Abstract
Rationale
Cyclic nucleotide phosphodiesterases (PDE) through the degradation of second messenger cyclic guanosine monophosphate (cGMP) play critical roles in maintaining cardiomyocyte homeostasis. Ca2+/CaM-activated cGMP-hydrolyzing PDE1 family may play a pivotal role in balancing intracellular Ca2+/CaM and cGMP signaling, however its function in cardiomyocytes is unknown.
Objective
Herein we investigate the role of Ca2+/CaM-stimulated PDE1 in regulating pathological cardiomyocyte hypertrophy in neonatal and adult rat ventricular myocytes (NRVM and ARVM) and in the heart in vivo.
Methods and Results
Inhibition of PDE1 activity using a PDE1 selective inhibitor IC86340 or downregulation of PDE1A using siRNA prevented phenylephrine (PE) induced pathological myocyte hypertrophy and hypertrophic marker expression in neonatal (NRVM) and adult (ARVM) rat ventricular myocytes. Importantly, administration of the PDE1 inhibitor IC86340 attenuated cardiac hypertrophy induced by chronic ISO infusion in vivo. Both PDE1A and PDE1C mRNA and protein were detected in human hearts, however PDE1A expression was conserved in rodent hearts. Moreover, PDE1A expression was significantly upregulated in vivo in the heart and myocytes from various pathological hypertrophy animal models and in vitro in isolated NRVM and ARVM treated with neurohumoral stimuli such as angiotensin II (Ang II) and ISO. Further, PDE1A plays a critical role in PE-induced reduction of intracellular cGMP and PKG activity, and thereby cardiomyocyte hypertrophy in vitro.
Conclusions
These results elucidate a novel role for Ca2+/CaM-stimulated PDE1, particularly PDE1A, in regulating pathological cardiomyocyte hypertrophy via a cGMP/PKG-dependent mechanism, thereby demonstrating Ca2+ and cGMP signaling cross-talk during cardiac hypertrophy.
Keywords: phosphodiesterase, cGMP, cardiomyocyte, cardiac hypertrophy
INTRODUCTION
Ca2+/CaM dependent signaling has been implicated in promoting pathological gene expression involved in hypertrophy and heart failure through the activation of Ca2+/CaM-dependent kinases, phosphatases, and ion channels1. Recently, a number of intrinsic negative regulators of cardiac growth have been identified which activate cGMP-dependent signaling2. Stimulation of cGMP synthesis through genetic upregulation of natriuretic peptide receptor (GC-A) prevents neurohumoral or pressure overload induced hypertrophy3, while disruption of cGMP synthesis results in enhanced hypertrophy and deteriorated cardiac function4. Likewise, chronic inhibition of cGMP metabolism by a PDE5 inhibitor prevents and reverses pressure overload induced cardiac hypertrophy5.
PDEs, by degrading cAMP and/or cGMP, regulate the amplitude, duration, and compartmentation of intracellular cyclic nucleotide signaling. PDEs constitute a superfamily of enzymes grouped into 11 broad families based on their distinct kinetic, regulatory, and inhibitory properties. PDE family members are also differentially expressed in various tissues and present within distinct sub-cellular domains. Together, these properties enable PDE enzymes to regulate the spatio-temporal, intracellular cAMP and cGMP gradients in response to various external stimuli. At least five PDE families, PDE1–5, have been reported in the heart, of which PDE1 and PDE5 are most likely responsible for cGMP hydrolysis. Logically alteration of cGMP-hydrolyzing PDE expression/activity may contribute to a pathological imbalance observed during prolonged stress.
Ca2+/CaM-stimulated PDE1 family constitutes three gene products: PDE1A, PDE1B, and PDE1C6. In vitro, PDE1A and PDE1B have been shown to preferentially hydrolyze cGMP with greater affinity than cAMP, while PDE1C hydrolyzes cAMP and cGMP with equally high affinity6. In vivo, PDE1A has been shown to preferentially hydrolyze cGMP in VSMCs7, 8. Although a recent study has reported that PDE1C is highly expressed in the human myocardium and present in human cardiomyocytes9, the physiologic and pathologic roles of PDE1 in the heart are still unknown. Here, we identified both PDE1A and PDE1C expression in human hearts, while PDE1A expression was more conserved in rodent hearts and in NRVM and ARVM. Using various loss-of function strategies in isolated NRVM and ARVM we demonstrated that PDE1A regulates cardiomyocyte hypertrophy, which is dependent on modulating cGMP/PKG signaling. We further showed that the PDE1 selective inhibitor IC86340 was able to reduce myocyte hypertrophy in an ISO-induced hypertrophy mouse model. Moreover, we observed that PDE1A expression was significantly upregulated in the heart and myocytes from various pathological hypertrophy rodent models and in isolated cardiomyocytes treated with hypertrophic stimuli. Together, our findings suggest a novel role for PDE1, particularly PDE1A, in regulating cardiomyocyte hypertrophic growth.
MATERIALS AND METHODS
Animal care and use was in accordance with institutional guidelines. Cardiac hypertrophy was induced in vivo by chronic infusion of angiotensin II (Ang II) at 0.7 mg/kg/day for 2 weeks in Sprague-Dawley rats or isoproterenol (ISO) at 30 mg/kg/day for 1 week in C57Bl/6J mice, as previously described10. TAC was performed in 8–10 week old male C57Bl/6J mice as previously described10, 11. IC86340 (3 mg/kg/day) or vehicle was administered daily via i.p injection.
An expanded Methods section is available in the online supplement.
