Abstract
Objectives
This study aimed to identify an early marker of functional impairment after an ICD shock as a predictor of heart failure progression.
Background
The ICD population has substantial risk of death due to progressive pump failure.
Methods
Near field (NF) bipolar right ventricular (RV) electrograms (EGMs) during induced ventricular fibrillation (VF) and 10 seconds after rescue ICD shock were analyzed in 310 patients (mean age 59±14.5 years, 219 male [71%]) with structural heart disease, NYHA class I–III, and implanted with a single- or dual-chamber Medtronic ICD for primary (245 patients, 79%) or secondary prevention of sudden cardiac arrest. A local injury current (LIC) on NF RV EGM was defined as a deviation of EGM potential ≥1 mV or ≥15% of the preceding R wave peak-to-peak amplitude.
Results
During mean follow-up of 29.3±15.0 months, the combined endpoint of death or hospitalization due to CHF exacerbation was documented in 40 patients (12.9%, or 5.3% per person-year of follow-up). LIC was observed in 106 patients. In multivariate risk analysis, after adjustment for baseline prognostic factors (ejection fraction, history of atrial fibrillation, diabetes mellitus) and appropriate ICD shocks during follow-up, patients with observed LIC after induced VF rescue ICD shock at ICD implantation were more likely to die or to be hospitalized (hazard ratio, 2.69; 95%CI, 1.41 –5.14; P=0.003).
Conclusions
Transient LIC on bipolar NF RV EGM after induced VF rescue ICD shock is associated with increased risk of CHF progression, future hospitalizations due to CHF exacerbation, and subsequent heart failure death.
Keywords: congestive heart failure, implantable cardioverter-defibrillator, ventricular tachyarrhythmia
ICDs improve survival of patients who are at risk for SCA.1,2;3,4 However, long-term follow-up of ICD patients with CHF has shown that both appropriate5–7 and inappropriate5;8 ICD shocks are associated with increased risk of death, predominantly from progressive heart failure. Thoughtful medical management of heart failure and programming of ICD therapies in this patients cohort might improve the prognosis, but no early markers of heart failure progression available at the time of ICD implantation are known.
Extensive data indicate that defibrillation shocks are accompanied by transient adverse effects. These adverse effects include (1) transient ectopy, tachycardia, or induction of ventricular fibrillation;9;10 (2) complete heart block and increased pacing thresholds;10;11 (3) atrial and ventricular mechanical dysfunction (stunning);12–15 (4) significant elevation of troponin I serum level;16 and (5) decrease of the myocardial lactate extraction rate by mitochondria.17 Whether transient signs of myocardial injury after an ICD shock could predict future progression of CHF remains unclear.
Changes of ECG and intracardiac EGMs during ICD implantation procedures were observed previously. Transient ST-segment elevation on surface ECG after induced VF rescue ICD shock was described in 19% of patients,18 but its prognostic significance was not studied. Other investigators have shown that a current of injury on intracardiac EGM within 10 minutes of lead fixation serves as a marker of adequate active lead fixation during an ICD or pacemaker implantation procedure.19;20 The prognostic significance of transient local injury current (LIC) on near-field (NF) RV EGM after induced VF rescue ICD shock is unknown. We hypothesized that LIC on bipolar NF RV EGM after induced VF rescue ICD shock predicts future CHF progression in patients with NYHA class I–III CHF.
Methods
The study protocol was approved by the Johns Hopkins University and the Washington University Human Studies Committees, and all patients gave written informed consent before entering the study.
Study population
This is prospective observational study. Male and female patients older than 18 years with structural heart disease and NYHA class I–III CHF were eligible for the study if they had a Medtronic transvenous single- or dual-chamber ICD device with dedicated bipolar ICD lead implanted for primary or secondary prevention of SCA within 1 week before enrollment. Exclusion criteria were indications for CRT-D and NYHA class IV, contraindications for DFT testing, pregnancy, inherited channelopathies, and concomitant conditions other than cardiac diseases that were associated with a high likelihood of death during 1 year after enrollment.
VT/VF was induced with a shock-on-T wave protocol. Stored intracardiac EGMs recorded during DFT testing (induced tachyarrhythmia and 10 seconds post-ICD shock) were extracted from the ICD memory 7 days after procedure, converted into digital format using proprietary Medtronic software, and further analyzed using custom Matlab software application. Control recordings of NF RV EGM at rest simultaneously with one-lead (lead II) surface ECG were obtained via Medtronic programmer 2090 using the NI USB-9215A portable data acquisition system (National Instruments, Austin, TX) 7 days after the procedure.
Programming of the ICD device was based on the attending electrophysiologist’s clinical evaluation. Patients were followed-up in the Washington University Arrhythmia Clinic and via the Internet-based CareLink© remote monitoring system. All ICD interrogation data were adjudicated by an ICD endpoint committee (attending electrophysiologist and 2 of the investigators [L.G.T and R.D.B.]). ICD shocks occurring for VT or VF were classified as appropriate.
