Summary
A novel oligoamine analog inhibitor of histone demethylases blocks colon tumor cell growth in association with histone methylation and gene re-expression. It also markedly potentiates the activity of hypomethylating agents in vitro and in vivo, suggesting that histone demethylase inhibitors may represent a valuable addition to armamentarium of epigenetic agents.
In this issue of Clinical Cancer Research, Huang et al., characterize the effects of novel histone demethylase inhibitors, either alone or in combination with inhibitors of DNA methyltransferases (DNMTIs), on the proliferation and survival of transformed cells 1. A widely accepted view holds that the origins of cancer lie, at least in part, in the silencing of genes responsible for cell death and/or differentiation, a phenomenon that generally stems from perturbations in the transcriptional regulatory machinery. In the broadest sense, silencing of such genes occurs at three fundamentally different but closely interrelated levels. For example, silencing frequently results from DNA methylation at the site of CpG islands by DNA methyltransferases (DNMTs) 2. Silencing can also stem from mutations in chromatin remodeling complexes (i.e., nucleosomal remodeling factors; NURFs) such as SWI/SNF. Finally, silencing can result from multiple post-translational covalent histone modifications, which collectively comprise the histone code, and which include such processes as acetylation, methylation, SUMOylation, glycosylation, ubiquitlylation, and phosphorylation 3. The therapeutic relevance of these events is underscored by accumulating evidence indicating that agents capable of reversing these processes e.g., DNMT inhibitors (DNMTIs) or histone deacetylase inhibitors (HDACIs) are clearly capable of inducing re-expression of silenced genes in association with induction of transformed cell death. Moreover, representative agents of these classes have recently been approved for the treatment of hematologic malignancies e.g., DNMTIs such as 5-azacytidine in the case of MDS, and HDACIs in the case of cutaneous T-cell lymphoma.
There is also evidence that these silencing mechanisms do not operate in a vacuum, but instead interact with each other, thereby providing a theoretical foundation for combinatorial approaches. For example, in preclinical studies, HDACIs have been shown to cooperate with DNMTIs to reverse gene silencing, and to block the proliferation and survival of transformed cells 4. Such findings have stimulated multiple clinical trials in which HDACIs are combined with hypomethylating agents, and preliminary results in certain diseases e.g., leukemia, appear potentially promising 5. However, whether such outcomes actually reflect gene reactivation remains to be determined.
While attempts to interfere with DNA methylation (e.g, by DNMTIs) and histone deacetylation (e.g., by HDACIs) have received the bulk of attention, recent efforts have begun to focus on pharmacologic disruption of other epigenetic regulatory processes. Histone methylation represents one such target. Histones can be methylated on arginine and lysine residues, and both the position and extent of methylation determine whether the mark stimulates or represses transcription 6. For example, methylation at H3K4, H3K36, and H3K79 is associated with enhanced transcription whereas methylation at H3K9 and H3K27 is associated with transcriptional repression 7. Methyl residues (mono- and di-) are removed by a member of amine oxidase family (LSD1), whereas trimethylated residues are removed by Jumonji family proteins (JmjC) 6. Clearly, intereference with these events would be expected to alter histone methylation status and as a consequence, modify gene transcription. However, the specific biologic effects of such interventions have not been extensively characterized.
This situation is now changing, at least in the case of histone methylation. Huang et al., describe the effects of a novel class of oligoamine analogs that function as potent inhibitors of LSD1, a FAD-dependent histone demethylase 6, in colon cancer cells 1. They report that these compounds substantially increased H3K4 mono- and di-methylation, events associated with enhanced transcription, and attenuated H3K9 dimethylation, a repressive mark. Notably, these events were associated with re-expression of aberrantly silent antagonists of the Wnt signaling pathway (i.e., secreted frizzled-related proteins; SFRPs). Interestingly, co-exposure of cells to oligoamines in conjunction with a hypomethylating agent (5-azacytidine) resulted in pronounced inhibition of colon tumor cell growth both in vitro and in vivo. The authors conclude that modulation of histone methylation status, particularly when combined with disruption of DNA methylation, may represent a novel epigenetic form of therapy with significant therapeutic potential.