RESULTS
PDE1 inhibitors reduce cardiomyocyte hypertrophy and hypertrophic gene expression in cardiomyocytes
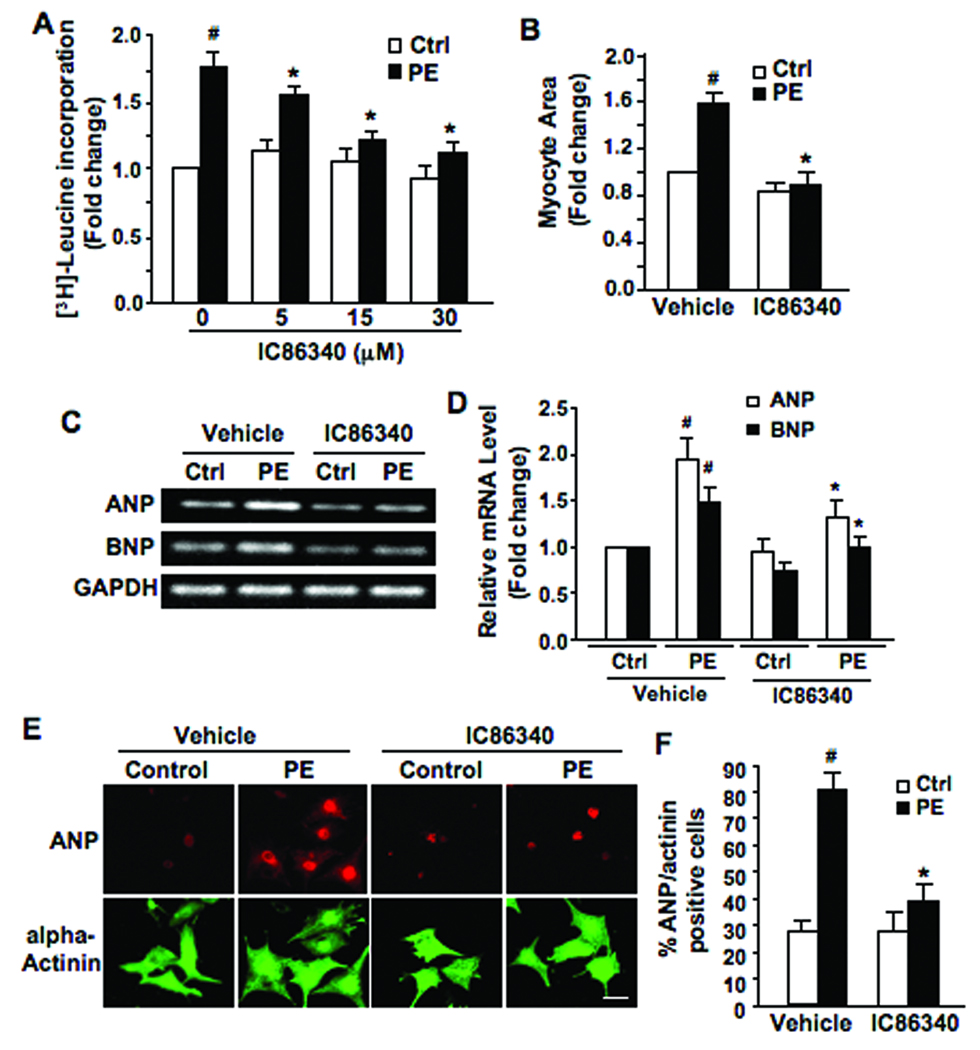
To determine the role of PDE1 in cardiomyocyte hypertrophy, we first used pharmacological inhibitors of PDE1 in isolated NRVM. Treatment of NRVM with PDE1 inhibitor, IC863408 dose-dependently attenuated the PE-induced protein synthesis assessed by leucine incorporation (Fig. 1A). A distinct PDE1 inhibitor, 8-MM-IBMX (8-methoxymethyl-isobutylmethylxanthine)12 used at 20 µmol/L, elicited similar inhibitory effects (data not shown). Furthermore, IC86340 treatment resulted in a substantial reduction in the PE-induced myocyte surface area (Fig. 1B). Similarly, we observed that IC86340 also blunted ISO-stimulated protein synthesis (data not shown). Based on previously reported IC50 values of IC86340 on inhibiting different PDE family members (Supplemental Table), the doses of IC86340 used in this study should preferentially hydrolyze PDE1.
Figure 1. Effects of PDE1 inhibitor on pathological NRVM hypertrophy.
(A) [3H]-leucine incorporation in NRVM pre-treated with IC86340 (5, 15, or 30 µmol/L) or vehicle for 30 min, followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 24–48 hours. (B) Total cardiomyocyte surface area was averaged from 100 alpha-actinin immuno-positive cells per condition. Myocytes were pretreated with IC86340 (30 µmol/L) or vehicle, followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 48 hours. (C) RT-PCR results showing ANP, BNP, skeletal alpha-actin, and GAPDH mRNA in NRVM pretreated with IC86340 (30 µmol/L) or vehicle, followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 24–48 hours. (D) Quantified RT-PCR results analyzed by densitometry in a linear range, showing the mRNA levels of ANP and BNP relative to GAPDH. (E) Representative micrographs of myocytes treated as abovementioned. Cells were subjected to immunostaining for ANP and sarcomeric alpha-actinin. Scale bar=50 µm. (F) ANP positive cells were measured by cells with perinuclear ANP staining as a percent of total alpha-actinin positive cells. (n=100 cells per condition). Values are mean±SD of triplicates. Data were normalized to sample (vehicle alone) that was arbitrarily set to 1.0. Values are mean±SD of at least three independent experiments performed in triplicates. #p<0.05 vs. vehicle alone, *p<0.05 vs. vehicle+PE.
The anti-hypertrophic effects of PDE1 inhibitor correlated with a reduction in the mRNA levels of hypertrophic markers, such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) (Fig. 1C and D). Moreover, we examined ANP expression via perinuclear ANP immunostaining as described previously13 (Fig. 1E and F). We found that PE markedly stimulated the percentage of ANP/actinin positive cells compared to the vehicle control, while IC86340 treatment abrogated these effects. Together, these results implicate PDE1 in regulating pathological myocyte hypertrophy in vitro.
PDE1 inhibitor prevents adult rat ventricular myocyte hypertrophy
We next examined the effects of PDE1 inhibition on ARVM hypertrophy by measuring myocyte surface area, width/length of myocytes, and protein synthesis. Indeed, PDE1 inhibition with IC86340 in ARVM significantly reduced the PE-induced myocyte surface area (Fig. 2A and B), myocyte width (Fig. 2C), and protein synthesis assessed by [3H]-leucine incorporation (Fig. 2D).
Figure 2. Effects of PDE1 inhibitor on PE-induced ARVM hypertrophy.
(A) Representative bright-field micrographs of myocytes treated with IC86340 (15 µmol/L) or vehicle, followed by PE (10 µmol/L) or vehicle (control) stimulation for 24 hours. (B and C) Changes in mean cell surface areas (B) and width (C) in ARVM pretreated with IC86340 (15 µmol/L), followed by PE (10 µmol/L) or vehicle (ctrl) stimulation for 24 hours. Data represent the average of a minimum of 100 myocytes per condition. (D) [3H]-leucine incorporation in ARVM treated as abovementioned. Data were normalized to the sample (vehicle alone) that was arbitrarily set to 1.0. Values are mean±SD of three independent experiments performed in triplicates. #p<0.05,vs. vehicle alone, *p<0.05 vs. vehicle+PE.
