Abstract
Objective
We sought to determine the physiologic actions and potential therapeutic applications of mutant atrial natriuretic peptide (mANP).
Background
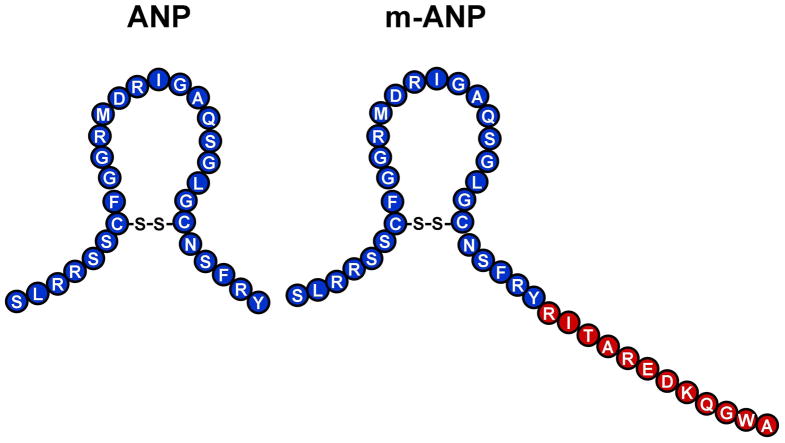
The cardiac hormone atrial natriuretic peptide (ANP) is a 28 amino acid (AA) peptide which consists of a 17 AA ring structure together with a 6 AA N-terminus and a 5 AA C-terminus. In a targeted scan for sequence variants within the human ANP gene, a mutation was identified which results in a 40 AA peptide consisting of native ANP(1-28) and a C-terminal extension of 12 AA. We have termed this peptide mutant ANP.
Methods
In vitro 3′,5′-cyclic guanosine monophosphate (cGMP) activation in response to mANP was studied in cultured human cardiac fibroblasts known to express natriuretic peptide receptor A. The cardiorenal and neurohumoral properties of mANP compared to ANP were assessed in vivo in normal dogs.
Results
We observed an incremental in vitro cGMP dose response with increasing concentrations of mANP. In vivo with high dose mANP (33 pmol/kg/min) we observed significantly greater plasma cGMP activation, diuretic, natriuretic, glomerular filtration rate enhancing, renin-angiotensin-aldosterone system inhibiting, cardiac unloading and blood pressure lowering properties when compared to native ANP. Low dose mANP (2 pmol/kg/min) has natriuretic and diuretic properties without altering systemic hemodynamics compared to no natriuretic or diuretic response with low dose native ANP.
Conclusions
These studies establish that mANP activates cGMP in vitro and exerts greater and more sustained natriuretic, diuretic, glomerular filtration rate, and renal blood flow enhancing actions than native ANP.
Keywords: natriuretic peptide, kidney, heart failure, blood pressure, aldosterone, angiotensin
INTRODUCTION
Atrial natriuretic peptide (ANP) is a 28 amino acid (AA) peptide which consists of a 17 AA ring formed by a disulfide bond together with a 6 AA N-terminus and a 5 AA C-terminus. Studies in animal models of altered ANP production or receptor function as well as studies in humans with ANP infusion have demonstrated that ANP plays an important role in integrated cardiorenal function. ANP possesses natriuretic, vasodilatory, lusitropic, renal enhancing, and renin-angiotensin-aldosterone system (RAAS) inhibiting properties via activation of the natriuretic peptide receptor A (NPR-A) and generation of the second messenger 3′,5′ cyclic guanosine monophosphate (cGMP) (1–6). By activating NPR-A ANP also is anti-hypertrophic and anti-fibrotic and genetic deletion of either the ANP gene (Nppa) or NPR-A results in hypertension, cardiac hypertrophy and fibrosis (7–12). Importantly, ANP is cleared from the circulation through degradation by neutral endopeptidase (NEP) and by a receptor mechanism following binding to the natriuretic peptide receptor C (NPR-C) (13,14).
Most recently mutations for the gene encoding proANP have been reported. Rubattu et al (15) reported that hypertensive subjects carrying an allelic variant in the ANP gene promoter demonstrated increased left ventricular mass index as compared with the wild-type genotype in association with lower plasma pro-ANP levels. In contrast, a recent report involving the Woman’s Health Study reported the presence of an ANP promoter polymorphism is associated with a decrease in the development and progression of hypertension (16).
We recently identified an ANP gene mutation in a Caucasian family with familial atrial fibrillation (17). Translation of the mutant gene results in a fusion protein consisting of the normal 28 AA mature native ANP plus an anomalous C-terminus possessing 12 additional residues (Figure 1). We have termed this 40 AA peptide mutant ANP (mANP).
Figure 1. Amino Acid Sequence of ANP and Mutant ANP.
Amino acid sequence and structure of atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP).
Previous studies have demonstrated that the C-terminus of native ANP enhances the biological actions of ANP based upon augmenting interaction with NPR-A (18). Further, Dendroaspis natriuretic peptide (DNP), which also binds NPR-A, possesses an extended C-terminus (15 AA). DNP possesses greater resistance to degradation by NEP compared to other natriuretic peptides demonstrating that the extended C-terminus contributes to the potent biological properties of DNP (19).
In the current studies we hypothesized that mANP would in vitro activate cGMP using cardiac fibroblasts which are known to highly express NPR-A (20). Most importantly, in in vivo models we hypothesized that mANP, compared to native ANP, would possess more sustained biological actions in the control of cardiorenal function and in suppression of the RAAS.