Measurement of LIC on the bipolar NF RV EGM after induced VF rescue ICD shock
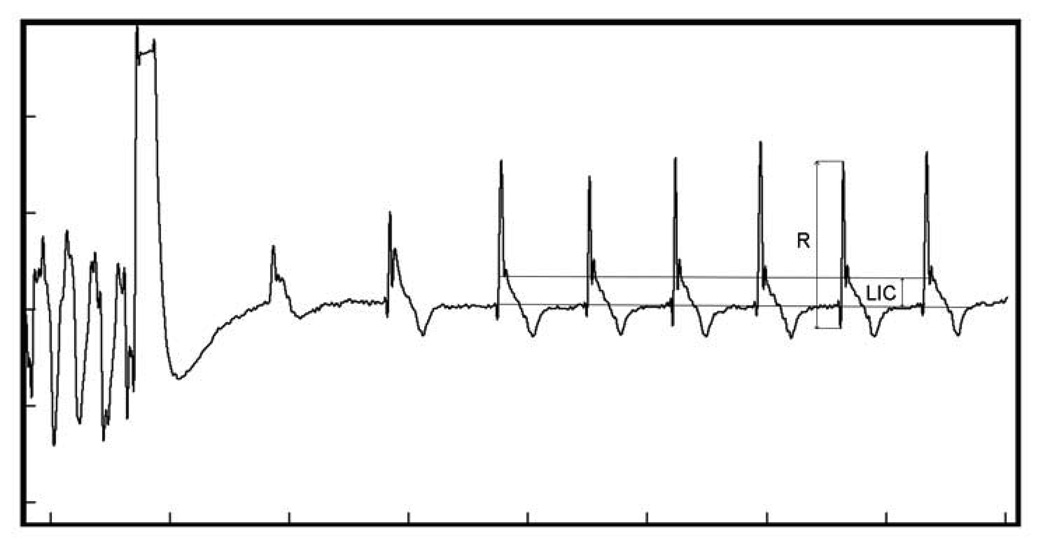
Endocardial NF RV EGM was recorded as the difference of potentials between the tip and the ring of the dedicated bipolar ICD lead implanted in the RV apex. The LIC was characterized as the magnitude of elevated or depressed potential immediately after the major fast EGM deflection (Figure 1), measured from the baseline (the isoelectric portion before the major EGM deflection) to its highest point in mV. Peak-to-peak amplitude of major fast EGM deflection (R wave) was measured to assess relative LIC on average representative beat. Significant LIC was defined as a deviation of EGM potential ≥ 1 mV or ≥ 15% of preceding R wave peak-to-peak amplitude. Digital EGM (bandpass filter 2–100 Hz) was magnified and measured after separate calibration of each recording (1 mV equal to 30–40 pixels, Screen Calipers 4.0 Iconico, Inc). The first 2 seconds after shock were excluded. LIC was measured on every sinus beat and averaged. Ventricular paced beats, distorted beats of undetermined origin and ectopic beats were excluded.
Figure 1. Measurement of local injury current (LIC) after induced VF rescue ICD shock.
Endpoints
Either of 2 major CHF events—death or hospitalization due to CHF exacerbation, whichever came first—served as the primary endpoint. We use the term “CHF event” to refer to this combined endpoint throughout the paper. Cases of death with clear confirmed non-cardiovascular cause were censored at the time of the last office visit. Time to event was measured from the day of ICD implantation.
Statistical analysis
Results are presented as mean±standard deviation (SD) for normally distributed variables, and as median and interquartile range for skewed distributions. Continuous variables were compared using the independent samples t test if normally distributed and the Wilcoxon rank sum test if skewed. The Pearson chi-square test was used to compare categorical variables. A P-value of <0.05 was considered significant. Kaplan-Meier survival analysis was used to compute mean and median survival time. The log-rank (Mantel-Cox) statistic was computed to test the equality of survival distributions. Cox multivariate regression model was used for adjustment by known predictors of CHF progression. Appropriate ICD shock for VT/VF at follow-up was treated as time-dependent covariate. SPSS 17.0.0 (SPSS Inc, Chicago, IL) and STATA 10 (StataCorp LP, College Station, TX) software packages were used for calculations.
Results
Study population
The study population consisted of 310 patients (mean age 59.0±14.5 years, 219[71%] men) who underwent ICD implantation for primary (245 patients, 79%) or secondary (65 patients, 21%) prevention of SCA. Ischemic cardiomyopathy with MI history was diagnosed in 187 (60.3%) patients and non-ischemic cardiomyopathy in 123 (39.7%). A single-chamber ICD was implanted in 175 (56.5%), and dual-chamber ICD in 135 (43.5%) patients. A new dedicated bipolar transvenous ICD lead was implanted in 264 (85.1%) patients, and an ICD generator change procedure was performed in 46 patients (14.9%) who had had an ICD lead implanted more than 1 year ago. Only the first induced VT/VF and EGM after the first rescue ICD shock was analyzed.