PDE1 inhibitor reduced myocyte hypertrophy in vivo in ISO-induced mouse hypertrophy model
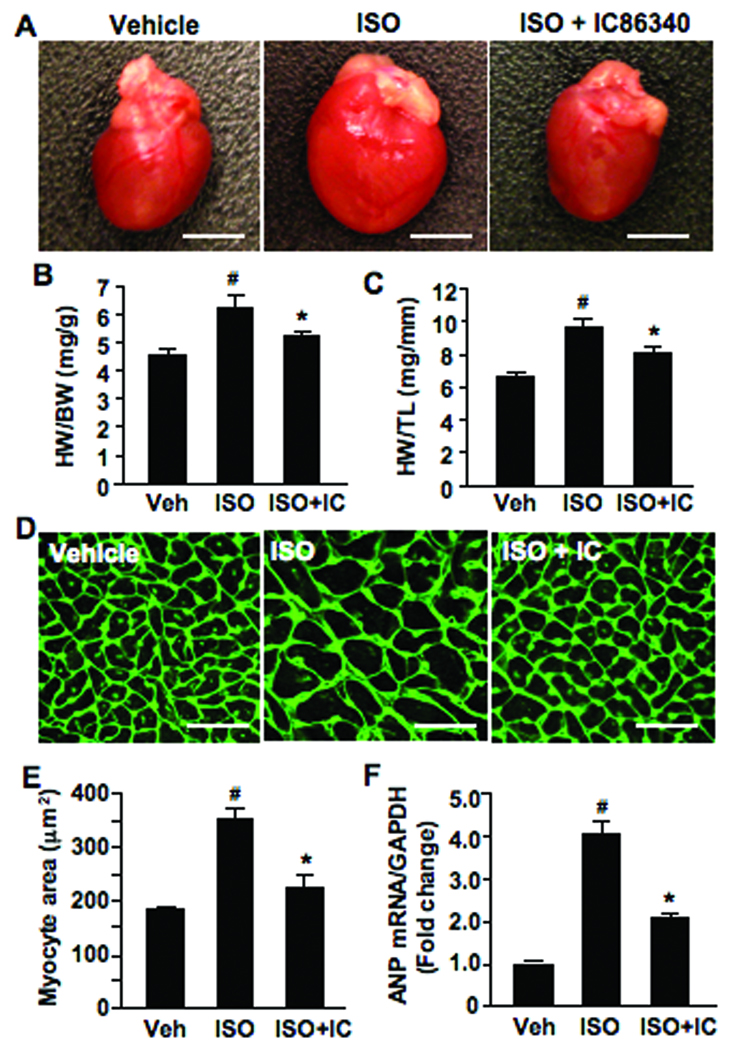
To further examine whether PDE1 inhibition attenuates adult cardiac hypertrophy in vivo, we administered the PDE1 inhibitor IC86340 in mice. We found that mice receiving daily IC86340 treatment at the doses of 3 and 6 mg/kg/day had normal heart and body weights. A small reduction of blood pressure ≈10–20 mmHg and no changes in heart rate were observed (Supplemental Fig. S1), which is consistent with previous findings that PDE1 regulates vascular reactivity7, 14. Therein, we examined the effects of IC86340 on cardiac hypertrophy using a mouse hypertrophy model induced by ISO infusion at 30 mg/kg/day for 7 days. It has been demonstrated that there is no correlation between ISO-induced cardiac hypertrophy and blood pressure15. We found that ISO infusion significantly induced cardiac hypertrophy analyzed by gross heart morphology (Fig. 3A), heart weight/body weight (HW/BW) ratio (Fig. 3B), and heart weight/tibia length (HW/TL) ratio (Fig. 3C), which were attenuated by IC86340 treatment (3 mg/kg/day). Further, we found that ISO enlarged cardiac myocyte cross-sectional area and ANP gene expression, which is significantly reduced by IC86340 treatment (Fig. 3D–F). We determined the plasma level of IC86340 by PDE assay to be approximately 12 µM (Supplemental methods). Taken together, these results support the notion that PDE1 plays a critical role in ISO-induced cardiac hypertrophy in vivo.
Figure 3. Effects of PDE1 inhibitor on ISO-induced cardiac hypertrophy in vivo.
(A) Representative gross heart images showing effects of PDE1 inhibitor on cardiac hypertrophy. Scale bars: 5mm. Animals were infused with vehicle or ISO (30mg/kg/d) for 7 days, and administered vehicle (20% DMSO) or IC86340 (3 mg/kg/d) daily via i.p injection for 10 days (3 days prior and 7 days during ISO infusion). (B) Quantified results of heart weight/ body weight ratio (mg/g). (C) Heart weight/ tibia length ratio (mg/mm). (D) Representative WGA-FITC (green) stained heart sections showing cardiomyocyte cross-sectional area. Scale bars: 25µm. (E) Quantified results of cardiomyocyte cross-sectional area (averaged from 200 random myocytes per section per animal). (F) Hypertrophic marker ANP mRNA expression normalized to GAPDH analyzed by quantitative real-time RT-PCR. Data represent mean±SD from vehicle (n=16), ISO (n=12) and ISO+IC86340 (n=12). #p<0.05,vs. vehicle alone, *p<0.05 vs. vehicle + ISO.
PDE1A, 1B and 1C are differentially expressed in adult human, rat, and mouse hearts, as well as in NRVM and ARVM
To evaluate the presence of Ca2+/CaM-stimulated PDE1 enzymes in the heart and in isolated cardiomyocytes, both mRNA and protein expression were examined in heart samples from various species relative to known PDE1-expressing tissues such as brain and testis. We observed PDE1A mRNA to be detected at nearly equivalent levels in human, rat and mouse hearts, while PDE1C was primarily detected in human and mouse hearts, and PDE1B was weakly detected overall in the heart (Fig. 4A–C). We observed that PDE1A protein levels were comparable in hearts from different species whereas PDE1B was not detectable in the hearts, consistent with the mRNA expression (Fig, 4D). However, mouse heart elicited much lower PDE1C protein expression compared with human, inconsistent with the mRNA expression level (Fig. 4D). The lower PDE1C protein in mouse heart is unlikely due to antibody insensitivity since the antibody strongly recognized mouse testis (Fig. 4D). Additionally, we observed PDE1A mRNA and protein in both NRVM and ARVM at comparable levels to that in adult rat heart, while PDE1B and PDE1C expression levels were significantly lower in these cells (Fig. 4E and F). Together these data indicate that both PDE1A and PDE1C isoforms are present in human hearts, while PDE1A expression is conserved in rodent hearts, particularly in rat cardiomyocytes.