To test these hypotheses, we synthesized mANP and performed studies in vitro and in vivo to determine the ability of mANP and native ANP to activate cGMP, the second messenger of ANP, and to characterize the cardiorenal and RAAS suppressing properties of mANP and native ANP. In vivo studies were performed in normal anesthetized dogs. The identification of this familial mutation provided the opportunity to better understand the biology of ANP and the important role of the C-terminus in mediating biological activities.
METHODS
Peptides
Mutant ANP was synthesized by the Mayo Protein Core Facility using solid phase methods as previously described (17, 21). Native ANP was purchased from Phoenix (Mountain View, CA). Structure of each peptide was confirmed by mass spectrometry and HPLC analysis confirmed purity of each peptide to be >90%.
Cell Culture
Human cardiac fibroblasts (ScienCell, San Diego, CA) were cultured in fibroblast media (ScienCell, San Diego, CA) and supplemented with fibroblast growth serum, fetal bovine serum, and Penicillin/Streptomycin as previously described (22). Cultured fibroblasts with one to four passages were treated with mANP or native ANP and cGMP generation was determined (23).
Intracellular cGMP
Cells were treated at 80–90% confluency as described previously (22). Briefly, cells were incubated in Hank’s balanced salt solution (Invitrogen, Carlsbad, CA) containing 20mmol/L N-[2-hydroxyethyl]piperazine-N′[2-ethanesulfonic acid], 0.1% bovine serum albumin, and 0.5 mmol/L 3-isobutyl-1-methylzanthine (Sigma, St. Louis, MO). Treated cells received no treatment (control), ANP (10−6 M), or mANP (10−6 M, 10−8 M, or 10−11M) for 10 minutes (Figure 2). Of note, we performed preliminary studies evaluating cGMP generation to various incubation times with the two peptides including 10, 30, and 60 minutes. We found that 10 minutes provided a maximal response in cGMP. Studies were performed in triplicate for each concentration of ANP, mANP, or control. Cells were then lysed in 6% TCA and sonicated for 10 min. The samples were ether extracted four times in ether, dried, and reconstituted in 500ml cGMP assay buffer. The samples were assayed using competitive radioimmunoassay cGMP (Perkin-Elmer, Boston, MA) as previously described (22).
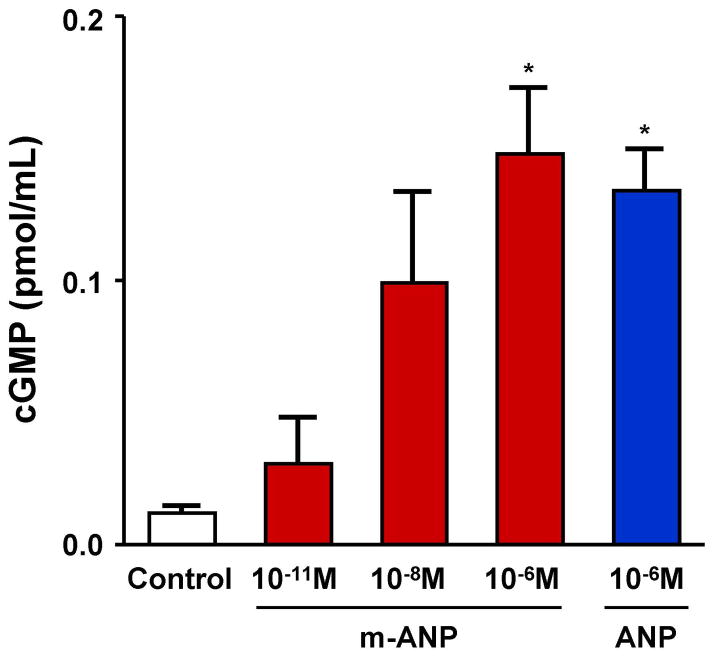
Figure 2. In Vitro cGMP Generation in Response to ANP and Mutant ANP.
In vitro 3′,5′cyclic guanosine monophosphate (cGMP) generation in cultured human cardiac fibroblasts in response to atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP) compared to controls (no treatment). Values are mean ± SEM. *P < 0.05 vs. control by unpaired t-tests.
In Vivo Experimental Protocol
Studies were performed in normal male mongrel dogs (21–26 kg) on a fixed sodium diet (58 mEq/day, Hill’s ID) for at least 15 days prior to experiments with free access to drinking water. The study was performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
The night before experimentation, dogs were fasted and given 300 mg of lithium carbonate for assessment of renal tubular function. All studies were initiated between 8:00 and 10:00 AM. On the day of the experiment, dogs were anesthetized with pentobarbital sodium (15 mg/kg intravenous), intubated, and mechanically ventilated with supplemental oxygen (Harvard respirator, Amersham, MA) at 12 cycles/min. A flow-directed balloon-tipped thermodilution catheter was advanced to the pulmonary artery via the external jugular vein for measurement of cardiac filling pressures and cardiac output. The femoral artery was cannulated for mean arterial blood pressure (MAP) monitoring and blood sampling. The femoral vein was cannulated for inulin and normal saline infusion. Via a left lateral flank incision the left kidney was exposed and the ureter was cannulated for urine sampling. A calibrated electromagnetic flow probe (Carolina Medical Electronics) was placed around the renal artery to measure renal blood flow (RBF). Supplemental non-hypotensive doses of pentobarbital were administered as needed during the experiment.
