Abstract
Previous studies suggest that activation of the CNS melanocortin system reduces appetite while increasing sympathetic activity and arterial pressure. The present study tested whether endogenous activity of the CNS melanocortin 3/4 receptors (MC3/4-R) contributes to elevated arterial pressure in the spontaneously hypertensive rat (SHR), a model of hypertension with increased sympathetic activity. A cannula was placed in the lateral ventricle of male SHR and Wistar (WKY) rats for chronic intracerebroventricular (ICV) infusions (0.5 μL/h). Mean arterial pressure (MAP) and heart rate (HR) were recorded 24 hour/d using telemetry. After 5-day control period, rats were infused with MC3/4-R antagonist (SHU-9119, 1 nmol/h-ICV) for 12 days, followed by 5-day posttreatment period. MC3/4-R antagonism increased food intake in SHR by 90% and in WKY by 125%, resulting in marked weight gain, insulin resistance, and hyperleptinemia in SHR and WKY. Despite weight gain, MC3/4-R antagonism reduced HR in SHR and WKY (≈40 bpm), while lowering MAP to a greater extent in SHR (−22±4 mm Hg) than WKY (−4±3 mm Hg). SHU9119 treatment failed to cause further reductions in MAP during chronic adrenergic blockade with propranolol and terazosin. These results suggest that endogenous activity of the CNS melanocortin system contributes to the maintenance of adrenergic tone and elevated arterial pressure in SHR even though mRNA levels for POMC and MC4R in the mediobasal hypothalamus were not increased compared to WKY. These results also support the hypothesis that weight gain does not raise arterial pressure in the absence of a functional MC3/4-R.
Keywords: leptin, melanocortin, hypertension, food intake, blood pressure, POMC, insulin
Previous studies from our laboratory and others indicate that leptin, an adipocyte derived cytokine, may be an important link between obesity, sympathetic activation, and hypertension. Chronic leptin infusion in rodents not only reduces appetite but also raises arterial pressure and heart rate1 by increasing sympathetic nervous system (SNS) activity.2–5 Although the mechanisms involved in mediating the effects of leptin on SNS activity and cardiovascular function have not been fully elucidated, activation of proopiomelanocortin (POMC) neurons in the arcuate nucleus (ARC) of the hypothalamus and stimulation of the melanocortin receptor types 3 and 4 (MC3/4-R) appear to play a key role. We recently showed that chronic blockade of the brain MC3/4R by intracerebroventricular (ICV) infusion of the antagonist, SHU-9119, completely prevented the effects of leptin to reduce food intake and to raise arterial pressure and heart rate.6 SHU-9119 also blocked leptin-induced increases in renal SNS activity.7,8 Furthermore, mice lacking a functional MC4-R receptor are totally unresponsive to the chronic effects of leptin to raise arterial pressure and reduce food intake.9 These results suggest that an intact central MC3/4-R is required for leptin to exert its effects on food intake and cardiovascular function.
In addition to the major role of the MC3/4-R in mediating the effects of leptin on appetite and arterial pressure, our previous studies suggest that the central melanocortin system may play an important role in the regulation of baseline sympathetic tone. Chronic activation of the MC3/4R using a synthetic agonist, Melanotan II (MTII), increased arterial pressure and heart rate,10 and this effect was abolished by α-and β-adrenergic receptor blockade.11 Conversely, chronic antagonism of the MC3/4-R with ICV infusion of SHU-9119 or agouti-related peptide (an endogenous antagonist of the MC3/4-R) in lean normotensive Sprague-Dawley rats markedly reduced heart rate and caused small reductions in arterial pressure despite doubling food intake and causing pronounced weight gain, which are usually associated with increases in arterial pressure and heart rate.10,12 Furthermore, MC4-R–deficient mice are hyperphagic, morbidly obese, hyperinsulinemic, and hyperleptinemic but do not develop hypertension and have lower heart rates compared to age-matched wild-type controls.9,13 These observations suggest that the endogenous activity of the central MC3/4-R may play a tonic role in the regulation of sympathetic tone, and this effect may be important in cardiovascular regulation even in normotensive animals with low to moderate baseline SNS activity.
Given the potential importance of MC3/4-R role in regulating sympathetic tone, we hypothesized that an elevated endogenous activity of the melanocortin system may contribute to increased sympathetic tone and hypertension and that chronic blockade of the MC3/4-R may have a greater antihypertensive action in animals with high sympathetic tone compared to animals with normal or low sympathetic tone. To test this hypothesis we chronically inhibited the central MC3/4-R in spontaneously hypertensive rat (SHR), a widely studied model of hypertension associated with elevated SNS activity, and in control normotensive Wistar rats (WKY). Our results show that blockade of the MC3/4-R reduces arterial pressure in SHR to a much greater extent than in WKY. Adrenergic blockade also caused a greater fall in arterial pressure in SHR compared to WKY, and MC3/4-R antagonism did not further reduce arterial pressure in SHR and WKY during chronic adrenergic receptor blockade. These observations suggest that endogenous tonic activity of the central melanocortin system contributes to the elevated adrenergic activity and hypertension in SHR.