Bipolar NF RV EGM changes after induced VF rescue ICD shock
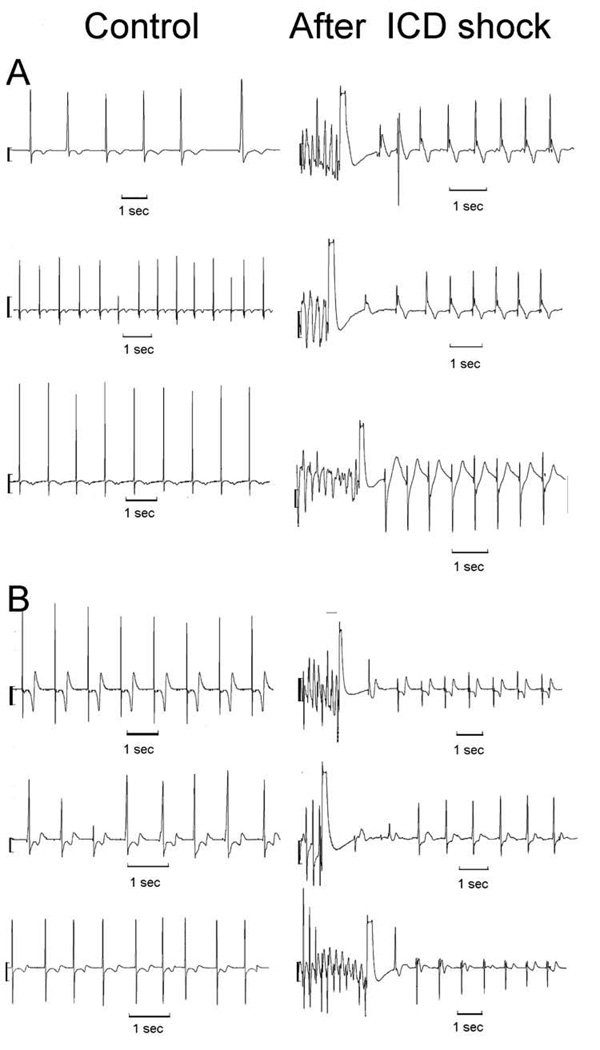
Significant LIC after 1st induced rescue ICD shock was found in 106 (34.2%) patients. The baseline characteristics of the patients are summarized in the Table 1, and ICD shock characteristics are summarized in the Table 2. Figure 2 shows examples of EGM changes after 1st induced VF rescue ICD shock. Control EGMs obtained 7 days after the procedure confirmed that observed changes were temporary and demonstrated isoelectric potential.
Table 1.
Clinical characteristics of patients with and without LIC after 1st ICD shock
| Characteristic | LIC (+) [n = 106] | LIC (−) [n = 204] | P |
|---|---|---|---|
| Mean age ±SD, y | 59.9±11.7 | 61.5±14.9 | 0.534 |
| Male, n(%) | 74(69.8) | 145(79.9) | 0.816 |
| African Americans, n (%) | 20(18.8) | 28(15.9) | 0.422 |
| Ischemic CM with MI history, n (%) | 72(68.8) | 115(64.6) | 0.389 |
| Primary prevention of SCD, n(%) | 82(77.4) | 163(79.9) | 0.602 |
| Single-chamber ICD, n(%) | 83(78.3) | 92(45.1) | < 0.0001 |
| LVEF at ICD implantation ±SD, % | 33.4±11.6 | 32.8±12.2 | 0.797 |
| NYHA class I, n (%) | 19 (18.4) | 54 (26.3) | 0.699 |
| NYHA class III, n (%) | 20(18.8) | 35(19.5) | 0.946 |
| Diabetes mellitus, n (%) | 46(43.8) | 62(34.6) | 0.197 |
| Hypertension, n (%) | 89(85.4) | 119(67.1) | 0.016 |
| CABG, n (%) | 37(35.4) | 62(34.6) | 0.535 |
| PTCA, n (%) | 37(35.4) | 53(29.6) | 0.312 |
| Beta blockers, n (%) | 87(83.3) | 160(90.1) | 0.195 |
| Digoxin, n(%) | 41(38.4) | 56(27.6) | 0.046 |
| Aldosterone antagonists, n(%) | 50(47.2) | 53(26.1) | <0.0001 |
| Nitrates, n(%) | 21(19.8) | 42(20.7) | 0.856 |
| Class III antiarrhythmics, n(%) | 85(80.2) | 154(75.5) | 0.350 |
| VT/VF with appropriate ICD shocks, n(%) | 29(27.4) | 49(24.0) | 0.465 |
| Renal failure, n (%) | 13(12.5) | 41(23.2) | 0.102 |
| History of atrial fibrillation, n(%) | 24(22.6) | 48(23.5) | 0.633 |
Table 2.
