SUMMARY
We describe a patient with an autoinflammatory disease in which the main clinical features are pustular rash, marked osteopenia, lytic bone lesions, respiratory insufficiency, and thrombosis. Genetic studies revealed a 175-kb homozygous deletion at chromosome 2q13, which encompasses several interleukin-1 family members, including the gene encoding the interleukin-1–receptor antagonist (IL1RN). Mononuclear cells, obtained from the patient and cultured, produced large amounts of inflammatory cytokines, with increasing amounts secreted after stimulation with lipopolysaccharide. A similar increase was not observed in peripheral-blood mononuclear cells from a patient with neonatal-onset multisystem inflammatory disorder (NOMID). Treatment with anakinra completely resolved the symptoms and lesions.
In most autoinflammatory disorders, systemic and local inflam-mation appear spontaneously at a young age.1 In one such disorder, neonatal-onset multisystem inflammatory disorder (NOMID), there is a rash at birth and eventual development of inflammatory arthropathy, meningitis, hearing loss, and signs of systemic inflammation.2,3 This disease is caused by mutations in NLRP3, which encodes cryopyrin, a protein that mediates the activation of caspase 1 and proteolytic conversion of pro–interleukin-1β to functional interleukin-1β.4-6 Mononuclear cells in NOMID produce high levels of inter leukin-1β, which appear in the serum.6 Treatment with the recombinant interleukin-1–receptor antagonist anakinra alleviates the symptoms and signs of NOMID.7
In this report, we describe an autoinflammatory syndrome caused by a deletion in the interleukin-1 family gene cluster including the interleukin-1–receptor antagonist. Clinical features, gene-expression analysis, and the pattern of cytokine production by peripheral-blood mononuclear cells distinguish this syndrome from NOMID. Treatment with anakinra resulted in complete resolution of all symptoms.
CASE REPORT
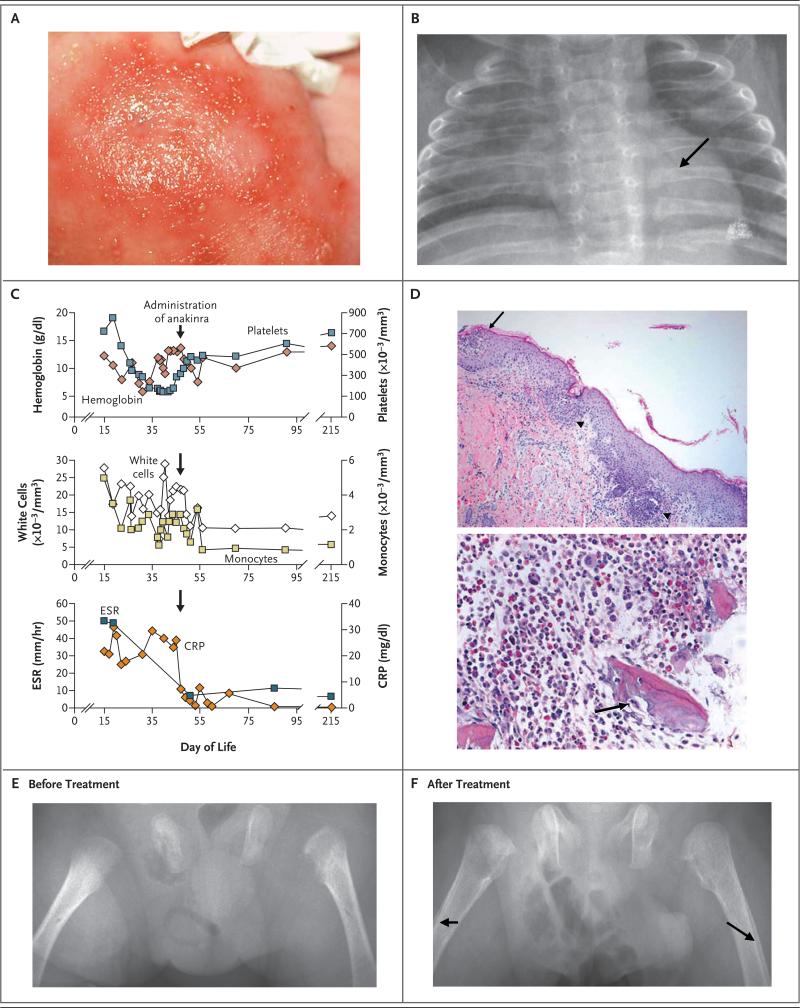
The affected male infant was born at 33 weeks’ gestation after an uncomplicated pregnancy and remained in the hospital for 5 days because of apnea. The parents have the same grandmother and are half first cousins. There was no family history of autoimmunity or inflammatory, skin, or bone disorders. An erythematous macular rash with central pustules appeared in the infant's groin, at approximately 10 days of age, and subsequently spread over his body (Fig. 1A). Because of the rash and continued apneic episodes, he was readmitted to the hospital at 15 days of age. Fever was not noted before or after admission to the hospital. Bacterial cultures of blood, urine, and cerebrospinal fluid specimens were negative, but culture of the pustular lesions was positive for methicillin-resistant Staphylococcus aureus (MRSA). Ultrasonography of the pelvis showed bilateral hip effusions, which were aspirated, but no organisms were cultured. Chest radiography showed diffuse osteopenia and abnormalities of the ribs, with flaring anteriorly near the costochondral junction (Fig. 1B). A skeletal survey showed corner fractures of the distal right femur and the proximal right tibia. Because of mild hypoxemia, computed tomography (CT) of the chest was performed, revealing localized ground-glass opacities and areas of atelectasis. Results of laboratory studies included leukocytosis, monocytosis, anemia, thrombocytosis, and an elevated erythrocyte sedimentation rate and C-reactive protein level (Fig. 1C).