Figure 4. PDE1 mRNA and protein expression in human, rat, and mouse ventricular tissues and isolated cardiomyocytes.
(A–C) RT-PCR results showing PDE1A, PDE1B, and PDE1C mRNA expression in adult human, rat, and mouse heart tissue compared to indicated-controls (mouse brain for PDE1A and 1B or mouse testis for PDE1C). RT-PCR data was quantified by densitometry in a linear range from three independent samples, which were normalized to GAPDH mRNA levels and expressed relative to human hearts (AU=arbitrary units). (D) Representative western blot showing relative PDE1A, PDE1B, and PDE1C protein levels in human, rat, and mouse hearts, compared to respective controls (Brain for PDE1A and PDE1B; Testis for PDE1C). GAPDH was used to normalize protein loading. (E and F) PDE1 expression in isolated neonatal and adult cardiomyocytes (NRVM and ARVM). RT-PCR showing relative PDE1A, 1B, and 1C mRNA levels in NRVM, ARVM, and rat hearts, compared to respective controls (E). Western blot depicting relative PDE1A, 1B, and 1C protein levels in NRVM, ARVM, compared to rat hearts and respective controls. GAPDH was used to normalize mRNA and protein expression.
Down-regulation of PDE1A expression inhibits cardiomyocyte hypertrophy in NRVM and ARVM
To specifically determine the contribution of PDE1A in myocyte hypertrophy, we used gene silencing to down-regulate PDE1A expression. PDE1A expression levels were significantly down-regulated in NRVM by a PDE1A siRNA specifically targeting the N-terminal sequence of rat PDE1A (Fig. 5A, inset). The negative control, a non-targeting control siRNA had no effect on PDE1A protein levels. As a specificity control, we found that PDE1A siRNA did not alter the expression of other PDE isoforms such as PDE1C and PDE5A (data not shown). We found that treatment with PDE1A siRNA significantly abrogated the PE-mediated increase in protein synthesis (Fig. 5A) and total myocyte surface area compared to controls in NRVM (Fig. 5B). We obtained similar results in NRVM treated with adenovirus encoding a different PDE1A shRNA, specifically targeting the C-terminal sequence of rat PDE1A (Supplement Fig. S2). These findings coincided with reduced ANP marker expression in NRVM (Fig. 5C). Downregulation of PDE1A followed by IC86340 treatment reduced NRVM protein synthesis to similar levels while the PDE5 inhibitor sildenafil potentiated these effects, suggesting that IC86340 effects are attributed to PDE1A (Fig. 5D). Ad-PDE1A shRNA also significantly downregulated PDE1A expression in ARVM (Fig. 5E, inset), in which we observed blunted PE-induced protein synthesis (Fig. 5E) and total myocyte area (Fig. 5F).
Figure 5. Effects of PDE1A down-regulation on PE-induced cardiomyocyte hypertrophy.
(A) Protein synthesis assessed by [3H]-leucine incorporation in NRVM transfected with off-targeting control siRNA or PDE1A siRNA followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 24 hours. (Inset), Representative blot showing PDE1A protein expression in NRVM transfected with control or PDE1A siRNA. (B) Total cell surface area of cardiomyocytes treated with siRNA as abovementioned. (C) Representative RT-PCR results showing ANP mRNA expression in NRVM treated with siRNA as abovementioned. Data were quantified by densitometry in a linear range and normalized to GAPDH mRNA levels. (D) [3H]-leucine incorporation in NRVM transduced with adenovirus expressing the shRNA targeting LacZ (Ad-LacZ shRNA, as a negative control) or shRNA targeting PDE1A (Ad-PDE1A shRNA), and treated with vehicle, IC86340 (30 µM), or sildenafil (1 µM), followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 24 hours. (E) [3H]-leucine incorporation in ARVM transduced with adenovirus as abovementioned, followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 24 hours. (Inset), Representative blot showing PDE1A protein expression in ARVM treated with adenovirus as abovementioned. (F) Total cell surface area of ARVM treated with adenovirus as abovementioned. Data were normalized to the sample (ctrl+control siRNA or shRNA) that was arbitrarily set to 1.0. Values are mean±SD from six (for A) or three (for C) independent experiments performed in triplicate, or triplicates from the same experiment (for D and E, similar results were obtained from at least three independent experiments). The total cell surface areas for (B and F) were averaged from 100 random alpha-actinin immuno-positive cells per condition.
Despite the relatively low PDE1C expression level in NRVM, lower PDE1C could be functionally relevant in rat myocytes. Therefore we also examined the effect of PDE1C siRNA in PE stimulated myocyte hypertrophy. We found that downregulation of PDE1C expression via siRNA did not attenuate PE-stimulated cardiomyocyte hypertrophy (Supplemental Fig. S3) or ANP gene expression (Supplemental Fig. S4). These results suggest that PDE1A but not PDE1C specifically regulates PE-stimulated hypertrophic growth.