Characteristics of induced VF events and ICD rescue shocks
| Characteristic | LIC (+) [n = 106] | LIC (−) [n = 204] | P |
|---|---|---|---|
| Cycle length of induced VF, ms | 199.5±26.8 | 208.6±29.8 | 0.031 |
| Duration of induced VF event, sec | 9.7±3.5 | 9.3±2.3 | 0.309 |
| Delivered shock energy, J | 25.5±2.6 | 24.6±2.6 | 0.070 |
Figure 2. RV EGMs: Control and after ICD shock.
A. Typical post-shock ICD recording of RV NF EGM in control, and after ICD shock in patients with presented LIC (A), and in patients with lack of injury (B).
Death, CHF hospitalizations, and appropriate ICD shocks
During mean follow-up of 29.3±15.0 months, the combined endpoint death or hospitalization due to CHF exacerbation was documented in 40 patients (12.9%, or 5.3% per person-year of follow-up). Appropriate ICD shocks were observed in 78 patients (25.2%, or 10.3% per person-year of follow-up); of these patients, 3 died median 113 days after appropriate ICD shocks, and 3 underwent successful heart transplantation. CHF events were twice as frequent among patients with appropriate ICD shocks (16 patients, 20.5%) compared with patients without sustained arrhythmia (24 of 232 patients, 10.3%; P=0.020). ICD shock preceded CHF event by median 132 days (inter-quartile range 6–627).
Risk of CHF progression associated with LIC at ICD implantation
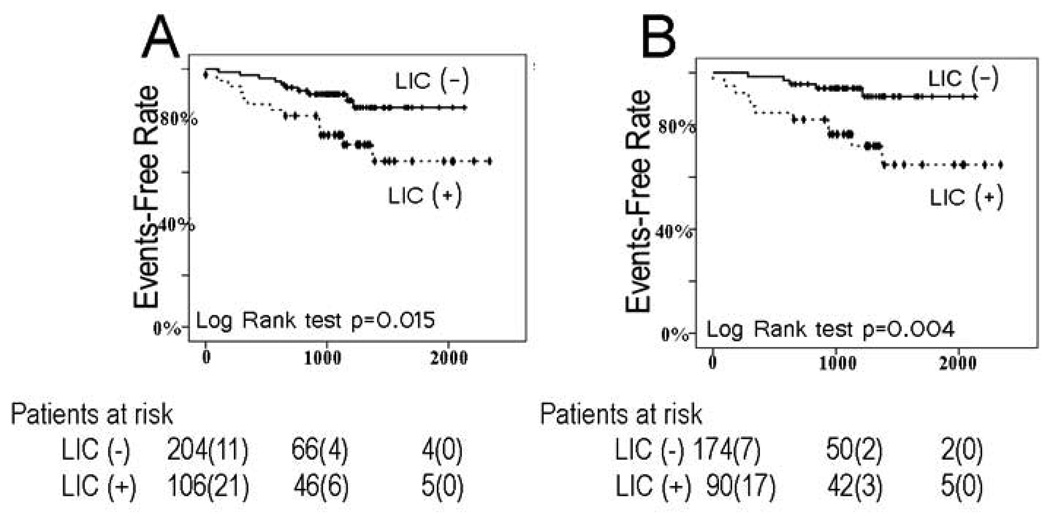
LIC (−) patients had a higher CHF event-free survival rate during follow-up (88.1% vs. 71.1%, P=0.015, Figure3A). Cox proportional hazards ratio for the newly implanted ICD lead subgroup was higher (HR 3.29, 95% CI 1.54–7.06, P=0.002) than for all patients (HR 2.61, 95% CI 1.37–4.99, P=0.004). Figure 3B shows Kaplan-Meier curves when the analysis was confined to patients with newly implanted leads. This effect was not significant for the chronic ICD lead subgroup (HR 0.78, CI 0.09–6.67, P=0.820). Multivariate Cox model that included LIC, time-dependent appropriate ICD shocks at follow-up, new/old lead factor, and interaction between LIC and the lead factor confirmed effect modification (P<0.0001).
Figure 3.
Kaplan-Meier curves for freedom from CHF events in patients with significant LIC (+) and those with the LIC (−) for all leads (A) and for newly implanted leads only (B).