Methods
Animal Surgeries
The experimental procedures and protocols of this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Blood Pressure Telemetry Probe Implantation and Femoral Vein Catheterization
Male SHR (n=12) and WKY rats (n=11) (Taconic Farms) weighing 275 to 325 g were anesthetized with 50 mg/kg sodium pentobarbital (Nembutal), and atropine sulfate (0.1 mg/kg) was administered to prevent excess airway secretions. Using aseptic techniques, a laparotomy was performed and the catheter of the pressure telemetry transmitter (Model TA11PAC40, Data Sciences International) was inserted into the abdominal aorta, distal to the kidneys according to the manufacturer’s instructions. The catheter was fixed in the aorta with a small drop of cyanoacrylate adhesive, and the transmitter was secured to the abdominal wall by sutures. Mean daily 24-hour BP data were derived from the average BP measured by bursts of 10 seconds every 10 minutes using the software (Dataquest 4.0) provided by the manufacturer. Through a left femoral vein incision, a sterile catheter was placed in the vena cava and exteriorized in the scapular region through a subcutaneously implanted stainless steel button.
Additional groups of SHR (n=5) and WKY (n=5) were implanted with arterial and venous catheters for blood sampling and for determination of glomerular filtration rate (GFR) at different time points.
Intracerebroventricular Cannulation
Immediately after telemetry probe and venous catheter implantation, a stainless steel cannula (26 gauge, 10 mm long) was implanted into the right lateral cerebral ventricle using the coordinates as previously described.6 The guide cannula was anchored into place with 2 stainless steel machine screws, a metal cap, and dental acrylic, and a stylet was inserted to seal the cannula until use. During stereotaxic manipulation, anesthesia was maintained with 0.5% isofluorane. Several days after recovery from surgery, accuracy of the cannula placement was tested by measuring the dipsogenic response (immediate drinking of at least 5 mL of water in 10 minutes) to an ICV injection of 100 ng of angiotensin II. After the experiment, the animals were killed and the brains removed and sectioned to confirm the placement of the cannula.
Immediately after surgery, rats were housed individually in metabolic cages for determination of daily food and water consumption. The venous catheter was connected to a syringe pump, via a single-channel infusion swivel (Instech), for continuous infusion of saline (0.9%, 20 mL/d). To maintain total sodium intake constant at ≈3.1 mEq/d despite potential changes in food intake the continuous saline infusion was combined with sodium-deficient rat chow (0.006 mmol sodium/g food, Teklad). Intravenous solutions were infused through a sterile filter (0.22 μm, Millipore) and infusion started immediately after placement of the rats into the metabolic cages. The rats were allowed to recover for 7 to 10 days before control measurements were initiated, and then we began monitoring mean arterial pressure (MAP) and heart rate (HR) continuously 24 hours/d using the telemetry data acquisition system from Data Science International.
Experimental Protocols
MAP, HR, and food and water intake were recorded daily. Fasting blood samples (1.5 mL) were collected once during the control period, on day 11 of the experimental period, and at the end of the recovery period for measurements of glomerular filtration rate (GFR), plasma renin activity (PRA), insulin, glucose, and leptin concentrations. The blood was replaced with 1.5 mL of 0.9% saline.