Figure 1. Clinical Phenotype of Our Patient with the Homozygous Deletion at the IL1RN Locus.
The patient's rash consisted of erythematous macules with pustules (Panel A). Chest radiography showed osteopenia and anterior rib flaring with osteolytic lesions (Panel B, arrow). Panel C shows laboratory values before and after the administration of anakinra. In Panel D (hematoxylin and eosin), a skin-biopsy specimen (top) shows subcorneal pustules (arrow), filled with neutrophils, and focal acantholysis. Neutrophils also infiltrated and destroyed the infundibulum of hair follicles (arrowheads). A bone-biopsy specimen (bottom) shows fragments of woven bone with scalloping of the edges and a focally prominent cement line. Osteoclasts are scattered throughout, including one at a scalloped edge of the bone (arrow). Radiographs of the femurs before (Panel E) and after (Panel F) administration of anakinra show healing bone, as well as periosteal reactions (arrows) in response to treatment. CRP denotes C-reactive protein, and ESR erythrocyte sedimentation rate.
Because of concerns about the MRSA infection and possible osteomyelitis and septic arthritis, a 4-week course of vancomycin and meropenem was given. Nevertheless, the elevated inflammatory markers, pustular rash, and respiratory distress continued. CT was performed again, showing new bone lesions in the proximal femurs and both humeri. A repeat skeletal survey on day 24 showed increased osteopenia, an increased number of lytic lesions in the ribs and proximal left femur, and new lesions of the distal left ulna and radius. On day 29, swelling of the right leg developed, and ultrasonography showed thrombosis of the right common femoral and right external iliac veins. Shortly thereafter, swelling of the left arm developed, and ultrasonography revealed a large thrombus of the left basilic, axillary, and subclavian veins in association with a peripherally inserted central catheter. Laboratory studies to rule out a hypercoagulable state (including prothrombin and factor V Leiden sequence analyses; tests of protein S activity, protein C activity, and antithrombin III activity; and measurement of lupus anticoagulant and antiphospholipid antibody levels) were negative, and heparin was started. A skin-biopsy specimen from the left side of the chest showed subcorneal pustules with infundibulofolliculitis (Fig. 1D). Bone-biopsy specimens from the left femur contained fragments of reactive woven bone with scalloped edges, scattered osteoclasts, and increased numbers of neutrophils (Fig. 1D). No evidence of cancer or Langerhans’-cell histiocytosis was found. Cultures of bone-tissue samples for anaerobes, aerobes, acid-fast bacilli, and fungi were all negative.