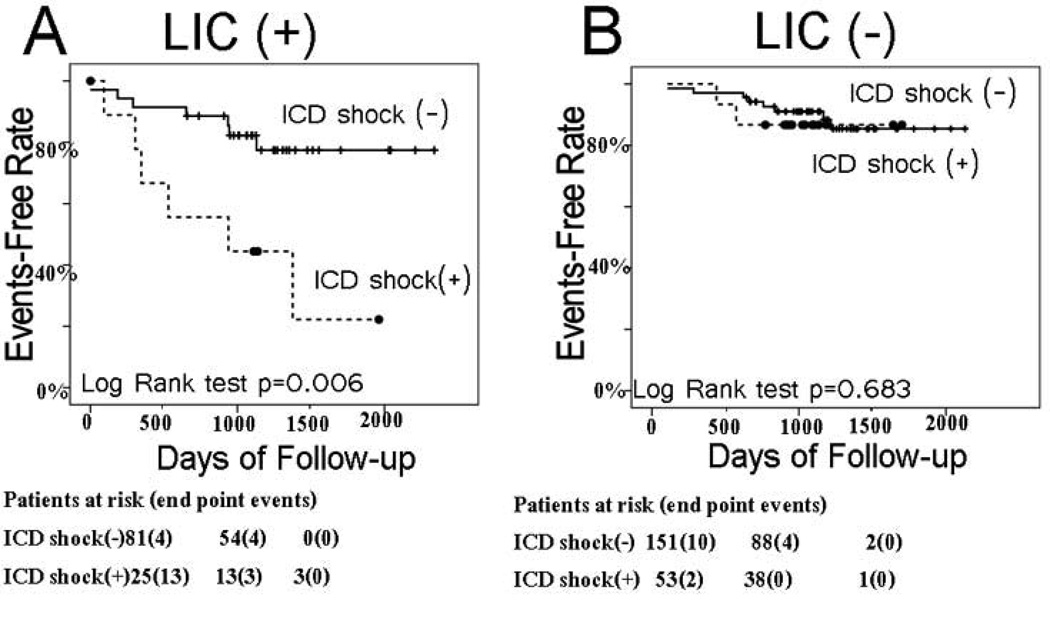
In patients with LIC, subsequent sustained VT/VF events with appropriate ICD shocks predicted CHF progression (Figure 4A, event-free survival 40% vs. 80%, P<0.006), whereas in patients without LIC, subsequent VT/VF was not predictive (Figure 4B, event-free survival 87% vs. 88%, P=0.683). Multivariate Cox regression model that included LIC, time dependent appropriate ICD shocks, and interaction between LIC and ICD shocks, confirmed significant effect modification (P=0.001).
Figure 4.
Kaplan-Meier curves for freedom from CHF events in patients with and without appropriate ICD shocks at follow-up among LIC (+) patients (A), and LIC (−) patients (B).
After adjustment for baseline factors (age, race, LVEF, NYHA class, history of diabetes mellitus, atrial fibrillation or flutter, renal failure, hypertension, use of digoxin and aldosterone antagonists), and time-dependent appropriate ICD shocks during follow-up, LIC signified a highly increased risk of subsequent CHF events. Each Cox model included LIC, time-dependent appropriate ICD shocks during follow-up, NYHA class and one by one other tested covariates as listed in the Table 3. LIC was significant predictor in all tested Cox models with hazard ratio from 2.2 to 2.6 (P<0.01). Hazard ratios of time-dependent appropriate ICD shock at follow-up ranged from 2.5 to 7.1. Time-dependent appropriate ICD shock at follow-up was not significant predictor in the models that included cycle length of VF and renal failure. NYHA class hazard ratios ranged from 2.7 to 3.1 and were significant in all models (P<0.001).
Table 3.
Univariate and multivariate hazard ratios of tested predictors
| Predictor | Unadjusted hazard ratio (95% CI), and P value |
Adjusted hazard ratio (95% CI), and P value |
|---|---|---|
| LIC | 2.61(1.37–4.99), P=0.004 | 2.50(1.31–4.79), P=0.005 |
| NYHA class | 2.69(1.99–3.63), P<0.0001 | 2.99(1.45–6.15), P=0.003 |
| Time-dependent appropriate | ||
| ICD shocks | 2.67(1.41–5.05), P=0.003 | 6.71(1.52–29.64), P=0.012 |
| LVEF | 0.95(0.92–0.97), P<0.0001 | 0.94(0.90–0.97), P<0.0001 |
| Diabetes mellitus | 1.73(1.09–2.72), P=0.019 | 1.50(0.78–1.58), P=0.221 |
| Single-chamber ICD device | 0.65(0.41–1.05), P=0.077 | 0.73(0.34–1.58), P=0.423 |
| History of Hypertension | 1.92(1.01–3.65), P=0.045 | 2.10(0.81–5.43), P=0.126 |
| Digitalis | 2.02(1.28–3.18), P=0.003 | 1.47(0.76–2.83), P=0.252 |
| Aldosterone antagonists | 2.02(1.28–3.18), P=0.003 | 1.70(0.87–3.34), P=0.122 |
| Cycle length of induced VF | 1.00(0.98–1.01), P=0.755 | 1.00(0.99–1.02), P=0.741 |
| Atrial Fibrillation | 3.15(1.68–5.91), P<0.0001 | 2.39(0.90–6.34), P=0.080 |
| African-American Race | 3.00(1.86–4.84), P<0.0001 | 1.91(0.93–3.89), P=0.077 |
| Renal Failure | 2.68(1.34–5.37), P=0.005 | 2.08(0.80–5.44), P=0.134 |
| Age | 1.00(0.99–1.02), P=0.476 | 1.00(0.97–1.02), P=0.725 |
Each multivariate test includes LIC, time-dependent appropriate ICD shocks, NYHA class, and then tested other covariates one by one. Ejection fraction was tested in the model without NYHA class. Hazard ratio of appropriate ICD shock at follow up indicates the relative risk of the death or hospitalization due to CHF exacerbation per year from the first appropriate ICD shock.