Chronic MC3/4R Antagonism
After a 5-day control period, the MC3/4-R antagonist, SHU-9119 (1 nmol/h, 0.5 μL/h, Polypeptide Laboratories) or vehicle (0.9% saline) were infused ICV for 12 days in SHR and WKY groups (n=6 per group) via osmotic minipump (model 2002, Durect Corp). The minipump was implanted subcutaneously in the scapular region and connected to the ICV cannula using a tygon tubing (Cole Parmer). The rate of SHU-9119 infusion was based on previous study showing that this dose effectively blocks the MC3/4-R.14,15
Chronic MC3/4R Antagonism During Adrenergic Receptor Blockade
After a 5-day control period, a mixture of the α1 adrenergic receptor antagonist, terazosin (10 mg/kg per day, Sigma), plus a β1/β2 adrenergic receptor antagonist, propranolol (10 mg/kg per day, Sigma), was infused intravenously in SHR (n=6) and WKY (n=5) for 20 days. The compounds were added to the intravenous saline infusion for continuous infusion 24-hour/d. Five days after starting the infusion of the α- and β-adrenergic receptor antagonists, rats received SHU-9119 (1 nmol/h, 0.5 μL/h, ICV) for 10 days. The doses of the adrenergic receptor antagonists were chosen based on our previous studies showing blockade of at least 90% to 95% of the blood pressure and heart rate responses to bolus injection of α- and β1/β2-adrenergic receptors agonists.2
POMC, MC4-R, MC3-R, Leptin Receptor, Neuropeptide Y (NPY), and Agouti-Related Peptide (AGRP) mRNA Message in Mediobasal Hypothalamus of SHR and WKY
Additional groups of noninstrumented 12-week-old SHR (n=12) and WKY (n=11) were euthanized with CO2. The brains were quickly removed and the mediobasal hypothalamus was dissected on an ice cold platform. Tissue samples were frozen immediately by immersion into liquid nitrogen (LN2) and subsequently stored in a “frozen tissue transition solution” (RNAlater-Ice, from Ambion) at −80°C. Total RNA was extracted with TRIZOL (Invitrogen) and the integrity of RNA was monitored using Agilent RNA 6000 kit (2100 Bioanalyzer, Agilent Technologies). First strand cDNA was generated by reverse transcription using iScript cDNA Synthesis Kit (Bio-Rad). Reverse-transcribed material was diluted 1:5 with DEPC-treated water, and 1-μL aliquots (representing approximately 10 ng of the initial total RNA) were used in real-time polymerase chain reaction (PCR) assays (iQ SYBR Green Supermix, Bio-Rad). PCR primers for rat POMC (Reference sequence accession number NM_139326), MC3-R (NM_001025270), MC4-R (NM_013099), NPY (NM_012614), AgRP (XM_001075738), ObRb (NM_012596) and for the reference gene hypoxanthine-guanine phosphoribosyl transferase (HPRT; NM_012583), were taken from the literature.14,15 Fidelity of each PCR amplification was confirmed by melt curve analysis and agarose gel electrophoresis. Relative concentration of mRNA was calculated by using the 2−ΔΔCt method16 and data presented as fold-change comparing WKY to SHR.
Analytic Methods
Plasma renin activity (PRA), plasma insulin, and leptin concentrations were measured by radioimmunoassay. Plasma glucose concentration was measured using the glucose oxidation method (Beckman glucose analyzer 2). GFR was calculated from the 24-hour clearances of [125I]-iothalamate, as previously described.1
Statistical Analysis
The data are expressed as mean±SEM and analyzed by using Student t test and 1-way ANOVA when appropriate. The Bonferroni post hoc test was used for comparisons between groups. Dunnett test was used for comparisons of experimental and control values within each group, when appropriate. Statistical significance was accepted at a level of P<0.05.
Results
Food Intake and Hormones During MC3/4-R Antagonism
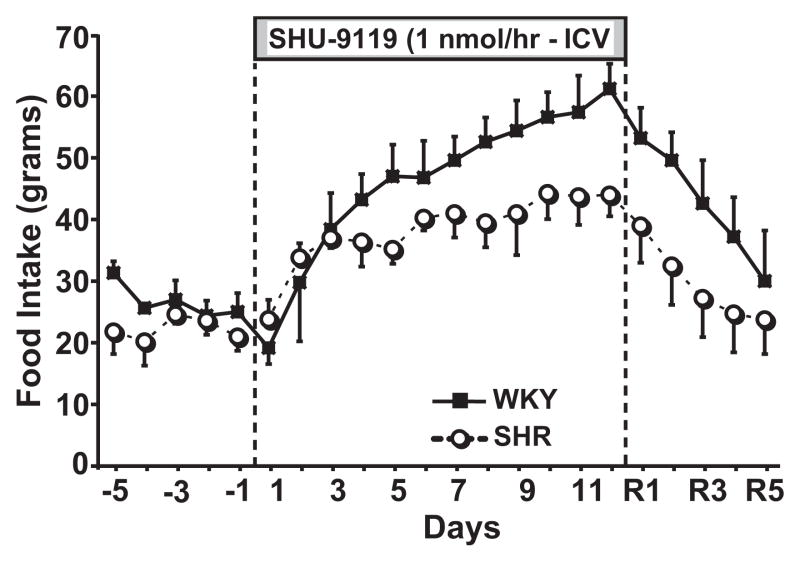
Although WKY and SHR were age matched and no major differences in food intake were observed (Figure 1), the control body weight of WKY rats was approximately 40% larger than that of SHR (Table). Despite the larger body weights of WKY, no differences were found in GFR, PRA, or fasting plasma insulin and glucose levels, and in fact, SHR had significantly higher circulating leptin levels compared to WKY (6.7±3.4 and 2.4±0.7 ng/mL, respectively; Table).
Figure 1.