This report provides the first evidence that histone demethylase inhibitors, as previously reported in the case of HDAC inhibitors 4, cooperate with DNMTIs to promote gene re-expression in transformed cells, a phenomenon accompanied by pronounced inhibition of cell growth and induction of cell death. Such findings argue, albeit indirectly, that targeting a single epigenetic aberration, by itself, may be insufficient to achieve the desired therapeutic outcome. Instead, targeting multiple cooperating abnormalities may be required. However, as in the case of DNMT/HDAC inhibitory strategies, numerous questions remain to be answered. For example, in the present study, combined treatment of colon cancer cells with a DNMTI and a histone demethylase inhibitor resulted in re-expression of aberrantly silenced SFRPs. While it is tempting to speculate that induction of antagonists of the Wnt signaling cascade, a pathway that has been implicated in colon carcinogenesis and in cancer stem cell survival 8, may be responsible for or contribute to the antiproliferative effects of the combination regimen, the functional significance of SRFP induction in this setting requires validation, Analogous questions have arisen regarding the functional role of p15INK4B re-expression in the antileukemic activity of combined DNMTI/HDACI regimens 5. Because the genetic changes induced by concomitant H3K4 methylation and DNMT inhibition are likely to very broad, identifying specific alterations in gene expression responsible for the antineoplastic activity of this strategy will not be trivial. However, gene array analysis should help to resolve this important issue.
Another question relates to the possibility that so-called epigenetic modulators may simultaneously act as cytotoxic agents. For example, both 5-aza-2’-deoxycytidine and 5-azacytine, currently viewed primarily as DNMTIs, were initially developed because of their direct cytotoxic activities 9. While combination strategies incorporating epigenetic modulators generally employ sub- or minimally toxic concentrations of these agents when administered individually, it is conceivable that their limited cytotoxicity might be substantially increased by alterations in gene expression (e.g., down-regulation of DNA repair or anti-apoptotic genes). In fact, the possibility that perturbations in gene expression may cooperate with more direct cytotoxic actions to promote cell death seems quite likely. Whether histone demethylase inhibitors exert direct cytotoxic actions in addition to their effects on the epigenome remains to be determined.
Aside from providing the first demonstration that blocking histone demethylation, as in the case of HDAC inhibition 4, may cooperate with DNMT inhibition to antagonize transformed cell growth both in vitro and in vivo, the present findings have broader therapeutic implications that extend beyond this particular setting. Although within the field of epigenetic therapy, HDAC and DNMT inhibitors have received the bulk of attention to date, attention is currently beginning to focus on inhibitors of other proteins involved in epigenetic regulation, including, as described in the present study, histone demethylases, as well as histone methyltransferases and histone acetyltransferases, among numerous others. In addition, efforts to target other epigenetic components of the transcriptional control machinery e.g., co-repressor proteins, are well underway 10. Such considerations raise the possibility that in the future, combination chemotherapy regimens may consist of, instead of purely cytotoxic agents, multiple inhibitors (or activators) targeting distinct regulatory components of the epigenome (summarized in Figure 1). Because our understanding of the epigenetic factors regulating gene expression, as well as our ability to target them, are advancing rapidly, the number of such regimens is potentially very large. Nevertheless, based in part on the report by Huang et al., 1, such efforts appear well justified.