Discussion
To our knowledge, this is the first description of local injury current on bipolar near-field RV EGM after ICD shock. Our results demonstrate for the first time that transient myocardial injury after induced VF rescue ICD shock manifesting as LIC on bipolar NF RV EGM is associated with increased risk of CHF progression, future hospitalizations due to CHF exacerbation, and heart pump failure death. LIC after induced VF rescue ICD shock was a predictor of adverse CHF outcomes after adjustment by traditional risk factors, including appropriate ICD shocks and LVEF or NYHA class, and provided additional prognostic information.
We propose a “triple-hit” hypothesis to explain the genesis of LIC on the NF RV EGM: (1) cardiac myocytes are fragile due to an underlying condition that leads to progressive CHF; (2) mechanical injury occurs due to lead placement; (3) a rescue ICD shock elicits LIC, especially if the first two “hits” are present.
CHF progression in ICD patients
High risk of death due to pump failure in ICD patient populations without or after appropriate ICD therapies remains an important health care problem. Several clinical factors elucidated to be prognostic for CHF progression in ICD patients in previous studies are appropriate and inappropriate ICD shocks5, renal failure21, NYHA class, and LVEF22. Our study is the first to show that the LIC phenomenon after induced VF rescue ICD shock carries an independent high risk, if observed in newly implanted ICD leads.
It is known that neurohumoral and cytokine activations contribute to the inflammatory and oxidative characteristics of CHF patients23. We speculate that these chronically activated pathways in at risk CHF patients result in a dramatic response to induced VF rescue ICD shock. Since patients without subsequent CHF were less likely to exhibit LIC, susceptibility for heart failure progression appears to be the “first hit” prerequisite for the LIC phenomenon we observed, and allows the appearance of LIC to serve as a marker of CHF risk.
Local mechanical myocardial injury and injury current on bipolar intracardiac electrogram
Transient LIC presenting on NF RV EGM during the acute placement of an ICD or pacemaker lead is well known. Several groups of investigators linked characteristics of LIC at the time of an active-fixation lead placement with subsequent adequate lead fixation19;20 and with lead perforation.24 Transvenous insertion of endocardial leads for permanent pacing25 or use with an ICD26 is accompanied by acute injury, followed by a sequence of cardiac histopathological changes starting with acute inflammation and leading eventually to the formation of a fibrous connective tissue scar.27;28 Maximum ventricular lead diameter, number of implanted leads,25 and CRT device LV lead placement29 were independent predictors of peak cardiac troponin I levels in patients undergoing conventional pacemaker/ICD implantation. Less frequently observed LIC in patients with chronic leads is a finding that suggests recent local mechanical injury is usually required as the “second hit” for LIC to occur. Future study is needed to determine the optimal prognostic time window from lead fixation to VT/VF induction.
Transient myocardial injury after ICD shock
According to the excitation theory of defibrillation, electrical shocks depolarize the membranes of most cardiac cells, resulting in resynchronization of electrical activity of the heart. If shock-induced changes in transmembrane potential are excessively large, they can cause transient cell membrane damage due to electroporation.30–32 Other potential causes of myocardial injury after an ICD shock include free radicals formation33;34 and conformation changes of the membrane ion channels.35 We speculate that enhanced LIC on bipolar NF RV EGM after an ICD shock in patients prone to subsequent CHF progression is produced by local voltage gradients, resulting from potential differences between electroporated myocardial cells and normal cells. In our study, LIC was observed after ICD shock, but not in subsequent control EGMs, thus supporting the importance of the shock and possibly the induced arrhythmia) for the “third hit.”
Our results show that appropriate ICD shock predicts future CHF exacerbation and death only in patients with significant LIC after rescue ICD shock. Conversely, patients without LIC and subsequent appropriate ICD shocks during follow-up had the same favorable course as patients without ICD shocks. This important clinical finding suggests that ICD shock does not cause, but rather unveils risk of progressive CHF.
Study limitations
Our observations were limited by the 10-second post-shock EGM recording storage. We were unable to determine a final recovery time point and duration of EGM changes. EGM after ICD lead fixation but before induced VT/VF was not available for analysis.
Specific filter settings on bipolar NF RV EGM may preclude analysis of other manufacturers’ electrograms. Small number of end-point events limited multivariate Cox regression analysis.
Small number of chronic leads in this study prompts further investigation of LIC after ICD shock in chronic leads to determine its predictive value for subsequent CHF exacerbation.
In this study, we did not test the effect of ICD shock alone, without preceding induced arrhythmia, on the genesis of LIC. Theoretically, this could have been assessed at device implant through the use of a protocol for upper limit of vulnerability (ULV) testing36–37 , instead of that used to determine DFT.
Clinical significance.