Worsening respiratory distress developed in association with progression of ground glass opacities on chest CT. An open lung biopsy showed alveoli filled with macrophages and some neutrophils. Because of the worsening clinical picture and similarities of this case to NOMID, anakinra was administered on day 49 (5 mg per kilogram of body weight, administered subcutaneously, daily). There was a rapid and sustained resolution of skin lesions and respiratory distress. Inflammatory markers returned to normal, and remained normal for over 6 months. After 2 months of anakinra therapy, bone mineral density increased and periosteal reactions were noted, indicating bone healing (Fig. 1E and 1F). After 6 months of therapy, the bone mineral density increased from 0.185 to 0.265 g per square centimeter. The patient is currently growing and developing normally: he is 18 months of age, weighs 9.3 kg (3rd percentile), and is 77 cm tall (18th percentile). He remains asymptomatic, while receiving anakinra daily, at a dose of 2.5 mg per kilogram. (Nine similar cases are reported in this issue of the Journal by Aksentijevich et al.8)
METHODS
CELL ISOLATION AND ANALYSIS
Mononuclear cells were isolated with the use of Ficoll-Paque (Amersham) and cultured in medium with or without lipopolysaccharide (10 μg per milliliter, Sigma) for 4 or 24 hours. Supernatants were analyzed for interleukin-8, interleukin-1β, interleukin-6, interleukin-10, tumor necrosis factor α (TNF-α), and interleukin-12 p70 with the use of a BD Cytometric Bead Array human inflammation kit (BD Biosciences) or by an interleukin-1β enzyme-linked immunosorbent assay kit (eBioscience). Peripheral-blood mononuclear cells, either stimulated with lipopolysaccharide or not stimulated, were stained with antibodies against CD14 and analyzed with the use of a flow cytometer (FACSCalibur, BD Biosciences).9
DNA ISOLATION AND RNA SYNTHESIS
Genomic DNA was isolated by means of a genomic DNA purification system (Wizard SV, Promega). Total RNA was obtained with the use of the Trizol reagent (Invitrogen), according to the manufacturer's instructions.
ANALYSIS OF COPY-NUMBER VARIATION
Genomewide analysis of copy-number variation was performed with the use of a single-nucleotide polymorphism (SNP) array (Genome-Wide Human SNP Array 6.0, Affymetrix). Copy-number analysis was performed by means of a genotyping console (Genotyping Console version 2.0, Affymetrix) and a reference model (GenomeWideSNP_6.hapmap270.422 data set). A total of 1.8 million probes are included on the array. All samples had contrast quality scores of more than 0.4. For the analysis, we selected the following standard settings: median absolute pairwise difference, more than 0.4; minimum number of markers, 5; and minimum genomic size of the segment, 100 kb.