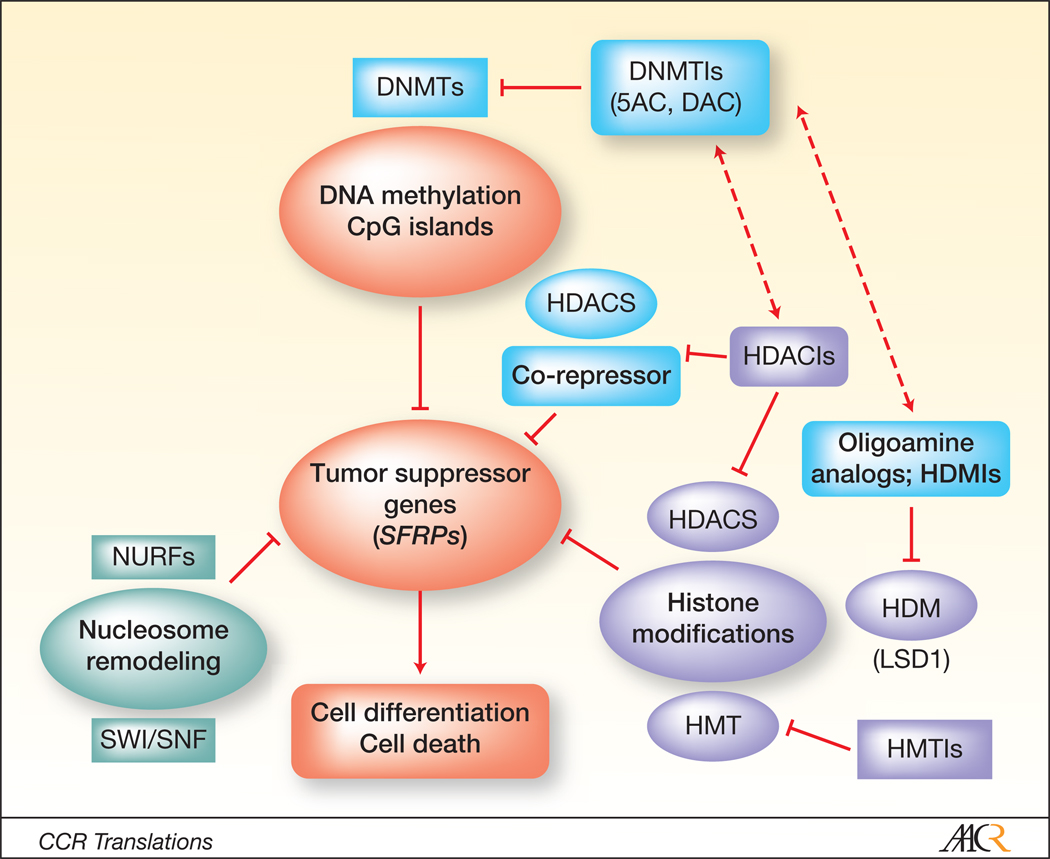
Figure 1.
Model of interactions between epigenetic agents in gene re-expression and tumor cell death. Tumor suppressor genes are silenced in transformed cells by multiple mechanisms, including aberrant methylation at promoter regions, mutations in nucleosome remodeling complex proteins, and diverse histone modifications, including acetylation, methylation, phosphorylation, SUMOylation, and glycosylation, among others. Inhibitors of DNMT such as 5-azacytine and 5-aza-2’-deoxycytidine reverse gene methylation and promote re-expression. Analogously, HDAC inhibitors lead to histone acetylation, resulting in a more open chromatin structure, and enhanced gene expression. They can also block the actions of co-repressor complexes. Oligoamine analog inhibitors of histone demethylases i.e., LSD1 induce positive methylation marks on H3K4, accompanied by re-expression of Wnt pathway antagonist genes (SRFPs). Combined exposure of tumor cells with two agents that act at different levels of the epigenome (e.g., HDACIs and hypomethylating agents or hypomethylating agents and histone methyltransferase inhibitors) may be particularly effective in counteracting gene silencing and triggering transformed cell death. Abbreviations: DNMT: DNA methyltransferase; DNMTI (DNA methyltransferase inhibitor); 5-AC: 5-azacytidine; DAC: 5-aza 2’-deoxyazacytine; HDM: histone demethylase; HDMI: histone demethylase inhibitor; HMT: histone methyltransferase; HMTI: histone methyltransferase inhibitor; LSD1: flavin-dependent amine oxidase histone demethylase; SFRPs: secreted frizzled-related proteins; SWI/SNF complex: mating-type switching/sucrose non-fermenting complex.
References
- 1.Huang Y, Murray-Stewart T, Wu Y, et al. Novel oligoamine analogues inhibit lysine-specific demethylase 1 (LSD1) and induce re-expression of epigenetically silenced genes. Clin Cancer Res. 2009;volume 15 doi: 10.1158/1078-0432.CCR-09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky SA, Klinge DM, Stidley CA, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 5.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 6.Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 9.Cihak A, Vesely J, Skoda J. Azapyrimidine nucleosides: metabolism and inhibitory mechanisms. Adv Enzyme Regul. 1985;24:335–354. doi: 10.1016/0065-2571(85)90085-8. [DOI] [PubMed] [Google Scholar]
- 10.Polo JM, Dell'Oso T, Ranuncolo SM, et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10:1329–1335. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]