Effect of chronic MC3/4R antagonism with SHU-9119 on food intake in SHR (n=6) and WKY (n=6).
Table.
Responses to Chronic MC3/4-R Blockade on Body wt, Plasma Hormones, and Renal Function in SHR and WKY Rats
| Experimental Groups | Body Weight, g | Glucose, mmol/L | Insulin, ng/mL | Leptin, ng/mL | PRA, ng AI/mL/h | GFR, mL/min |
|---|---|---|---|---|---|---|
| SHR (n=5) | ||||||
| Control | 329±10† | 6.2±0.3 | 5.3±1.3 | 6.7±3.4 | 5.4±3.4 | 3.8±0.3 |
| SHU-9119 | 397±16*† | 6.4±0.4 | 17.4±1.3* | 12.8±3.7* | 9.6±1.2*† | 4.7±0.3*† |
| Recovery | 386±10*† | 6.1±0.3 | 4.9±1.0 | 2.2±0.1*† | 9.1±0.8*† | 4.5±0.2* |
| WKY (n=5) | ||||||
| Control | 462±14 | 6.3±0.2 | 3.8±0.8 | 2.4±0.7 | 5.7±0.8 | 3.8±0.4 |
| SHU-9119 | 593±22* | 6.4±0.6 | 15.2±1.3* | 18.8±2.3* | 6.9±0.6 | 6.4±0.6* |
| Recovery | 588±28* | 6.4±0.4 | 7.3±2.4 | 12.3±3.1*† | 5.9±0.4 | 4.8±0.3* |
PRA indicates plasma renin activity; GFR, glomerular filtration rate. Values were determined on day 5 of control period, day 11 of SHU-9119 treatment, and day 5 of recovery period.
P<0.05 vs control;
P<0.05 vs WKY.
Chronic antagonism of the MC3/4-R for 12 days markedly increased appetite and food intake doubled by the end of the MC3/4-R blockade period (Figure 1). This increased food consumption was associated with a rapid and pronounced weight gain in SHR (21% gain) and WKY (29% gain) in the short period of 12 days. Along with the increased body weight during SHU-9119 infusion, we observed marked insulin resistance as evidenced by a 3- to 4-fold increase in fasting plasma insulin levels whereas glucose levels remained unchanged. Weight gain was also associated with hyperleptinemia and increased GFR in both groups, whereas PRA increased significantly only in SHR (Table).
After stopping SHU-9119 infusion, food intake gradually decreased and completely returned to baseline control values by day 4 or 5 of recovery. However, body weight remained elevated at the end of the 5-day recovery period, compared to the control values (Table). Plasma insulin and leptin levels returned toward control levels at the end of the recovery period in both groups, although plasma leptin remained significantly elevated in WKY, compared to control values. GFR also decreased during the recovery period in WKY reaching an intermediary value between the values observed for the control and experimental period. In the SHR group GFR remained significantly elevated, compared to control values, 5 days after stopping SHU-9119 infusion (Table). PRA also remained elevated in SHR 5 days after the MC3/4-R antagonist infusion was stopped.
Effects of MC3/4-R Antagonism on MAP and HR
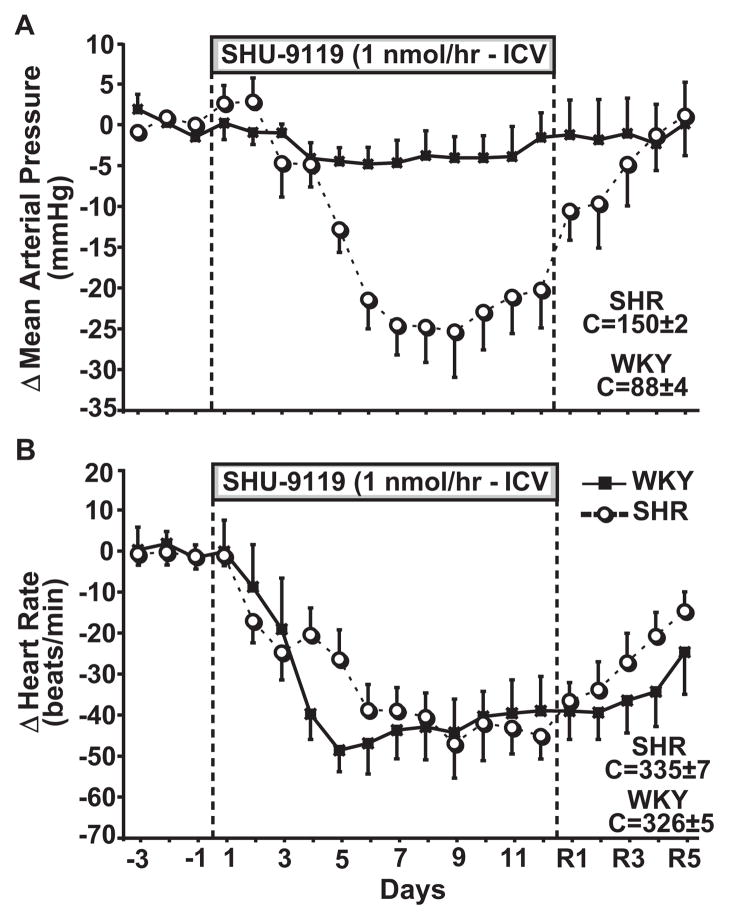
Control MAP was higher in SHR (150±2 mm Hg) compared to WKY (88±4 mm Hg), whereas no differences were found in HR (335±7 and 326±5 bpm, respectively). Chronic MC3/4-R blockade reduced MAP in both groups. However, the reduction in MAP was much greater in SHR (−22 to −25 mm Hg) than in WKY (−4 to −5 mm Hg, average of the last 7 days of SHU-9119 infusion; Figure 2A). HR decreased similarly in both groups during chronic MC3/4-R antagonism (−40 to −45 bpm, Figure 2B).