The study protocol started with the administration of a weight-adjusted inulin bolus. Continuous inulin and saline infusions at a rate of 1 mL/min each were started. After 60 minutes of equilibrium, a baseline clearance was performed. All clearances lasted 30 minutes and consisted of urine collection over 30 minutes. Arterial blood sampling and hemodynamic measurements were measured midway through each clearance. After the baseline clearance, the saline infusion was replaced by either high dose native ANP (n = 7), high dose mANP (n = 7), low dose native ANP (n = 7), or low dose mANP (n = 7). High dose was defined as 33 pmol/kg/min and low dose as 2 pmol/kg/min. Peptides were infused for a total of 45 minutes which included a 15 minute lead-in period followed by a 30 minute clearance. Peptide infusion was then discontinued and four 30-minute clearances were performed (washout, recovery 1, recovery 2, and recovery 3).
Cardiovascular parameters measured included MAP, cardiac output (CO), and pulmonary capillary wedge pressure (PCWP). CO was measured by thermodilution (Cardiac output computer model 9510-A, American Edwards Laboratories). Systemic vascular resistance (SVR) was calculated as (MAP – RAP)/CO. Glomerular filtration rate (GFR) was measured by inulin clearance.
Neurohormonal and Electrolyte Analysis
Plasma and urine ANP were measured by radioimmunoassay as previously described (24). There is high cross reactivity for mANP with the above ANP assay. Plasma and urinary samples for cGMP were measured by RIA using the method of Steiner et al (23). Plasma renin activity (25), angiotensin II (26), and aldosterone (27) were determined by commercially available radioimmunoassays as described previously. Inulin concentrations were measured using the anthrone method for GFR analysis as previously described (28). Electrolytes including lithium were measured by flame photometry (IL943, Instrumentation Laboratory) and GFR was measured by the clearance of inulin. Employing the lithium clearance (CLLi) technique, we calculated the proximal fractional reabsorption of sodium (PFRNa) according to the equation PFRNa = [1 − (CLLi/GFR)] × 100, and we calculated the distal fractional reabsorption of sodium (DFRNa) according to the equation DFRNa = [(CLLi − CLNa)/CLLi] × 100, in which CLLi = [(urine Li × urine flow)/plasma Li] and CLNa = [(urine Na × urine flow)/plasma Na].
Statistical Analysis
Results are expressed as means ± standard error. Student’s unpaired t-tests were employed for single comparisons between groups. Comparisons within a group were made by one-way analysis of variance (ANOVA) for repeated measures followed by Dunnett’s posttest analysis. The baseline measurement was used as the “control” in Dunnett’s analysis. Two-way ANOVA was used to compare the main group effects of mutant ANP vs. native ANP and group differences at specific time points were evaluated by Bonferroni posttest analysis. GraphPad Prism software was used for the above calculations. Statistical significance was accepted as P < 0.05.
RESULTS
Cyclic GMP Generation in Cardiac Fibroblasts
In vitro studies measuring cGMP generation after exposure to ANP and mANP were performed in cultured human cardiac fibroblasts and results are shown in Figure 2. We observed an incremental cGMP generation dose response with increasing concentrations of mANP consistent with activation of NPR-A. There was no significant difference in cGMP generation with ANP (10−6M) and mANP (10−6M).
Cardiorenal and Neurohormonal Function – High Dose
Systemic, renal hemodynamics and cGMP responses are reported in Table 1. There was an overall greater and more sustained decrease in MAP with mANP compared to native ANP. Despite the greater reduction in MAP there was a greater and more sustained increase in renal blood flow (RBF) and GFR with mANP compared to native ANP. There was a trend (P=0.058) towards greater suppression of PCWP with mANP compared to ANP. There also was greater activation of plasma cGMP with mANP compared to native ANP with a trend (P=0.064) for greater urinary cGMP activation.
Table 1.
Cardiovascular, Renal Hemodynamics and cGMP Activating Properties with High Dose (33 pmol·kg−1·min−1) mANP and Native ANP
| Peptide | Baseline | High Dose Infusion | 30 min post infusion | 120 min post infusion | |
|---|---|---|---|---|---|
| MAP†, mmHg | mANP | 133±6 | 120±5* | 120±5* | 122±6* |
| Native ANP | 136±4 | 127±4* | 132±3 | 134±4 | |
| CO†, L/min | mANP | 3.9±0.3 | 3.6±0.3 | 2.9±0.3* | 2.8±0.2* |
| Native ANP | 3.8±0.3 | 3.6±0.3 | 3.5±0.3 | 3.3±0.2 | |
| PCWP, mmHg | mANP | 4.8±0.6 | 2.3±0.7* | 2.3±0.7* | 3.7±1.0* |
| Native ANP | 4.7±0.5 | 2.9±0.4* | 3.3±0.4 | 5.0±0.9 | |
| SVR, mmHg·L−1·min−1 | mANP | 33.8±2.1 | 33.8±2.8 | 42.4±3.7* | 43.0±3.1* |
| Native ANP | 35.7±3.7 | 35.0±3.6 | 37.7±4.0 | 40.6±4.1 | |
| RBF†, ml/min | mANP | 251±30 | 333±21* | 317±16* | 305±15* |
| Native ANP | 245±25 | 288±22* | 282±21* | 280±20* | |
| GFR†, ml/min | mANP | 41.5±5.2 | 65.4±6.6* | 53.2±5.7‡ | 49.1±2.8 |
| Native ANP | 36.1±4.3 | 54.1±4.3* | 33.9±3.9 | 43.1±6.4 | |
| Plasma ANP, pg/ml | mANP | 38.9±2.6 | 313.8±25.7* | 53.7±1.3 | 35.4±2.4 |
| Native ANP | 33.4±2.8 | 478.1±103.8* | 31.8±4.7 | 38.2±4.1 | |
| Plasma cGMP†, nmol/ml | mANP | 12.8±2.7 | 52.4±3.0* | 33.0±4.1* | 10.6±1.4 |
| Native ANP | 11.6±1.5 | 47.1±3.6* | 22.7±2.3* | 11.6±2.4 | |
| Urine cGMP, pmol/min | mANP |
946±100 | 8684±1433* | 6281±1374* | 1451±266 |
| Native ANP | 1000±155 | 6521±1105* | 4487±773* | 1358±223 | |
Values are mean ± SE. ANP, atrial natriuretic peptide; mANP, mutant atrial natriuretic peptide; MAP, mean arterial pressure, CO, cardiac output; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance; RBF, renal blood flow; GFR, glomerular filtration rate; cGMP, 3′,5′-cyclic guanosine monophosphate.