GENE-EXPRESSION ANALYSIS
Purified RNA (in aliquots of approximately 100 ng) was amplified by means of a two-cycle complementary-DNA synthesis kit, and complementary RNA was synthesized, labeled, fragmented, and hybridized to a hybridization array (GeneChip Human Genome U133 Plus 2.0 Array, Affymetrix). The hybridization arrays were washed and stained (with the GeneChip Hybridization Wash and Stain Kit, Affymetrix) and scanned. The image data were analyzed (with GeneChip Operating Software, Affymetrix) and normalized with the use of Robust Multichip Analysis to determine the signal intensity ratios. Hierarchical clustering was conducted with the use of Genesis software (version 1.7.2).10
RESULTS
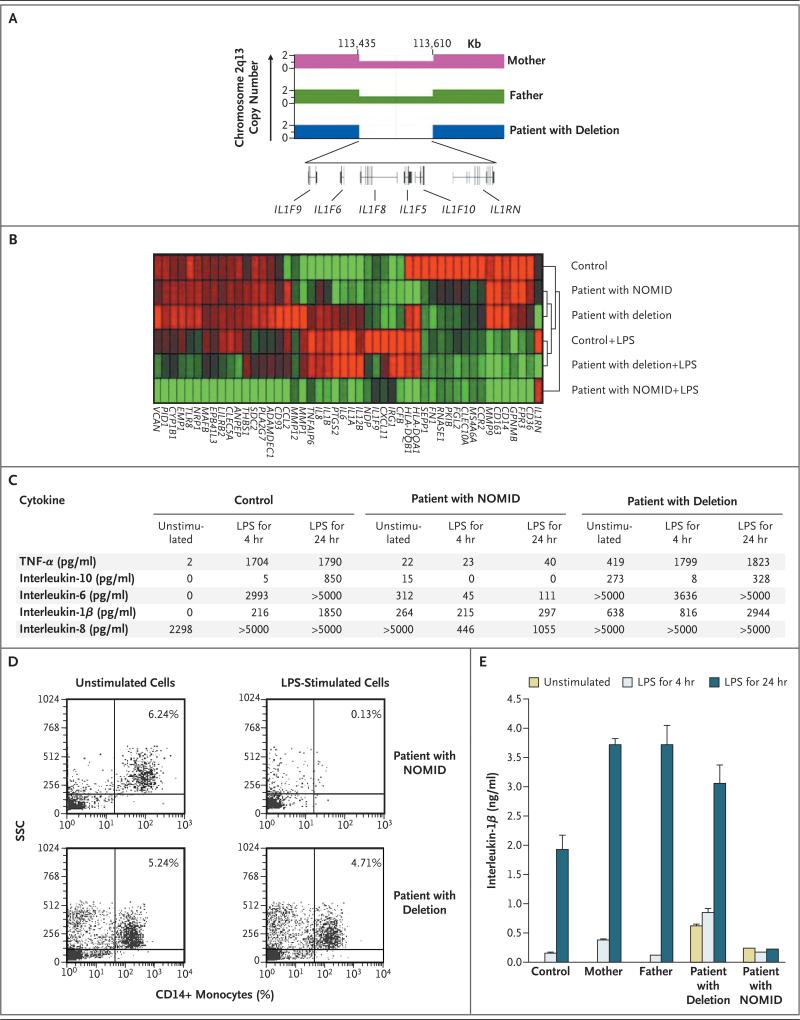
The unusual features and severity of the disease in this infant, who was born to consanguineous parents, prompted us to perform genomewide analysis of single-nucleotide polymorphisms. We found a 175-kb deletion at chromosome 2q13 that encompasses six interleukin-1 family members: IL1RN, IL1F5, IL1F6, IL1F8, IL1F9, and IL1F10 (Fig. 2A). The parents were heterozygous for this deletion and were healthy (Fig. 2A).
Figure 2. Genetic and Gene-Expression Characteristics of Our Patient with a Deletion at the IL1RN Locus and a Patient with Neonatal-Onset Multisystem Inflammatory Disease (NOMID).
Panel A shows the variation in genomic copy number in chromosome 2q13 for the patient and his mother and father, showing a homozygous 175-kb deletion in the patient and heterozygosity in his parents. The deletion includes genes encoding six members of the interleukin-1 family: the interleukin-1–receptor antagonist (IL1RN) and interleukin-1 family members 5, 6, 8, 9, and 10 (IL1F5, IL1F6, IL1F8, IL1F9, and IL1F10, respectively). The gene-expression profiles of peripheral-blood mononuclear cells from a control, our patient, and a patient with NOMID are shown in Panel B, before and after stimulation with lipopolysaccharide (LPS) for 4 hours. Red represents an increase in transcription, and green a decrease, with the intensity of the color indicating the degree of increase or decrease. The dendogram to the right of the microarray reflects the relatedness of the gene signatures between the samples. Panel C shows levels of inflammatory proteins in unstimulated and LPS-stimulated peripheral-blood mononuclear cells from a control, our patient, and a patient with NOMID. Panel D shows the numbers of CD14+ monocytes in peripheral-blood mononuclear cells from a patient with NOMID and in our patient before and after stimulation with LPS for 24 hours. The percentages of CD14+ monocytes remaining in the cultures are shown, according to side-scatter (SSC) analysis. Panel E shows mean levels of interleukin-1β in unstimulated and LPS-stimulated peripheral-blood mononuclear cells from our patient, his mother and father, a patient with NOMID, and a control subject. T bars indicate the standard deviation (not visible on some bars). TNF-α denotes tumor necrosis factor α.