Figure 2.
Effect of chronic MC3/4R antagonism with SHU-9119 on (A) mean arterial pressure, (B) delta mean arterial pressure, (C) heart rate, and (D) delta heart rate in SHR (n=6) and WKY (n=6).
The bradycardia and the fall in arterial pressure in both groups during chronic MC3/4-R blockade occurred despite increased food intake and pronounced weight gain which are usually associated with increases in arterial pressure and heart rate. These results, therefore, indicate that endogenous activity of the central melanocortin system contributes to the maintenance of elevated arterial pressure in the SHR, a strain with high sympathetic tone, and also support the concept that weight gain can be separated from increases in arterial pressure and heart rate when the central MC3/4-R are inhibited.
Effects of MC3/4-R Antagonism on MAP and HR During Chronic Adrenergic Receptor Blockade
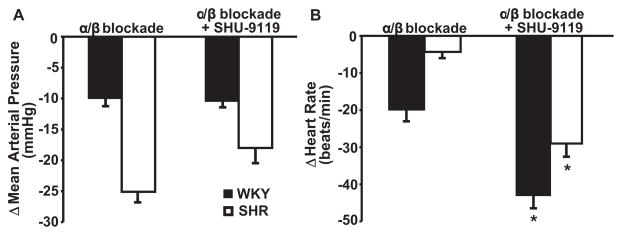
As expected, chronic intravenous infusion of propranolol and terazosin decreased arterial pressure and heart rate in SHR and WKY groups. However, adrenergic blockade reduced MAP to a greater extent in SHR (−25±3 mm Hg) than in WKY (−10±2 mm Hg; Figure 3A), whereas it caused a greater reduction in HR in WKY (−20±4 bpm) than in SHR (−5±2 bpm; Figure 3B). To test whether the fall in arterial pressure and heart rate caused by MC3/4-R antagonism resulted from reduction in adrenergic activity, we infused SHU-9119 after chronic blockade of α1- and β1/β2-adrenergic receptors. Confirming our hypothesis, blockade of the central melanocortin receptors had no additional effects to decrease arterial pressure in rats treated with propranolol and terazosin (Figure 3A). MC3/4-R antagonism, however, did cause a further reduction in HR during adrenergic blockade. These results suggest that the blockade of the endogenous activity of the MC3/4-R lowers MAP mainly by inhibiting adrenergic activity whereas the bradycardia is caused only in part by suppression of adrenergic activity.
Figure 3.
Effect of chronic MC3/4R antagonism with SHU-9119 during α- and β-adrenergic receptor blockade with propranolol and terazosin on (A) mean arterial pressure and (B) heart rate in SHR (n=6) and WKY (n=5). The delta change in mean arterial pressure and heart rate represents the average of the last 5 days of SHU-9119 infusion. *P<0.05 vs α/β blockade alone.