PDE1A expression is upregulated with hypertrophic stimulation in vivo and in isolated cardiomyocytes in vitro
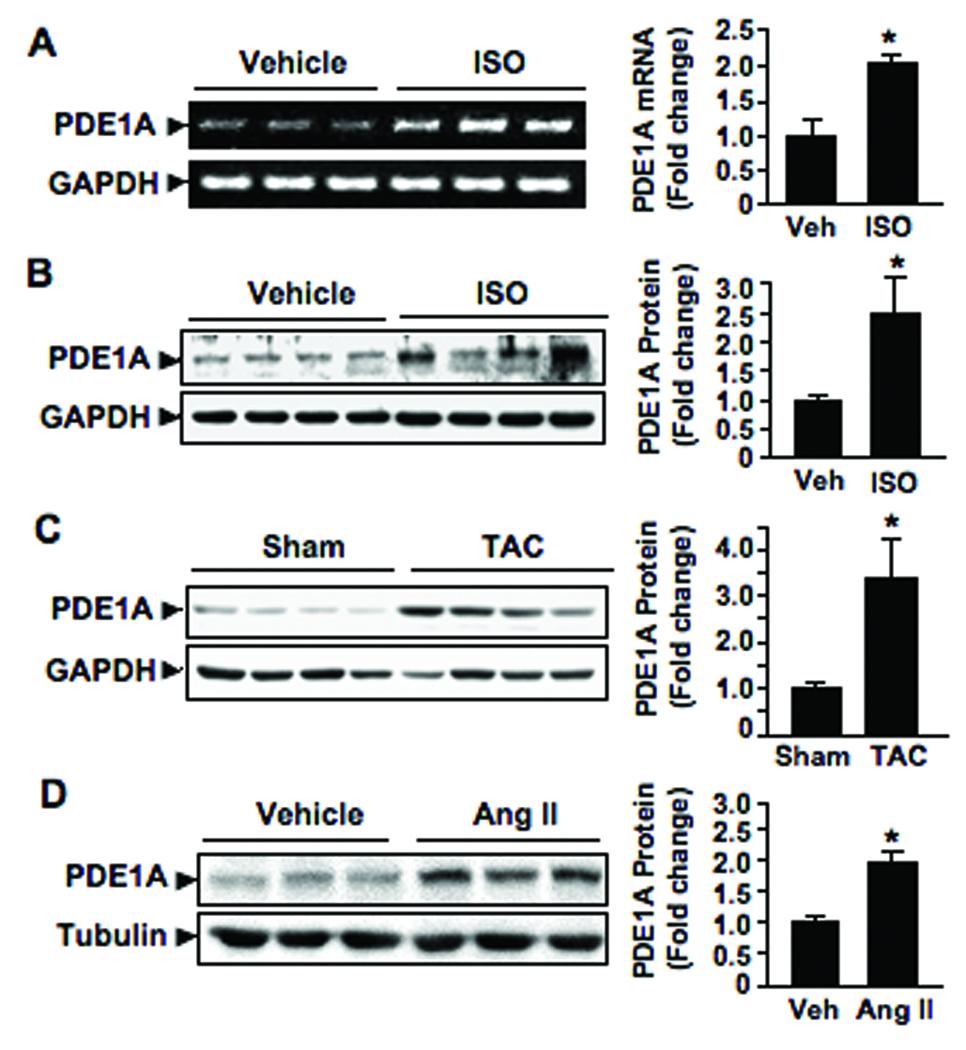
In order to further assess the patho-physiological role of PDE1A in the heart we measured PDE1A expression levels in the ventricle during pathological cardiac hypertrophy in vivo. Interestingly, we observed an approximate 2-fold increase in PDE1A mRNA levels (Fig. 6A) and 2.5-fold increase in PDE1A protein levels (Fig. 6B) in mouse hearts with ISO infusion (30 mg/kg/day) for 7 days relative to the vehicle control group. We also found significant upregulation of PDE1A protein levels in mouse hearts after chronic pressure overload via TAC for 3 weeks (Fig. 6C) and in rat hearts subjected to chronic Ang II infusion (0.7 mg/kg/day) for 2 weeks (Fig. 6D).
Figure 6. PDE1A expression is upregulated with cardiac hypertrophy both in vivo and in vitro.
(A and B) RT-PCR (A) and Western blot (B) showing PDE1A mRNA and protein levels, respectively, in ventricular tissues from mice subjected to vehicle or ISO infusion (30 mg/kg/d) for 7 days. (C and D) Western blot showing PDE1A protein levels in ventricular tissues from mice with pressure overload by TAC or sham operation for 3 weeks (C), or from rats subjected to vehicle or chronic Ang II infusion (0.7 mg/kg/d) for 14 days (D). Bar graphs represent quantitative results of blots analyzed by densitometry, showing PDE1A levels relative to loading controls (GAPDH or Tubulin). Data were normalized to vehicle or control treatment that was arbitrarily set to 1.0. Values are mean±SD (n=3 or 4). *p<0.05 vs. vehicle or control.
To determine whether the PDE1A induction occurs in cardiomyocytes in vivo, we performed immunohistochemical analyses of PDE1A in heart sections from control and hypertrophic rodent hearts. As shown in figure 7A, we found that PDE1A staining is significantly increased in the myocardium of hypertrophic hearts induced by TAC compared with sham controls. Obviously, the increase of PDE1A was detected in cardiomyocytes although it may not be exclusive (Fig. 7A, right panel). We obtained very similar observations in mouse hearts with chronic ISO infusion (Fig. 7B) and rat hearts with Ang II infusion (Fig. 7C). The specificity of the antibody was verified as shown in Supplemental Fig. S5.
Figure 7. PDE1A expression is upregulated in cardiac myocytes during hypertrophy both in vivo and in vitro.
(A–C) Immunohistochemistry (IHC) staining demonstrates PDE1A expression in myocardium of mouse hearts with pressure overload by TAC or sham operation for 3 weeks (A), mouse hearts with vehicle or ISO infusion (30mg/kg/day) for 1 week (B), and rat hearts with vehicle or AngII infusion (0.7 mg/kg/day) for 2 week (C). Inset images depict myocyte specific PDE1A immunostaining. (D and E) Western blot showing PDE1A protein expression in isolated NRVM treated with ISO (10 µmol/L) or vehicle (ctrl) for up to 48 hours (D), or in ARVM treated with ISO (1 µmol/L), Ang II (100 nmol/L), or vehicle (ctrl) for 24 hours (E). Bar graphs represent quantitative results of blots analyzed by densitometry within a linear range, showing PDE1A levels relative to GAPDH loading controls. Data were normalized to vehicle or control treatment that was arbitrarily set to 1.0. Values are mean±SD (n=3 independent experiments for in vitro). *p<0.05 vs. vehicle or control.
To further determine whether this PDE1A induction occurs in isolated NRVM and ARVM. We found that in NRVM, ISO treatment for up to 48 hours increased PDE1A protein levels relative to control (Fig. 7D). Similarly, we observed ISO or Ang II treatment of ARVM for 24 hours resulted in increased PDE1A protein levels (Fig. 7E). Together, these data suggest that PDE1A expression can be upregulated in cardiomyocytes via hypertrophic stimuli both in vivo and in vitro.