P < 0.05 vs. baseline (1-way ANOVA),
P < 0.05 for main group effect of mANP vs. native ANP (2-way ANOVA),
P < 0.05 for mANP vs. ANP at a specific time point (2-way ANOVA and Bonferroni posttests).
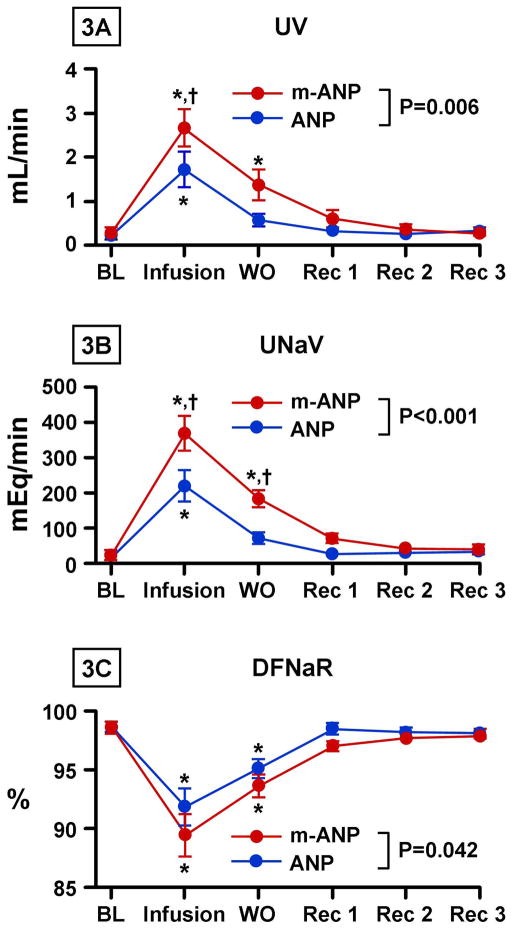
Figures 3A and 3B illustrate urine flow and urinary sodium excretion with high dose (33pmol/kg/min) infusion of mANP and native ANP. There was a greater peak and an overall greater increase in urine flow and sodium excretion with mANP infusion when compared to native ANP. The increased natriuresis with mANP infusion was localized to the distal nephron where there was a greater decrease in DFRNa with mANP infusion compared to native ANP (Figure 3C). There was no difference in PFRNa between the two peptides.
Figure 3. Renal Excretory Response to High Dose ANP and Mutant ANP.
Urine flow (UV) (3A), urine sodium excretion (UNaV) (3B), and distal tubular fractional sodium reabsorption (DFNaR) (3C) after infusion of high dose (33 pmol/kg/min) atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP) in normal dogs. BL represents baseline; Infusion, infusion of high dose (33 pmol/kg/min) of mANP or ANP; WO, washout (0–30 minutes post infusion); Rec 1, 30–60 minutes post infusion; Rec 2, 60–90 minutes post infusion; Rec 3, 90–120 minutes post infusion. Values are mean ± SEM. *P < 0.05 vs. baseline by 1-way ANOVA. †P < 0.05 for mANP vs. ANP at a specific time point as measured by 2-way ANOVA and Bonferroni posttests. P value shown in figure represents the main group effect between between ANP and mANP as measured by 2-way ANOVA.
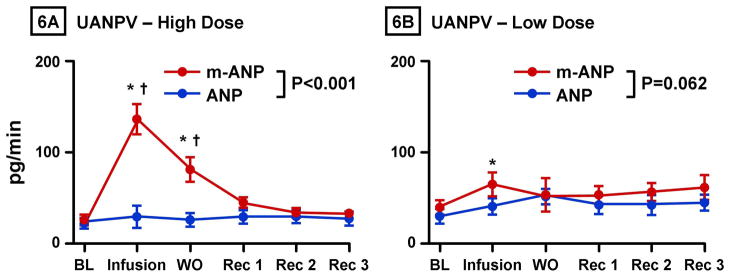
Assessment of the RAAS is reported in Figure 4. There was no significant difference in baseline values of plasma renin, angiotensin II, or aldosterone between mANP and native ANP groups as measured by Student’s unpaired t-test. Overall, there was a greater decrease in plasma renin activity with mANP infusion compared to native ANP (Figure 4A). Further, there was a significantly greater and sustained decrease in both angiotensin II (Figure 4B) and aldosterone (Figure 4C) from baseline with mANP infusion compared to native ANP. Figures 6A illustrates the significantly greater increase in urinary excretion of ANP immunoreactivity with mANP compared to native ANP.
Figure 4. Renin-Angiotension-Aldosterone Response to High Dose ANP and Mutant ANP.