Relative Abundance of POMC, MC4-R, MC3-R, Leptin Receptor, NPY, and AGRP Message in the Mediobasal Hypothalamus of SHR and WKY
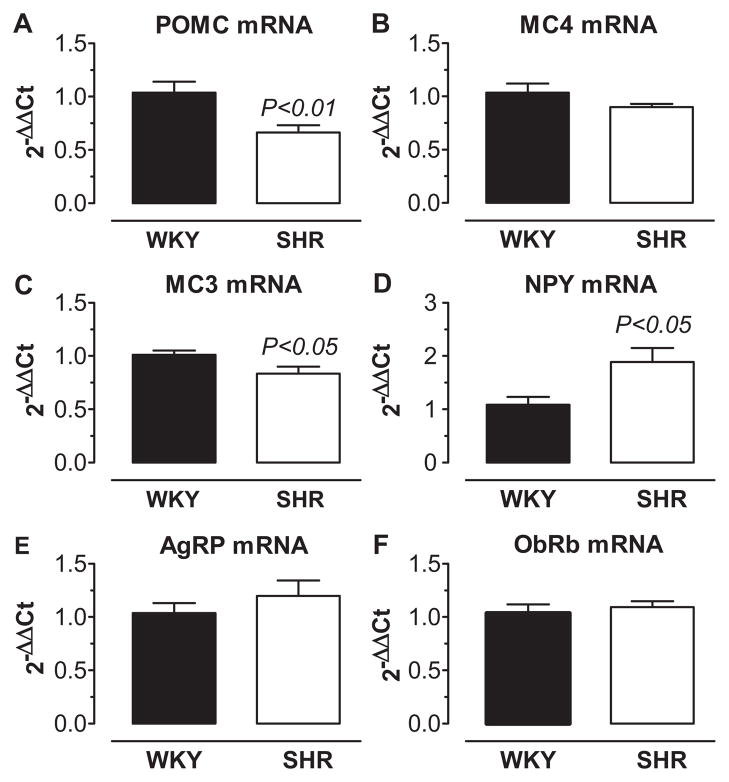
The observation that endogenous tonic activity of the central MC3/4-R appears to play a greater role in the regulation of arterial pressure in SHR compared to WKY led us to hypothesize that the POMC-MC3/4-R pathway is upregulated in the SHR compared to the WKY strain. Therefore, we also investigated whether the relative mRNA abundance for the components of the hypothalamic melanocortin pathway were upregulated in the mediobasal hypothalamus (which includes the arcuate nucleus [ARC]), of SHR compared to WKY. We also determined whether the messages for other neuropeptides (NPY and AGRP) that antagonize the actions of the melanocortin system were downregulated in the SHR. Contrary to what we anticipated, POMC and MC3-R message was reduced and NPY message was increased in SHR compared to WKY (Figure 4). MC4-R message also tended to be reduced in SHR but did not reach statistical significance. No difference was observed for the long-form of the leptin receptor and AGRP message between groups (Figure 4).
Figure 4.
Relative abundance of mRNA message by real-time RT-PCR in mediobasal hypothalamic sections of SHR (n=12) and WKY (n=11) for (A) pro-opiomelanocortin (POMC), (B) melanocortin 4 receptor (MC4), (C) melanocortin 3 receptor (MC3), (D) neuropeptide Y (NPY), (E) agouti-related peptide (AGRP), and (F) the long-form of the leptin receptor (ObRb).
Discussion
An important finding of this study is that chronic blockade of the MC3/4-R markedly reduced arterial pressure in SHR while causing only a small reduction in arterial pressure of normotensive WKY rats. MC3/4-R antagonism also markedly reduced heart rate, but to a similar extent in SHR and WKY. Furthermore, this study demonstrated that the reductions in arterial pressure and heart rate caused by MC3/4R blockade occurred despite hyperphagia and pronounced weight gain in both groups. This suggests that blood pressure in SHR is controlled, at least in part, by the central MC3/4-R, and supports our previous findings that weight gain is not associated with increased arterial pressure and heart rate in the absence of a functional central melanocortin system.10
The fact that blockade of the melanocortin system caused a greater fall in blood pressure in SHR, a hypertensive strain of rats known for its sympathetic overactivity, and that MC3/4-R antagonism failed to lower arterial pressure in the presence of adrenergic receptor blockade suggest: (1) that the endogenous activity of the central melanocortin system plays an important role in the maintenance of high adrenergic tone and elevated arterial pressure in the SHR, and (2) that a reduction in adrenergic activity may mediate the antihypertensive effects of chronic MC3/4-R blockade. In addition, because the kidneys play a pivotal role in long-term blood pressure regulation, the fall in arterial pressure during chronic MC3/4-R antagonism likely reflect a reduction in sympathetic outflow to the kidneys.
The bradycardia observed during MC3/4-R antagonism, however, does not appear to be mediated exclusively via suppression of SNS activity to the heart because chronic adrenergic blockade did not prevent SHU-9119 from further reducing HR. This raises the possibility that there is increased parasympathetic nervous system (PNS) outflow to the heart during MC3/4R blockade. In fact, previous studies demonstrated that acute injection of SHU-9119 into the fourth ventricle near the dorsal vagal complex tended to lower HR in rats.17 Additionally, retrograde tracing of nerve tracts showed innervation of the dorsal vagal complex by POMC neurons from the arcuate nucleus.17 These observations are consistent with the possibility that chronic MC3/4-R blockade may also lower HR by augmenting cardiac PNS outflow via direct modulation of the dorsal vagal complex or indirectly via projections from the hypothalamus. However, further studies would be required to directly test this hypothesis.
