.Worm HC, Wurzer H, Frosner G. Sporadic hepatitis E in Austria. N Engl J Med. 1998;339:1554–5. doi: 10.1056/NEJM199811193392115. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann WJ, Frosner GG, Eichenlaub D. Transmission of hepatitis E in Germany. Infection. 1998;26:409. doi: 10.1007/BF02770849. [DOI] [PubMed] [Google Scholar]
- 29.Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. Acute hepatitis E infection acquired in California. Clin Infect Dis. 2000;30:618–9. doi: 10.1086/313730. [DOI] [PubMed] [Google Scholar]
- 30.Zanetti AR, Schlauder GG, Romano L, et al. Identification of a novel variant of hepatitis E virus in Italy. J Med Virol. 1999;57:356–60. doi: 10.1002/(sici)1096-9071(199904)57:4<356::aid-jmv5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Meng XJ, Dea S, Engle RE, et al. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;59:297–302. [PubMed] [Google Scholar]
- 32.Drobeniuc J, Favorov MO, Shapiro CN, et al. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis. 2001;184:1594–7. doi: 10.1086/324566. [DOI] [PubMed] [Google Scholar]
- 33.Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–22. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88:912–7. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- 35.Bouwknegt M, Lodder-Verschoor F, van der Poel WH, Rutjes SA, de Roda Husman AM. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J Food Prot. 2007;70:2889–95. doi: 10.4315/0362-028x-70.12.2889. [DOI] [PubMed] [Google Scholar]
- 36.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–3. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–5. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- 39.Saad MD, Hussein HA, Bashandy MM, et al. Hepatitis E virus infection in work horses in Egypt. Infect Genet Evol. 2007;7:368–73. doi: 10.1016/j.meegid.2006.07.007. [DOI] [PubMed] [Google Scholar]