The anti-hypertrophic effect of PDE1A is mediated by a cGMP/PKG-dependent mechanism
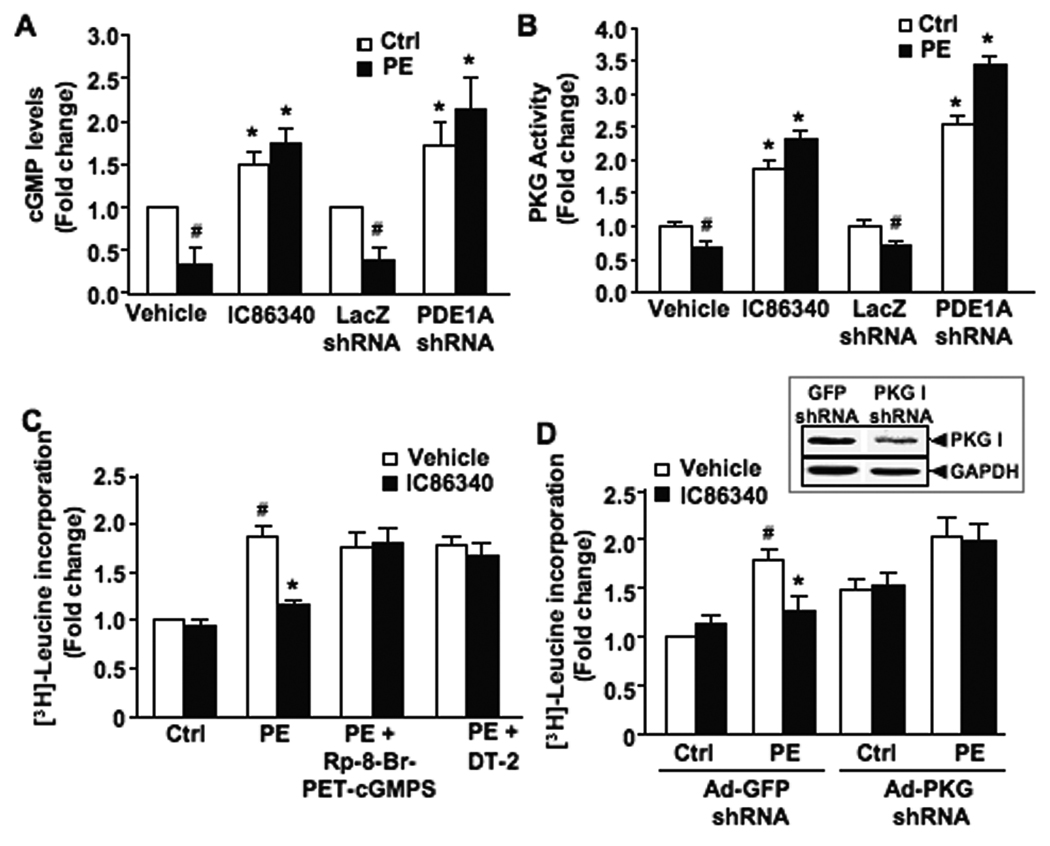
Intracellular cGMP levels were significantly decreased upon PE stimulation in NRVM, which was prevented by the PDE1 inhibitor IC86340 or PDE1A shRNA (Fig. 8A and B). However, the cAMP level was increased upon PE treatment and IC86340 did not significantly change the cAMP level (Supplemental Fig. S6A). We next assessed cGMP-activated PKG and cAMP-activated PKA activation. We found that PKG activity is decreased upon PE stimulation, and IC86340 or PDE1A shRNA treatment diminished PE effects and enhanced PKG activity (Fig. 8C), which was consistent with cGMP changes. PKA activity however was not significantly altered by PDE1 inhibition (Supplemental Fig. S6B).
Figure 8. The effect of PDE1 inhibitor on cardiomyocyte hypertrophy is PKG-dependent.
(A) Total cGMP levels measured by radioimmunoassay in NRVM pretreated with IC86340 (15 µmol/L) for 30 minutes or separately transduced with 100 MOI Ad-LacZ or Ad-PDE1A shRNA for 48 hours, followed by PE (50 µmol/L) stimulation for 5 minutes. (B) Net PKG activity (DT-2 inhibited) in NRVM treated as described above. (C) [3H]-leucine incorporation in NRVM pretreated with the PKG inhibitor, Rp-8-Br-PET-cGMPS (50 µmol/L) or DT-2 (250 nmol/L) for 30 minutes, in the presence of IC86340 (30 µmol/L) or vehicle, followed by PE (50 µmol/L) or vehicle (ctrl) stimulation for 48 hours. (D) Protein synthesis assessed via [3H]-leucine incorporation in NRVM transduced with 100 MOI Ad-PKG shRNA or Ad-GFP shRNA. (Inset) Representative blot showing PKG I protein expression in NRVM treated as described. Data were normalized to the sample (with vehicle or GFP-shRNA alone) that was arbitrarily set to 1.0. Values are mean±SD of at least three independent experiments performed in triplicates for A, C, and D, or triplicates in the same experiment for B (similar observations confirmed by two independent experiments). #p<0.05 vs. vehicle or control, *p<0.05 vs. vehicle+PE.
We then investigated the involvement of PKG I. Inhibition of PKG activity by either Rp-8-Br-PET-cGMPs or the peptide inhibitor DT-2, significantly blocked the inhibitory effect of IC86340 on [3H]-leucine incorporation (Fig. 8E). To further confirm the involvement of PKG, we downregulated PKG I expression using Ad-PKG I shRNA (Fig. 8F, inset). We found that PKG I shRNA abolished the effects of IC86340 on myocyte hypertrophy (Fig. 8F), similar to the effects of PKG inhibitors. We also examined the role of PKA using an adenovirus expressing the PKA inhibitor (PKI). We found that PKA inhibition via PKI reduced PE-stimulated myocyte hypertrophy (Supplemental Fig. S7C), consistent with previous findings that PKA activation is pro-hypertrophic16. Importantly, when PKA was inhibited by PKI, IC86340 still reduced myocyte hypertrophy (Supplemental Fig. S6C). These results suggest that the anti-hypertrophic effects of PDE1 inhibition is mediated through PKG I but not PKA activation.