To differentiate our patient's disease from NOMID, we compared global gene-expression profiles of mononuclear cells isolated from him, a control subject, and a patient with NOMID due to a mutation in NLRP3 (Phe525Leu). Since baseline gene expression varied considerably among these three subjects, we normalized the data to the overall mean log2 signal intensity for all conditions. Hierarchical clustering was conducted with the use of any probe set that exhibited a minimum difference by a factor of 16 among the three subjects. We found that the transcriptomes from unstimulated mononuclear cells from our patient with the deletion and the patient with NOMID were similar, but both differed from the control subject's transcriptome (Fig. 2B). After lipopolysaccharide stimulation of mononuclear cells, the transcriptome of mononuclear cells from the our patient was more similar to that of the control than that of the patient with NOMID. The transcription of genes encoding interleukin-1β, interleukin-1α, interleukin-8, and interleukin-6 increased in cells from our patient with the deletion and the control but not in those from the patient with NOMID.
To confirm the results of the microarray experiments, we measured the amounts of interleukin-1β, interleukin-6, interleukin-8, interleukin-10, and TNF-α in the supernatants from unstimulated and lipopolysaccharide-stimulated mononuclear cells. Elevated amounts of interleukin-1β, interleukin-6, and interleukin-8 and TNF-α were found in unstimulated cells from the patient with NOMID and our patient (Fig. 2C). After lipopolysaccharide stimulation, these cytokines increased markedly in the supernatants from cultured cells from the control and our patient but not in cells from the patient with NOMID.
Previous investigations showed that monocytes from patients with NOMID undergo apoptosis in response to lipopolysaccharide.11 We tested whether lipopolysaccharide stimulation also caused the death of monocytes from our patient with the deletion. Figure 2D shows dramatic loss of monocytes after lipopolysaccharide stimulation of mononuclear cells from the patient with NOMID, whereas no such loss was found in cultures of cells from controls (data not shown) and our patient.
Since both parents of our patient carried the deletion, we tested whether haploinsufficiency of the gene cluster on chromosome 2q13 increased the production of interleukin-1β. Interleukin-1β levels were measured in supernatants from lipopolysaccharide-stimulated mononuclear cells from the patient, both parents, a control, and a patient with NOMID. Cultures of unstimulated mono-nuclear cells from both parents and the control produced no interleukin-1β, whereas cultures of mononuclear cells from the patient with NOMID and our patient with the deletion showed elevated levels of interleukin-1β (Fig. 2E). After lipopolysaccharide stimulation, mononuclear cells from the control, mother, father, and our patient markedly up-regulated interleukin-1β, with the highest levels after 24 hours, whereas the cells from the patient with NOMID failed to up-regulate interleukin-1β beyond the levels before stimulation.
DISCUSSION
We describe an autoinflammatory disease due to a homozygous deletion of the interleukin-1 locus encompassing IL1RN and five other interleukin-1 family members. This disease shares some clinical features with NOMID — signs of systemic inflammation and rash, from birth — but it has a more severe clinical course and clinical features that are not observed in NOMID. These include severe osteopenia, lytic bone lesions, respiratory involvement, and thrombotic episodes. Whether the premature birth of the affected child was related to the inflammatory condition is unknown, but there is a suggestion that interleukin-1 may have a role in premature birth.12
Treatment with anakinra rapidly resolved all signs of the disease, including the severe osteopenia and bone lesions. The rapid healing of bone lesions in response to anakinra confirms the importance of interleukin-1 in osteoclast activation and bone loss.13 Lytic bone lesions do not develop in patients with NOMID, despite increased interleukin-1β production. We found that the production of inflammatory proteins by mono-nuclear cells in response to lipopolysaccharide was less in patients with NOMID than in our patient with the deletion; this difference could account for the milder NOMID phenotype. In this regard, the tendency of monocytes in patients with NOMID to undergo apoptosis in response to inflammatory stimuli may limit the amount of interleukin-1β and other inflammatory cytokines the monocytes can produce.