Measurement of the renin-angiotensin-aldosterone system after infusion of high dose (33 pmol/kg/min) atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP) in normal dogs. A, plasma renin activity; B, angiotensin II; C, aldosterone. BL represents baseline; Infusion, infusion of high dose (33 pmol/kg/min) of mANP or ANP; WO, washout (0–30 minutes post infusion); Rec 1, 30–60 minutes post infusion; Rec 2, 60–90 minutes post infusion; Rec 3, 90–120 minutes post infusion. Values are mean ± SEM. *P < 0.05 vs. baseline by 1-way ANOVA. P value shown in figure represents the main group effect between between ANP and mANP as measured by 2-way ANOVA.
Figure 6. Urinary ANP Immunoreactivity Excretory Response to ANP and Mutant ANP.
Urinary atrial natriuretic peptide immunoreactivity excretion (UANPV) after infusion of high dose (33 pmol/kg/min) (6A) and low dose (2 pmol/kg/min) (6B) of atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP). BL represents baseline; Infusion, infusion of low dose or high dose mANP or ANP; WO, washout (0–30 minutes post infusion); Rec 1, 30–60 minutes post infusion; Rec 2, 60–90 minutes post infusion; Rec 3, 90–120 minutes post infusion. Values are mean ± SEM. *P < 0.05 vs. baseline by 1-way ANOVA. †P < 0.05 for mANP vs. ANP at a specific time point as measured by 2-way ANOVA and Bonferroni posttests. P value shown in figure represents the main group effect between between ANP and mANP as measured by 2-way ANOVA.
Cardiorenal and Neurohormonal Function – Low Dose
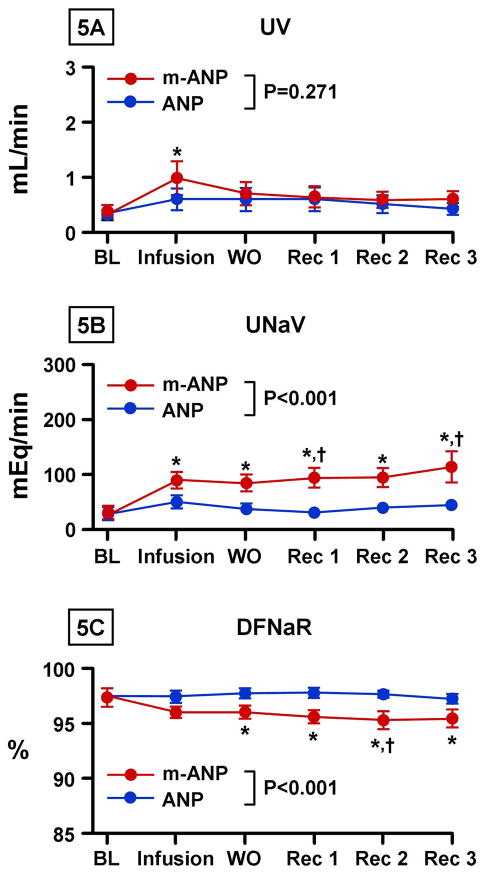
Figures 5A and 5B illustrate urine flow and urinary sodium excretion with low dose (2 pmol/kg/min) infusion of mANP and ANP. A significant increase in urine flow was observed only after mANP administration. There was a significantly greater overall increase in sodium excretion with mANP infusion when compared to native ANP and this increase was sustained for 120 minutes post-infusion. Again, the greater natriuresis was localized to the distal nephron with a greater decrease in DFRNa with mANP infusion (Figure 5C).
Figure 5. Renal Excretory Response to Low Dose ANP and Mutant ANP.
Urine flow (UV) (5A), urine sodium excretion (UNaV) (5B), and distal tubular fractional sodium reabsorption (DFNaR) (5C) after infusion of low dose (2 pmol/kg/min) atrial natriuretic peptide (ANP) and mutant atrial natriuretic peptide (mANP) in normal dogs. BL represents baseline; Infusion, infusion of low dose (2 pmol/kg/min) of mANP or ANP; WO, washout (0–30 minutes post infusion); Rec 1, 30–60 minutes post infusion; Rec 2, 60–90 minutes post infusion; Rec 3, 90–120 minutes post infusion. Values are mean ± SEM. *P < 0.05 vs. baseline by 1-way ANOVA. †P < 0.05 for mANP vs. ANP at a specific time point as measured by 2-way ANOVA and Bonferroni posttests. P value shown in figure represents the main group effect between between ANP and mANP as measured by 2-way ANOVA.
The systemic and renal hemodynamics with low dose infusion are reported in Table 2. In contrast to high dose infusion there was no decrease in MAP after low dose mANP infusion. Further, there was no difference in MAP between mANP and native ANP.
Table 2.