The observed LIC phenomenon predicts progression of CHF in ICD patients with appropriate ICD shocks, and with otherwise stable NYHA class I–III CHF. Early awareness of the high risk of CHF exacerbation and thoughtful medical management may improve CHF prognosis in ICD patients.
Acknowledgments
We thank Jane Chen, Timothy Smith, Marye Gleva and Bruce Lindsay for providing medical care for study participants, and Judy Osborn for help with collection of follow-up data.
Financial support & relationships with industry:
This study was supported by Medtronic, Inc. as an Investigator-initiated Research Project (awarded to Drs. Berger and Tereshchenko). Dr. Faddis has served as a consultant for Boston Scientific Corp., Stereofaxis, Inc., and St. Jude Medical, Inc. Dr. Efimov has been a stock owner and Scientific Advisory Board Chair with CARDIALEN; and has received NIH grants HL67322, HL074283, HL082729.
Abbreviations
- SCA
sudden cardiac arrest
- CHF
congestive heart failure
- ICD
implantable cardioverter-defibrillator
- EGM
electrograms
- RV
right ventricular
- NYHA
New York Heart Association
- DFT
defibrillation threshold test
- ECG
electrocardiogram
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- CRT-D
cardiac resynchronization therapy defibrillator
- LVEF
left ventricular ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Anderson JL, Hallstrom AP, Epstein AE, et al. The AVID Investigators. Design and results of the antiarrhythmics vs implantable defibrillators (AVID) registry. Circulation. 1999;99:1692–1699. doi: 10.1161/01.cir.99.13.1692. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, et al. Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–2817. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 8.Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 9.Fast VG, Cheek ER. Optical mapping of arrhythmias induced by strong electrical shocks in myocyte cultures. Circ Res. 2002;90:664–670. doi: 10.1161/01.res.0000013403.24495.cc. [DOI] [PubMed] [Google Scholar]
- 10.Waldecker B, Brugada P, Zehender M, Stevenson W, Welens HJ. Ventricular arrhythmias after precordial electric shock. Am J Cardiol. 1986;57:120–123. doi: 10.1016/0002-9149(86)90963-x. [DOI] [PubMed] [Google Scholar]
- 11.Eysmann SB, Marchlinski FE, Buxton AE, Buxton AE, Josephson ME. Electrocardiographic changes after cardioversion of ventricular arrhythmias. Circulation. 1986;73:73–81. doi: 10.1161/01.cir.73.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Sparks PB, Kulkarni R, Vohra JK, et al. Effect of direct current shocks on left atrial mechanical function in patients with structural heart disease. J Am Coll Cardiol. 1998;31:1395–1399. doi: 10.1016/s0735-1097(98)00121-1. [DOI] [PubMed] [Google Scholar]
- 13.Sparks PB, Jayaprakash S, Mond HG, Vohra JK, Grigg LE, Kalman JM. Left atrial mechanical function after brief duration atrial fibrillation. J Am Coll Cardiol. 1999;33:342–349. doi: 10.1016/s0735-1097(98)00585-3. [DOI] [PubMed] [Google Scholar]
- 14.Grimm RA, Stewart WJ, Arheart K, Thomas JD, Klein AL. Left atrial appendage "stunning" after electrical cardioversion of atrial flutter: an attenuated response compared ith atrial fibrillation as the mechanism for lower susceptibility to thromboembolic events. Am Coll Cardiol. 1997;29:582–589. doi: 10.1016/s0735-1097(96)00551-7. [DOI] [PubMed] [Google Scholar]
- 15.Kam RM, Garan H, McGovern BA, Ruskin JN, Harthorne JW. Transient right bundle ranch block causing R wave attenuation postdefibrillation. Pacing Clin Electrophysiol. 1997;20:130–131. doi: 10.1111/j.1540-8159.1997.tb04823.x. [DOI] [PubMed] [Google Scholar]
- 16.Hasdemir C, Shah N, Rao AP, et al. Analysis of troponin I levels after spontaneous implantable cardioverter defibrillator shocks. J Cardiovasc Electrophysiol. 2002;13:144–150. doi: 10.1046/j.1540-8167.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 17.Osswald S, Trouton TG, O'Nunain SS, Holden HB, Ruskin JN, Garan H. Relation between shock-related myocardial injury and defibrillation efficacy of monophasic and biphasic shocks in a canine model. Circulation. 1994;90:2501–2509. doi: 10.1161/01.cir.90.5.2501. [DOI] [PubMed] [Google Scholar]
- 18.Gurevitz O, Lipchenca I, Yaacoby E, et al. ST-segment deviation following implantable cardioverter defibrillator shocks: incidence, timing, and clinical significance. Pacing Clin Electrophysiol. 2002;25:1429–1432. doi: 10.1046/j.1460-9592.2002.01429.x. [DOI] [PubMed] [Google Scholar]