Several cytokines are not produced because of deletion of several genes within chromosome 2q13, and the absence of the interleukin-1–receptor antagonist probably accounts for many of the clinical and pathologic manifestations of our patient. Arteritis, arthritis, and skin inflammation develop in mice deficient in interleukin-1–receptor antagonist.14-16 Contributions by the other interleukin-1 family members may also be involved. Interleukin-1F5 and interleukin-1F10 have 40% amino acid sequence homology to interleukin-1– receptor antagonist and may have similar regulatory roles.17,18 The function of the other interleukin-1 family members is unclear, but interleukin-1F6, interleukin-1F8, and interleukin-1F9 also have similarities in amino acid sequence to interleukin-1β and can activate nuclear factor-κB.17-20 Inflammatory skin disease develops in transgenic mice expressing interleukin-1F6 in basal keratinocytes, and the skin lesions are worse in offspring of a cross between these animals and interleukin- 1F5–deficient mice.21 The skin in this animal model has intracorneal and intraepithelial pustules, reminiscent of the lesions in our patient, suggesting that the loss of interleukin-1F5 contributes to his rash. The consequences of the loss of interleukin-1F6, interleukin-1F8, and interleukin-1F9 are unclear, although we have not observed any increased susceptibility to infection or other immune problems in our patient after anakinra therapy.
In summary, we describe an autoinflammatory disease due to a homozygous deletion in chromosome 2q13 that encompasses six interleukin-1 family members, including the interleukin-1–receptor antagonist. Although other interleukin-1 family members may be involved in the pathogenesis of this disease, we hypothesize that the deficiency of interleukin-1–receptor antagonist is the primary cause.
Acknowledgments
We thank Drs. Jack Routes, Calvin Williams, William Grossman, and Talal Chatila for the careful review of a previous draft of this manuscript and Dr. Patti-Marie Young and the physicians at the Children's Hospital of Wisconsin for the outstanding clinical care of this patient.
Supported by grants from the National Institute of Allergy and Infectious Diseases (RO1AI078713 and U19AI062627), the Research and Education Foundation of the American College of Rheumatology, and the Children's Hospital of Wisconsin Foundation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hull KM, Shoham N, Chae JJ, Aksentijevich I, Kastner DL. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61–9. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr. 1981;99:79–83. doi: 10.1016/s0022-3476(81)80961-4. [DOI] [PubMed] [Google Scholar]
- 3.Prieur AM, Griscelli C, Lampert F, et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome: a specific entity analysed in 30 patients. Scand J Rheumatol Suppl. 1987;66:57–68. doi: 10.3109/03009748709102523. [DOI] [PubMed] [Google Scholar]
- 4.Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multi-system inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agostini L, Martinon F, Burns K, Mc-Dermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 8.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–8. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Nishikomori R, Kambe N, et al. Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood. 2008;111:2132–41. doi: 10.1182/blood-2007-06-094201. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 13.Strand V, Kavanaugh AF. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):iii10–iii16. doi: 10.1093/rheumatology/keh202. [DOI] [PubMed] [Google Scholar]
- 14.Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd J, Little MC, Nicklin MJ. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol. 2004;122:665–9. doi: 10.1111/j.0022-202X.2004.22305.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SL, Renshaw BR, Garka KE, Smith DE, Sims JE. Genomic organization of the interleukin-1 locus. Genomics. 2002;79:726–33. doi: 10.1006/geno.2002.6752. [DOI] [PubMed] [Google Scholar]
- 18.Nicklin MJ, Barton JL, Nguyen M, FitzGerald MG, Duff GW, Kornman K. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics. 2002;79:718–25. doi: 10.1006/geno.2002.6751. [DOI] [PubMed] [Google Scholar]
- 19.Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–88. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 20.Debets R, Timans JC, Homey B, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–6. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg H, Dinh H, Trueblood ES, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–14. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]