Cardiovascular, Renal Hemodynamics and cGMP Activating Properties with Low Dose (2 pmol·kg−1·min−1) mANP and Native ANP
| Peptide | Baseline | Low Dose Infusion | 30 min post infusion | 120 min post infusion | |
|---|---|---|---|---|---|
| MAP, mmHg |
mANP |
133±3 | 137±3 | 137±3 | 136±3 |
| Native ANP | 131±6 | 131±6 | 132±6 | 135±7 | |
| CO, L/min |
mANP |
3.1±0.1 | 2.6±0.2 | 2.6±0.3 | 2.7±0.2 |
| Native ANP | 3.3±0.4 | 2.6±0.3* | 2.3±0.2* | 2.5±0.3* | |
| PCWP, mmHg |
mANP |
4.1±1.0 | 4.1±0.9 | 4.5±1.1 | 5.0±1.1 |
| Native ANP | 3.6±0.4 | 3.8±0.5 | 4.5±0.5 | 6.5±1.5* | |
| SVR, mmHg·L−1·min−1 |
mANP |
48.5±3.1 | 54.2±5.2 | 56.0±6.7 | 54.0±5.8 |
| Native ANP | 52.4±5.7 | 55.9±4.8 | 59.9±5.3 | 58.9±6.1 | |
| RBF, ml/min |
mANP |
255±13 | 256±6 | 258±13 | 265±14 |
| Native ANP | 251±32 | 219±14 | 223±17 | 232±20 | |
| GFR, ml/min |
mANP |
41.9±4.2 | 50.1±5.1 | 49.2±5.5 | 45.9±6.5 |
| Native ANP | 37.1±5.2 | 51.0±4.0 | 42.0±4.8 | 46.2±6.5 | |
| Plasma ANP, pg/ml |
mANP |
55.1±3.9 | 81.8±7.8* | 63.1±7.4 | 69.8±11.3 |
| Native ANP | 51.9±2.4 | 121.6±7.1* | 69.6±3.4 | 70.9±5.7* | |
| Plasma cGMP†, nmol/ml |
mANP |
10.4±0.7 | 14.1±1.0* | 10.9±1.0 | 10.4±1.0 |
| Native ANP | 12.1±0.7 | 19.9±1.9* | 14.2±1.2 | 12.3±1.2 | |
| Urine cGMP, pmol/min | mANP |
1271±192 | 2013±241* | 1665±265 | 1436±228 |
| Native ANP | 1221±164 | 2505±345* | 2007±320 | 1402±143 | |
Values are mean ± SE. ANP, atrial natriuretic peptide; mANP, mutant atrial natriuretic peptide; MAP, mean arterial pressure, CO, cardiac output; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance; RBF, renal blood flow; GFR, glomerular filtration rate; cGMP, 3′,5′-cyclic guanosine monophosphate.
P < 0.05 vs. baseline (1-way ANOVA),
P < 0.05 for main group effect of mANP vs. native ANP (2-way ANOVA),
P < 0.05 for mANP vs. ANP at a specific time point (2-way ANOVA and Bonferroni posttests).
Plasma levels of renin, angiotensin II, and aldosterone levels were not significantly different between the two peptides (data not shown). Plasma cGMP was greater with ANP infusion but was increased with both peptides. Urinary cGMP excretion was increased with both peptides (Table 2). Figure 6B illustrates that there was no difference in urinary excretion of ANP immunoreactivity with mANP and native ANP at low dose.
DISCUSSION
In this study we have demonstrated that mANP, comprised of native ANP and a 12 AA addition to the C-terminus, activates cGMP in vitro thus indicating the mANP is capable, despite its extended C-terminus, to interact with its particulate guanylyl cyclase receptor, NPR-A. High dose mANP in normal dogs demonstrated greater blood pressure lowering properties together with greater diuretic, natriuretic, GFR enhancing, and RAAS inhibiting properties when compared to native ANP. These enhanced cardiorenal and neurohumoral properties observed with mANP were associated with a greater increase in plasma cGMP than native peptide. We also found that low dose mANP, in normal dogs at non-hypotensive concentrations, has natriuretic and diuretic properties which were not observed with native ANP.
We and others have pursued in the past the molecular design of chimeric natriuretic peptides which combine selected AA sequences from the native natriuretic peptides so as to produce novel designer hormones whose biological actions go beyond those of the native natriuretic peptides. In the current study, we have utilized information from an ANP gene mutation found in a Caucasian family with familial atrial fibrillation (17). Specifically, this new ANP is a result of the translation of the mutant gene resulting in a fusion protein consisting of the normal 28 AA mature native ANP plus an anomalous C-terminus possessing 12 additional residues (Figure 1).
Our in vitro assay clearly demonstrated that mANP can activate natriuretic peptide receptors linked to particulate guanylate cyclase as cGMP generation increased with mANP in cultured human fibroblasts which are known to express NPR-A (20). Increasing mANP concentrations resulted in incremental increases in cGMP generation. These in vitro results establish that mANP, characterized by the 12 AA extension to native ANP, maintains biological activity. When we compared cGMP generation of mANP to ANP in cultured fibroblasts there was no significant difference between the two peptides. This suggests that the in vivo differences seen between mANP and ANP are likely secondary to altered degradation of mANP and not enhanced activation of NPR-A. Further studies will be needed to clarify this speculation.
It is well documented that native ANP promotes diuresis and natriuresis through its direct actions on the kidneys and inhibition of the RAAS together with renal vasodilatory properties (29–32). In this study using normal dogs, we observed a greater natriuretic response with both high and low dose mANP when compared to native ANP. The greater natriuretic properties of both high and low dose mANP appear to be at least partially localized to the distal nephron, where NPR-A is highly expressed (31), with a significantly greater reduction in DFRNa when compared to native ANP. We also demonstrated a greater increase in GFR and RBF with mANP compared to native ANP. This too is consistent with high expression of the NPR-A in the glomerulus and renal vasculature (33).
Despite a decrease in MAP we observed a significant inhibition of the RAAS with high dose mANP as compared to native ANP. The reduction in renin secretion was likely secondary to increased sodium delivery to the macula densa as previous studies have demonstrated an inverse relationship between sodium delivery to the macula densa and renin secretion in view of the enhanced GFR with mANP (34). A direct cGMP dependent action of ANP has also been demonstrated in JGC which may be an alternative mechanism for greater renin suppression (35). The reduction in angiotensin II most likely results from suppression of renin but may also be secondary to increased renal perfusion. Regarding aldosterone, native ANP is known to directly inhibit both the basal and angiotensin II induced secretion of aldosterone from the zona glomerulosa where there is a high concentration of NPR-A receptors (33,36,37). The suppression of aldosterone activation observed with mANP is likely multifactorial including reduced angiotensin II levels as well as greater or prolonged activation of the NPR-A receptors in the adrenal glands and may have contributed to the greater diuresis and natriuresis of mANP.