Whereas we and others have shown that chronic or acute stimulation of the MC3/4-R causes tachycardia and raises renal SNS activity and arterial pressure in normotensive animals,7,8,11,18 Pavia et al observed that acute microinjection of α-MSH or MTII into the NTS produced a rapid decrease in blood pressure and heart rate in anesthetized Sprague-Dawley rats.19 A depressor response to acute stimulation of the MC3/4-R in the NTS of SHR has also been demonstrated by Tai et al.20 In this latter study, the authors suggested that activation of inducible nitric oxide synthase (iNOS) mediates the cardiovascular actions of MC3/4-R in the NTS by showing attenuation of the hypotension and bradycardia induced by α-MSH in the presence of iNOS inhibitor, aminoguanidine.20 Kuo et al, however, found augmented tachycardic responses to chronic ICV leptin infusion in Sprague-Dawley rats that were pretreated with L-NAME.21 Taken together, these conflicting results may suggest a differential response to activation of the leptin-POMC-MC3/4-R axis in different regions of the brain that may produce opposite cardiovascular responses. Yet, the fact that most previous studies and the present study show bradycardia and hypotensive responses when the MC3/4-R antagonist is delivered via the brain lateral ventricle, which results in concomitant blockade of the MC3/4-R in several brain regions, suggests that that the net result of central MC3/4-R antagonism is marked reduction in HR and arterial pressure. However, acute inhibition of the MC3/4R may produce different responses than chronic MC3/4-R antagonism. There have been no previous studies, to our knowledge, that have tested the chronic effects of MC3/4-R antagonism in a model with high SNS activity such as the SHR. Our observations indicate that chronic CNS MC3/4-R antagonism has potent antihypertensive actions in SHR.
Based on the greater reduction in blood pressure in SHR during chronic MC3/4-R blockade, we hypothesized that the POMC-MC3/4-R pathway may have higher endogenous baseline expression in SHR compared to WKY. However, the relative abundance of mRNA levels of POMC and of the melanocortin 3 and 4 receptors were lower in mediobasal hypothalamus of SHR compared to WKY, whereas higher expression levels of NPY and AGRP, orexigenic peptides that antagonize the actions of the melanocortin system, were observed in the SHR. We are not aware of previous studies that have examined the expression of these orexogenic and anorexogenic peptides in the hypothalamus of SHR and WKY, but POMC expression appears to be reduced in the pituitary gland of SHR22,23 and NPY expression is increased in the arcuate nucleus of the hypothalamus of SHR, compared to WKY.24 Whether the expression of POMC or of MC3/4R are increased in other areas of the brain that also play an important role in mediating the cardiovascular effects of the leptin-melanocortin pathway is unknown and will require further investigation. One potential explanation for a greater fall in arterial pressure in the face of reduced expression of the melanocortin system in the brain of SHR is increased sensitivity of the melanocortin receptors to its endogenous ligands. Furthermore, reduced mRNA expression may not necessarily translate into reduced protein levels. Also, we measured mRNA levels only in the mediobasal hypothalamus, and it is possible that the melanocortins system in other regions of the brain may play an important role in blood pressure regulation. Future studies are needed to determine the levels of POMC, α-MSH, and MC3/4R proteins in specific regions of the brain that could contribute to the greater blood pressure responsiveness to blockade of the MC3/4R in SHR.
It is also important to note that not all of the expected effects of MC3/4-R blockade were augmented in SHR. In fact, the orexigenic actions of SHU-9119 were slightly attenuated in SHR compared to WKY, leading to a 40% greater weight gain in the latter. Balthasar et at recently showed that rescuing MC4R expression only in the paraventricular nucleus of the hypothalamus of MC4R knockout mice prevented the development of obesity mainly by reducing food intake without altering the impaired thermogenic phenotype of these mice.25 These observations suggest differential control of food intake and energy expenditure by MC4R activation in different areas of the CNS. Our results showing different blood pressure and appetite responses to chronic MC4R antagonism in SHR and WKY support the concept of divergent control of cardiovascular function by the melanocortin system, and also lead us to speculate that MC4R activation in areas located outside of the mediobasal hypothalamus may play a role in the regulation of blood pressure. Despite the larger weight gain observed in the WKY during MC3/4-R antagonism, the associated increases in circulating leptin and insulin levels were similar. This suggests that although smaller, SHRs may have similar or higher adiposity compared to the WKY, or that the leptin and insulin responses to increasing adiposity may differ in these 2 strains. However, we did not quantify whole body fat or visceral fat in this study.