DISCUSSION
Previous studies have demonstrated that intracellular Ca2+/CaM-dependent signaling promotes maladaptive hypertrophic gene expression in cardiomyocytes through various effectors such as the protein phosphatase calcineurin, Ca2+/CaM-dependent kinase II (CaMKII), and transcription factors myocyte enhancer factor 2 (MEF2) and nuclear factor of activated T-cells (NFAT)17. Endogenous cGMP-dependent signaling is able to negatively regulate cardiac hypertrophy both in vitro and in vivo, by suppressing Gq/11 activation and normalizing Ca2+ signaling5, 18. The present study identifies a novel function of Ca2+/CaM-dependent PDE1 in regulating pathological cardiomyocyte hypertrophy. Given that PDE1 is a Ca2+/CaM stimulated PDE capable of hydrolyzing cGMP, we hypothesized that PDE1 may play a pivotal role in the crosstalk of Ca2+ and cGMP signaling to promote cardiac growth. We demonstrated that inhibition of PDE1 activity, particular PDE1A, blocked PE- or ISO-induced attenuation of cGMP/PKG signaling and cardiomyocyte hypertrophy in vitro. Administration of the PDE1 inhibitor IC86340 significantly also reduced cardiac hypertrophy in a mouse hypertrophy model in vivo. Moreover, PDE1A expression was significantly upregulated in the heart and myocytes from various pathological hypertrophy animal models in vivo and in isolated NRVM and ARVM treated with neurohumoral stimuli in vitro. Together these findings suggest that Ca2+, by activating PDE1A, decreases cGMP levels and PKG activity and thus leads to potentiated cardiomyocyte hypertrophy. Upregulation of PDE1A expression upon neurohumoral or biomechanical stress during cardiac hypertrophy further enhances PDE1A activity and attenuates cGMP/PKG signaling (proposed model in Supplemental Fig. S9).
The PDE1 family is comprised of Ca2+/CaM-stimulated PDEs, including PDE1A, 1B and 1C. It was previously shown that Ca2+/CaM-stimulated PDE1 represents the majority of the cGMP-hydrolyzing PDE activity in the human myocardium, however this study did not identify the cardiac cell type or the PDE1 isoform responsible for this activity19. An early report by Bode et al supposed that Ca2+/CaM-stimulated PDE1 activity was absent from isolated ARVM and restricted to the non-myocyte fraction of the whole rat ventricle20. PDE1A mRNA expression and/or activity has been described in cardiac tissue from several species including human21, bovine22, canine23, and rat24. Since most of these studies used whole heart lysates, it is still unclear if these isoforms are attributed to cardiomyocytes or other cardiac cell types. More recently, it has been shown that PDE1C is expressed in cardiomyocytes of the human myocardium, while PDE1A protein was not detected in these human heart samples9. Herein, we identified both PDE1A and PDE1C protein and mRNA in human hearts (Fig. 4), with PDE1A expression conserved in rat and mouse hearts. In fact, a previous study using immunoprecipitation and PDE assay demonstrated that PDE1A and PDE1C represent approximately 80% and 20% of total PDE1 activity in the rat heart, respectively25. The inconsistencies of PDE1A expression with previous reports may be rationalized by differences in tissue sample preparation or antibody cross-reactivity. Likewise, the discrepancy between PDE1C mRNA and protein level in mouse heart should be further characterized.
To address the specific contribution of PDE1A and PDE1C in mediating the effects of selective PDE1 inhibition on cardiomyocyte hypertrophy, we used siRNA specifically targeting PDE1A and PDE1C in NRVM (Fig. 5 and Supplemental Fig. S3). These data support a critical role for PDE1A but not PDE1C in mediating the anti-hypertrophic effect of PDE1 inhibition in NRVM. The negligible effect of PDE1C downregulation on myocyte hypertrophy may be due to the lower expression level of PDE1C (Supplemental Fig. S3) or may suggest an alternative role of PDE1C in rat myocytes. In fact, studies from cells highly expressing endogenous PDE1C elicited increased intracellular cAMP upon inhibition of PDE1C26, 27, suggesting that PDE1C is able to regulate cAMP signaling. However, specific downregulation of PDE1A primarily elevated cGMP levels in both VSMCs8and cardiomyocytes (Fig. 8, S6), suggesting PDE1A and PDE1C play distinct roles in intracellular cyclic nucleotide signaling. Our data that IC86340 prevents adverse cardiac hypertrophy in a mouse ISO infusion model suggest an important role for PDE1A in regulating cardiac hypertrophy, however, we cannot entirely rule out the contribution of PDE1C in mouse heart. Generation of PDE1A and PDE1C-deficient animal models will be indispensable to further examine their respective roles in adult cardiomyocyte function in vivo, which is currently under investigation.
PDE5 is another cGMP-hydrolyzing PDE expressed in cardiac tissues. Earlier reports demonstrated a low level of expression of PDE5 in hearts from various species and isolated cardiomyocytes19. Nonetheless, a recent study using PDE5A shRNA in isolated cardiomyocytes demonstrated an important role for PDE5A in these cells28, which may contribute to the cardioprotective effects elicited by sildenafil in preventing adverse cardiac remodeling and dysfunction from pressure overload5. PDE5 and PDE1 enzymes are likely coupled to distinct intracellular cGMP-dependent pathways and regulatory mechanisms. For example, PDE5 activity is primarily stimulated upon NO induced cGMP/PKG activation, which leads to a rapid decline in intracellular cGMP. Thus PDE5 plays an important role in the negative feedback regulation of NO/cGMP signaling. However, PDE1 activity is stimulated by Ca2+-elevating stimuli (i.e. Ang II, ET-1, and α-AR agonists), and PDE1-dependent cGMP hydrolyzing activity is predominant during elevations in [Ca2+]i29. In fact we have observed that the effects of PDE1 inhibitor on cGMP elevation were Ca2+/CaM-dependent (Supplemental Fig. S7). Thus PDE1 plays a critical role in the Ca2+-mediated negative regulation of cGMP signaling30. However, the source of the Ca2+ to which PDE1 alters cGMP-dependent signaling is still not clear and is currently under investigation. We also determined the additive or redundant effects of PDE1 and PDE5 on cardiomyocyte hypertrophy. We found that both IC86340 and PDE5 inhibitor sildenafil individually attenuated PE-induced NRVM hypertrophy, while the combination of PDE inhibitors potentiated these effects (Supplemental Fig. S8). Moreover, we found that the inhibitory effect of PDE1A shRNA on cardiomyocyte hypertrophy could be potentiated by sildenafil but not IC86340 (Fig. 5D). These findings together suggest that PDE1A and PDE5A are likely coupled to unique cGMP pools and PDE1A may regulate specific cGMP pools with elevations of [Ca2+]i during hypertrophic stimulation. PKG appears to be a critical mediator for the antihypertrophic effects resulted from both PDE1 (Fig. 8) and PDE5 inhibition31. However, the role and mechanism of PKG in cardiac hypertrophy in vivo deserves further investigation. In summary, the present findings elucidate a novel role of PDE1, particularly PDE1A in regulating cardiomyocyte hypertrophy and myocardial remodeling during cardiac disease.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Paul Brookes at the University of Rochester for providing ARVM. We also thank Dr. Anindita Das and Dr. Rakesh Kukreja from Virginia Commonwealth University for providing the Ad-PKG I shRNA.