A hallmark of the natriuretic peptides especially ANP, B-type natriuretic peptide (BNP) and Dendroaspis natriuretic peptide (DNP) is their ability to unload the heart by arterial and venodilatation together with a reduction in preload through diuresis and natriuresis. Of note, mANP demonstrated a more sustained reduction in PCWP compared to native ANP. Further, as stated above, reduction in arterial pressure and increase in RBF were greater with mANP compared to native ANP. Thus, despite a greater reduction in arterial pressure with mANP at the high dose employed with the current study, renal function was more enhanced. This feature is unique compared to other conventional vasodilators that tend to reduce renal perfusion and thus might have highly favorable characteristic with clinical implications.
With low dose mANP we did not observe significant MAP reduction in this study, nor did we demonstrate an inhibition of the RAAS or changes in GFR or RBF. However, it should be noted that low dose mANP resulted in a significant and sustained natriuresis when compared to native ANP. Indeed, this sustained effect on sodium excretion and DFRNa was more prolonged than at high dose underscoring the intrinsic natriuretic properties of mANP and the importance of renal perfusion in modulating the renal response to natriuretic peptides.
Regarding the mechanisms of the greater and sustained actions of mANP, it is possible that the elongated C-terminus of mANP renders the peptide more resistant to degradation by either NEP or clearance by NPR-C. Kinetic studies have shown the rank order for hydrolysis by NEP is C-type natriuretic peptide > ANP > BNP suggesting that the longer the C-terminus the greater resistance to NEP degradation by the peptide (19,38). Indeed, studies show that DNP with a 15 AA C-terminus is highly resistant to NEP degradation and potently natriuretic which has been attributed to resistance to degradation by NEP (19,39,40). As mANP has a 17 AA C terminus, longer than the 15 AA C-terminus of DNP, resistance to hydrolysis by NEP thereby potentiating mANP’s actions is plausible. Importantly, we noted that high dose mANP resulted in significantly greater urinary ANP excretion compared to native ANP consistent with greater resistance to renal degradation by NEP. Alternatively, Shimekake and coworkers (18) demonstrated that the C-terminus of ANP enhances ANP interactions with the NPR-A resulting in greater cGMP activation, enhanced vasorelaxing actions and augmented renal responses. It is possible that the extended C-terminus of mANP also enhances ligand-receptor interactions. Further studies are needed to address this issue including defining the biological actions of the novel entire C-terminus itself. The concept that the C-terminus itself has biological actions are consistent with the report that the 15 AA C-terminus of DNP has intrinsic natriuretic and diuretic actions (21).
Native ANP is currently approved for the treatment of heart failure in Japan. The greater and sustained properties of mANP underscore its own therapeutic potential. Recent studies demonstrate the increasing prevalence of systolic hypertension in the setting of acute heart failure (41). Thus, mANP may provide a reduction in blood pressure while improving renal hemodynamics, natriuresis, and diuresis with suppression of the RAAS. Alternatively, in acute heart failure patients with low blood pressure and subsequent renal compromise, low dose mANP could increase natriuresis without affecting blood pressure.
There are several limitations to the current study including the lack of time controls for the in vivo studies. It is therefore possible that the experimental protocol may account for some of the changes seen with either ANP or mANP infusion. However, we believe that the hemodynamic and neurohumoral data from washout and recovery periods suggest that the changes are secondary to peptide infusion and not the experimental protocol. Further anesthesia may alter the response to ANP and mANP and extrapolation of the data to conscious subjects (animals and humans) should be done so cautiously. Finally, in this study we sought to define the pharmacodynamics of mANP and future studies will need to be performed to define the pharmacokinetics of mANP
In summary, these studies highlight the important biology of the C-terminus of ANP especially the novel properties of a C-terminus defined by a human ANP mutation. Here we demonstrate the ability in vitro of mANP to activate the NPR-A linked to cGMP. This novel mANP, which possesses a longer C-terminus (17 AA) than native ANP and indeed the longest C-terminus of known natriuretic peptides, exhibits greater and more sustained natriuretic, diuretic, GFR, and RBF enhancing actions together with cardiac unloading and RAAS suppressing properties as compared to native ANP. The greater cardiorenal and neurohumoral actions of mANP may be secondary to increased resistance to NEP degradation and/or clearance by NPR-C. Additionally, greater interactions with NPR-A are also possible. These biological properties underscore the therapeutic potential of mANP in cardiorenal disease syndromes and warrant further studies.
Acknowledgments
Financial support: This work was supported by grants from the National Institute of Health (RO1 HL36634 and PO1 HL76611), Stanley J. Sarnoff Endowment for Cardiovascular Science and the Mayo Foundation.
This work was supported by grants from the National Institutes of Health (RO1 HL36634 and PO1 HL76611), Stanley J. Sarnoff Endowment for Cardiovascular Science, and Mayo Foundation. The authors acknowledge the outstanding support of Lynn Harstad, Gail Harty, Denise Heublein, and Sharon Sandberg.