- 19.Saxonhouse SJ, Conti JB, Curtis AB. Current of injury predicts adequate active lead fixation in permanent pacemaker/defibrillation leads. J Am Coll Cardiol. 2005;45:412–417. doi: 10.1016/j.jacc.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 20.Redfearn DP, Gula LJ, Krahn AD, Skanes AC, Klein GJ, Yee R. Current of injury predicts acute performance of catheter-delivered active fixation pacing leads. Pacing Clin Electrophysiol. 2007;30:1438–1444. doi: 10.1111/j.1540-8159.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 21.Schefer T, Wolber T, Binggeli C, Holzmeister J, Brunckhorst C, Duru F. Long-term predictors of mortality in ICD patients with non-ischaemic cardiac disease: impact of renal function. Europace. 2008;10:1052–1059. doi: 10.1093/europace/eun186. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–2415. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Tang WH, Francis GS. The year in heart failure. J Am Coll Cardiol. 2008;52:1671–1678. doi: 10.1016/j.jacc.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Van Gelder BM, Bracke FA. Current of injury (COI) pattern recorded from catheter delivered active fixation pacing leads. Pacing Clin Electrophysiol. 2008;31:786–787. doi: 10.1111/j.1540-8159.2008.01089_3.x. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaou NI, Spanodimos SG, Tsaglis EP, et al. Biochemical evidence of cardiac damage following transvenous implantation of a permanent antibradycardia pacemaker lead. Pacing Clin Electrophysiol. 2005;28:1174–1181. doi: 10.1111/j.1540-8159.2005.50136.x. [DOI] [PubMed] [Google Scholar]
- 26.Hurst TM, Hinrichs M, Breidenbach C, Katz N, Waldecker B. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol. 1999;34:402–408. doi: 10.1016/s0735-1097(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 27.Ford SE, Manley PN. Indwelling cardiac catheters. An autopsy study of associated endocardial lesions. Arch Pathol Lab Med. 1982;106:314–317. [PubMed] [Google Scholar]
- 28.Beyersdorf F, Schneider M, Kreuzer J, Falk S, Zegelman M, Satter P. Studies of the tissue reaction induced by transvenous pacemaker electrodes. I. Microscopic examination of the extent of connective tissue around the electrode tip in the human right ventricle. Pacing Clin Electrophysiol. 1988;11:1753–1759. doi: 10.1111/j.1540-8159.1988.tb06306.x. [DOI] [PubMed] [Google Scholar]
- 29.Altin T, Akyurek O, Vurgun K, et al. Effect of transvenous cardiac resynchronization therapy device implantation on cardiac troponin I release. Pacing Clin Electrophysiol. 2007;30:1356–1362. doi: 10.1111/j.1540-8159.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 30.Al Khadra A, Nikolski V, Efimov IR. The role of electroporation in defibrillation. Circ Res. 2000;87:797–804. doi: 10.1161/01.res.87.9.797. [DOI] [PubMed] [Google Scholar]
- 31.Nikolski VP, Sambelashvili AT, Krinsky VI, Efimov IR. Effects of electroporation on optically recorded transmembrane potential responses to high-intensity electrical shocks. Am J Physiol Heart Circ Physiol. 2004;286:H412–H418. doi: 10.1152/ajpheart.00689.2003. [DOI] [PubMed] [Google Scholar]
- 32.Nikolski VP, Efimov IR. Electroporation of the heart. Europace. 2005;7 Suppl 2:146–154. doi: 10.1016/j.eupc.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Caterine MR, Spencer KT, Pagan-Carlo LA, Smith RS, Buettner GR, Kerber RE. Direct current shocks to the heart generate free radicals: an electron paramagnetic resonance study. J Am Coll Cardiol. 1996;28:1598–1609. doi: 10.1016/s0735-1097(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 34.Ravingerova T, Slezak J, Tribulova N, Dzurba A, Uhrik B, Ziegelhoffer A. Free oxygen radicals contribute to high incidence of reperfusion-induced arrhythmias in isolated rat heart. Life Sci. 1999;65:1927–1930. doi: 10.1016/s0024-3205(99)00449-x. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Lee RC. Altered ion channel conductance and ionic selectivity induced by large imposed membrane potential pulse. Biophys J. 1994;67:603–612. doi: 10.1016/S0006-3495(94)80520-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day JD, Doshi RN, Belott P, et al. Inductionless or limited shock testing is possible in most patients with implantable cardioverter- defibrillators/cardiac resynchronization therapy defibrillators: results of the multicenter ASSURE Study (Arrhythmia Single Shock Defibrillation Threshold Testing Versus Upper Limit of Vulnerability: Risk Reduction Evaluation With Implantable Cardioverter-Defibrillator Implantations) Circulation. 2007;115:2382–2389. doi: 10.1161/CIRCULATIONAHA.106.663112. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow CD, Shehata M, Chen PS. Using the upper limit of vulnerability to assess defibrillation efficacy at implantation of ICDs. Pacing Clin Electrophysiol. 2007;30:258–270. doi: 10.1111/j.1540-8159.2007.00659.x. [DOI] [PubMed] [Google Scholar]