SOURCES OF FUNDING
This work was supported by the American Heart Association (AHA) Establishing Investigator Award 0740021N (CY), National Institutes of Health (NIH) HL088400 and HL077789 (CY), and AHA Predoctoral Fellowship 0815730D (CLM).
ABBREVIATIONS
- Ang II
angiotensin II
- ANP
atrial natriuretic peptide
- ARVM
adult rat ventricular myocytes
- CaM
calmodulin
- GC
guanylyl cyclase
- ISO
isoproterenol
- NRVM
neonatal rat ventricular myocytes
- PDE
cyclic nucleotide phosphodiesterase
- PE
phenylephrine
- PKA
cAMP-dependent protein kinase
- PKG
cGMP-dependent protein kinase
- RIA
radioimmunoassay
- TAC
thoracic aortic constriction
- VSMC
vascular smooth muscle cells
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63(3):500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF. Expression of constitutively active guanylate cyclase in cardiomyocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem. 2003;278(48):47694–47699. doi: 10.1074/jbc.M309661200. [DOI] [PubMed] [Google Scholar]
- 4.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111(9):1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 6.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93(4):280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 7.Ahn HS, Crim W, Romano M, Sybertz E, Pitts B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol. 1989;38(19):3331–3339. doi: 10.1016/0006-2952(89)90631-x. [DOI] [PubMed] [Google Scholar]
- 8.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98(6):777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, Radwanski PB, Florio V, Movsesian MA. Cyclic Nucleotide Phosphodiesterase PDE1C1 in Human Cardiac Myocytes. J Biol Chem. 2007;282(45):32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- 10.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111(19):2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, Berk BC, Yan C. A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci U S A. 2005;102(41):14771–14776. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115(17):2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273(10):5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 14.Souness JE, Brazdil R, Diocee BK, Jordan R. Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY-5445, vinpocetine and 1-methyl-3- isobutyl-8-(methylamino)xanthine. Br J Pharmacol. 1989;98(3):725–734. doi: 10.1111/j.1476-5381.1989.tb14599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadane N, Alpert L, Chalifour LE. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol. 1999;127(5):1165–1176. doi: 10.1038/sj.bjp.0702676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan C, Miller CL, Abe J. Regulation of phosphodiesterase 3 and inducible cAMP early repressor in the heart. Circ Res. 2007;100(4):489–501. doi: 10.1161/01.RES.0000258451.44949.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99(17):11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83(5A):3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 20.Bode DC, Kanter JR, Brunton LL. Cellular distribution of phosphodiesterase isoforms in rat cardiac tissue. Circ Res. 1991;68(4):1070–1079. doi: 10.1161/01.res.68.4.1070. [DOI] [PubMed] [Google Scholar]
- 21.Loughney K, Martins TJ, Harris EA, Sadhu K, Hicks JB, Sonnenburg WK, Beavo JA, Ferguson K. Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3',5'-cyclic nucleotide phosphodiesterases. J Biol Chem. 1996;271(2):796–806. doi: 10.1074/jbc.271.2.796. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenburg WK, Seger D, Beavo JA. Molecular cloning of a cDNA encoding the "61-kDa" calmodulin-stimulated cyclic nucleotide phosphodiesterase. Tissue-specific expression of structurally related isoforms. J Biol Chem. 1993;268(1):645–652. [PubMed] [Google Scholar]
- 23.Clapham JC, Wilderspin AF. Cloning of dog heart PDE1A - a first detailed characterization at the molecular level in this species. Gene. 2001;268(1–2):165–171. doi: 10.1016/s0378-1119(01)00413-9. [DOI] [PubMed] [Google Scholar]
- 24.Yanaka N, Kurosawa Y, Minami K, Kawai E, Omori K. cGMP-phosphodiesterase activity is up-regulated in response to pressure overload of rat ventricles. Biosci Biotechnol Biochem. 2003;67(5):973–979. doi: 10.1271/bbb.67.973. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenburg WK, Rybalkin SD, Bornfeldt KE, Kwak KS, Rybalkina IG, Beavo JA. Identification, quantitation, and cellular localization of PDE1 calmodulin-stimulated cyclic nucleotide phosphodiesterases. Methods. 1998;14(1):3–19. doi: 10.1006/meth.1997.0561. [DOI] [PubMed] [Google Scholar]
- 26.Dunkern TR, Hatzelmann A. Characterization of inhibitors of phosphodiesterase 1C on a human cellular system. FEBS J. 2007;274(18):4812–4824. doi: 10.1111/j.1742-4658.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 27.Han P, Werber J, Surana M, Fleischer N, Michaeli T. The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem. 1999;274(32):22337–22344. doi: 10.1074/jbc.274.32.22337. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O'Rourke B, Champion HC, Crow MT, Kass DA. Expression, activity, and prohypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu PJ, Tetzloff G, Ahn HS, Sybertz EJ. Comparative effects of vinpocetine and 8-Br-cyclic GMP on the contraction and 45Ca-fluxes in the rabbit aorta. Am J Hypertens. 1988;1(3 Pt 1):262–268. doi: 10.1093/ajh/1.3.262. [DOI] [PubMed] [Google Scholar]
- 30.Yan C, Nagel DJ, Jeon KI. Regulation and function of cyclic nucleotide phosphodiesterases in vascular smooth muscle and vascular diseases. In: Beavo J, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton, FL: CRC Press; 2007. pp. 465–484. [Google Scholar]
- 31.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, Mendelsohn ME, Kass DA. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119(2):408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.