Footnotes
Financial disclosure/Relationship with Industry: Mayo has licensed natriuretic peptides to Nile Therapeutics and Mayo and authors (T. Olson and J. Burnett) have filed patent for mANP. J. Burnett is Chair of Scientific Advisory Board of Nile Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 2.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12(2):131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 3.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;(191):341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cody RJ, Atlas SA, Laragh JH. Physiologic and pharmacologic studies of atrial natriuretic factor: a natriuretic and vasoactive peptide. J Clin Pharmacol. 1987;27(12):927–936. doi: 10.1002/j.1552-4604.1987.tb05592.x. [DOI] [PubMed] [Google Scholar]
- 5.Cody RJ, Atlas SA, Laragh JH, et al. Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion J Clin Invest. 1986;78(5):1362–1374. doi: 10.1172/JCI112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86(4):1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- 7.Patel JB, Valencik ML, Pritchett AM, Burnett JC, Jr, McDonald JA, Redfield MM. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2005;289(2):H777–H784. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- 8.Holtwick R, van EM, Skryabin BV, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111(9):1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101(4):812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vellaichamy E, Kaur K, Pandey KN. Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides. 2007;28(4):893–899. doi: 10.1016/j.peptides.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Oparil S, Feng JA, et al. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42(1):88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 12.Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94(26):14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny AJ, Stephenson SL. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988;232(1):1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- 14.Charles CJ, Espiner EA, Nicholls MG, et al. Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Physiol. 1996;271(2 Pt 2):R373–R380. doi: 10.1152/ajpregu.1996.271.2.R373. [DOI] [PubMed] [Google Scholar]
- 15.Rubattu S, Bigatti G, Evangelista A, et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48(3):499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 16.Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY. Natriuretic peptide precursor a gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50(6):1114–1119. doi: 10.1161/HYPERTENSIONAHA.107.097634. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson-Zingman DM, Karst ML, Zingman LV, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359(2):158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimekake Y, Kawabata T, Nakamura M, Nagata K. The role of the C-terminal region of rat brain natriuretic peptide in receptor selectivity. FEBS Lett. 1992;309(2):185–189. doi: 10.1016/0014-5793(92)81091-y. [DOI] [PubMed] [Google Scholar]
- 19.Chen HH, Lainchbury JG, Burnett JC., Jr Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J Am Coll Cardiol. 2002;40(6):1186–1191. doi: 10.1016/s0735-1097(02)02127-7. [DOI] [PubMed] [Google Scholar]
- 20.Huntley BK, Sandberg SM, Noser JA, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209(3):943–949. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- 21.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52(1):60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuruda T, Boerrigter G, Huntley BK, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91(12):1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 23.Steiner AL, Parker CW, Kipnis DM. The measurement of cyclic nucleotides by radioimmunoassay. Adv Biochem Psychopharmacol. 1970;3:89–111. [PubMed] [Google Scholar]
- 24.Burnett JC, Jr, Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231(4742):1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 25.Haber E, Koerner T, Page LB, Kliman B, Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- 26.Lisy O, Redfield MM, Jovanovic S, et al. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function In vivo. Circulation. 2000;102(3):338–343. doi: 10.1161/01.cir.102.3.338. [DOI] [PubMed] [Google Scholar]
- 27.Sancho J, Haber E. A direct microassay for aldosterone in plasma extracts. J Clin Endocrinol Metab. 1978;47(2):391–396. doi: 10.1210/jcem-47-2-391. [DOI] [PubMed] [Google Scholar]
- 28.Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med. 1963;62:351–356. [PubMed] [Google Scholar]
- 29.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247(5 Pt 2):F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 30.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sciences. 1981;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 31.Gunning ME, Ballermann BJ, Silva P, Brenner BM, Zeidel ML. Characterization of ANP receptors in rabbit inner medullary collecting duct cells. Am J Physiol. 1988;255(2 Pt 2):F324–F330. doi: 10.1152/ajprenal.1988.255.2.F324. [DOI] [PubMed] [Google Scholar]
- 32.Drexler H, Hirth C, Stasch HP, Lu W, Neuser D, Just H. Vasodilatory action of endogenous atrial natriuretic factor in a rat model of chronic heart failure as determined by monoclonal ANF antibody. Circ Res. 1990;66(5):1371–1380. doi: 10.1161/01.res.66.5.1371. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox JN, Augustine A, Goeddel DV, Lowe DG. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol. 1991;11(7):3454–3462. doi: 10.1128/mcb.11.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis JO, Freeman RH. Mechanisms regulating renin release. Physiol Rev. 1976;56(1):1–56. doi: 10.1152/physrev.1976.56.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz A, Della BR, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986;83(13):4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atarashi K, Mulrow PJ, Franco-Saenz R. Effect of atrial peptides on aldosterone production. J Clin Invest. 1985;76(5):1807–1811. doi: 10.1172/JCI112172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atarashi K, Mulrow PJ, Franco-Saenz R, Snajdar R, Rapp J. Inhibition of aldosterone production by an atrial extract. Science. 1984;224(4652):992–994. doi: 10.1126/science.6326267. [DOI] [PubMed] [Google Scholar]
- 38.Dussaule JC, Stefanski A, Bea ML, Ronco P, Ardaillou R. Characterization of neutral endopeptidase in vascular smooth muscle cells of rabbit renal cortex. Am J Physiol. 1993;264(1 Pt 2):F45–F52. doi: 10.1152/ajprenal.1993.264.1.F45. [DOI] [PubMed] [Google Scholar]
- 39.Lisy O, Jougasaki M, Heublein DM, et al. Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 1999;56(2):502–508. doi: 10.1046/j.1523-1755.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 40.Lisy O, Lainchbury JG, Leskinen H, Burnett JC., Jr Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension. 2001;37(4):1089–1094. doi: 10.1161/01.hyp.37.4.1089. [DOI] [PubMed] [Google Scholar]
- 41.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]