Plasma renin activity increased significantly during SHU-9119 treatment only in SHR, which could be explained by a compensatory activation of the renin-angiotensin system attributable to the greater reduction in MAP in the SHR.
Perspectives
Chronic inhibition of the MC3/4-R causes bradycardia and lowers arterial pressure in WKY and SHR despite hyperphagia, rapid weight gain, hyperinsulinemia, and hyperleptinemia, all of which usually cause increases in blood pressure. This implies that a functional MC3/4-R is required for all of these metabolic abnormalities to raise blood pressure. The greater reduction in arterial pressure in SHR during MC3/4-R antagonism and the lack of a further antihypertensive effect after adrenergic blockade suggests that endogenous activation of the MC3/4-R may contribute to the maintenance of elevated adrenergic activity and arterial pressure in SHR. The mechanisms responsible for enhanced blood pressure response to blockade of MC3/4-R in SHR do not appear to be related to increased POMC or MC3/4-R expression in the mediobasal hypothalamus and remain an important topic for future investigation. It would also be of interest to determine whether activation of MC3/4-R contributes to SNS activation and increased blood pressure in other experimental models of hypertension associated with sympathetic activation, or in human essential hypertension which is often associated with SNS activation. Our study also demonstrates differential control of appetite and cardiovascular function by the central MC3/4-R in SHR and WKY. Therefore, development of drugs that differentially target the cardiovascular and metabolic effects of the central melanocortin system could be important tools for understanding the complex interrelationships between obesity, sympathetic activation, and hypertension.
Acknowledgments
We thank Haiyan Zhang for radioimmunoassay of plasma insulin concentration and renin activity in these studies and Calvin Torrey for the measurement of plasma glucose concentration.
Sources of Funding
The authors’ research was supported by a National Heart, Lung, and Blood Institute grant PO1HL-51971 and by a Scientist Development Grant from the American Heart Association to Alexandre A. da Silva.
Footnotes
This paper was sent to Allyn L. Mark, consulting editor, for review by expert referees, editorial decision, and final disposition.
Disclosures
None.
References
- 1.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 3.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 4.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 5.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 7.Haynes WG, Morgon DA, Djalali A, Sivitz WI, Mark AL. Interaction between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 8.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 10.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 11.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 12.Tallam LS, Kuo JJ, da Silva AA, Hall JE. Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension. 2004;44:853–858. doi: 10.1161/01.HYP.0000148993.47498.b2. [DOI] [PubMed] [Google Scholar]
- 13.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 14.Lindqvist N, Napankangas U, Lindblom J, Hallbook F. Proopiomelanocortin and melanocortin receptors in the adult rat retinotectal system and their regulation after optic nerve transaction. Eur J Pharmacol. 2003;482:85–94. doi: 10.1016/j.ejphar.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Rocha M, Bing C, Williams G, Puerta M. Physiologic estradiol levels enhance hypothalamic expression of the long form of the leptin receptor in intact rats. J Nutr Biochem. 2004;15:328–334. doi: 10.1016/j.jnutbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Regul Integr Comp Physiol. 2005;289:R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 18.Silva AA, Kuo JJ, Tallam LS, Liu J, Hall JE. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension. 2006;47:259–264. doi: 10.1161/01.HYP.0000198458.70351.e0. [DOI] [PubMed] [Google Scholar]
- 19.Pavia JM, Schioth HB, Morris MJ. Role of the MC4 receptors in the depressor and bradycardic effects of alpha-MSH in the nucleus tractus solitarii of the rat. Neuroreport. 2003;15:703–707. doi: 10.1097/00001756-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Tai MH, Weng WT, Lo WC, Chan JY, Lin CJ, Lam HC, Tseng CJ. Role of nitric oxide in alpha-melanocyte-stimulating hormone-induced hypotension in the nucleus tractus solitarii of the spontaneously hypertensive rats. J Pharmacol Exp Ther. 2007;321:455–461. doi: 10.1124/jpet.106.118299. [DOI] [PubMed] [Google Scholar]
- 21.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension. 2001;37:670–676. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- 22.Braas KM, Hendley ED. Anterior pituitary proopiomelanocortin expression is decreased in hypertensive rat strains. Endocrinology. 1994;134:196–205. doi: 10.1210/endo.134.1.8275934. [DOI] [PubMed] [Google Scholar]
- 23.Felder RA, Garland DS. POMC biosynthesis in the intermediate lobe of the spontaneously hypertensive rat. Am J Hypertens. 1989;2:618–628. doi: 10.1093/ajh/2.8.618. [DOI] [PubMed] [Google Scholar]
- 24.Sweerts BW, Jarrott B, Lawrence AJ. The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J Neuroendocrinol. 2001;13:608–617. doi: 10.1046/j.1365-2826.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- 25.Balthasar N, DAlgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang C, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]