Abstract
G protein-coupled receptors (GPCRs) mediate signaling from extracellular ligands to intracellular signal transduction proteins1. Methuselah (Mth) is a class B (secretin-like) GPCR, a family typified by their large, ligand-binding, N-terminal extracellular domains2. Down-regulation of mth increases the lifespan of Drosophila melanogaster3— inhibitors of Mth signaling would thus be expected to enhance longevity. We used mRNA display selection4,5 to identify high affinity (KD = 15 to 30 nM) peptide ligands that bind to the N-terminal ectodomain of Mth. The selected peptides are potent antagonists of Mth signaling, and structural studies suggest that they perturb the interface between the Mth ecto- and transmembrane (TM) domains. Flies constitutively expressing a Mth antagonist peptide exhibit a robust lifespan extension, suggesting that the peptides inhibit Mth signaling in vivo. Our work thus provides novel lifespan-extending ligands for a metazoan and a general approach for the design of modulators of this important class of GPCRs.
Because of their participation in numerous cell processes, GPCRs are the targets of approximately half of marketed drugs, and new GPCR ligands continue to be pursued and developed6. Naïve approaches toward GPCR ligand identification typically involve high-throughput screening of a molecular library (102 to 105 unique members) in functional, cell-based assays6–8. A powerful, alternative approach for the rapid isolation of novel ligands is in vitro peptide selection using mRNA display, which allows access to very high library complexities (>1013) in a robust format4. High affinity peptide ligands for RNA, small molecule, and protein targets have been identified by mRNA display selection5.
The crystal structure of the hexahistidine-tagged Mth ectodomain was previously determined, demonstrating that the mature, N-terminal extracellular domain of Mth is a stably folded, glycosylated protein of 195 residues9. Since the ectodomains of other class B GPCRs maintain recognition of their cognate ligands independently of their TM cores10,11, we targeted the Mth ectodomain for in vitro selection to isolate putative modulators of Mth signaling. We expressed and purified a specifically biotinylated construct of the Mth ectodomain12 to avoid using the weak hexahistidine epitope as an immobilization tag for selection13. Hence, the Mth ectodomain was homogeneously presented, perhaps mimicking the juxtaposition of the ectodomain and TM domain in the context of the full-length receptor.
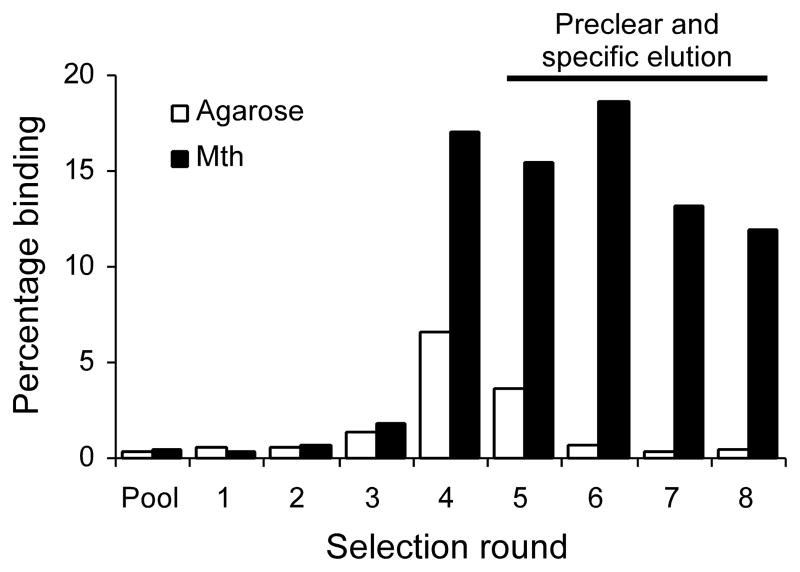
We constructed a random, 27-mer peptide mRNA display library. After eight rounds of selection, with the final four rounds including preclearing steps on matrix without target and specific elution with free, non-biotinylated Mth, we obtained a final 8th round pool that exhibited high activity for Mth and negligible non-specific binding (Fig. 1). DNA sequencing of individual clones from the final selection round revealed a highly conserved consensus, [R/P]xxWxxR, which we term the RWR motif (Table 1). This motif was not found in the recently identified Mth peptide agonist, Stunted14, and the shortness of the consensus precluded any statistically significant homology to the Drosophila proteome.
Fig. 1.
Selection of a 27-mer peptide library against the Mth ectodomain. RNase-treated, 35S-methionine–labeled mRNA displayed peptides from each round of selection were assayed for binding to immobilized Mth (black) or to matrix alone (white). Preclearing and competitive elutions were performed in the 5th through 8th rounds to eliminate non-specific binding peptides.
Table 1.
Peptide ligands for the Mth ectodomain. RWR motif residues are in bold. The C-terminal constant region (TSGGLRASAI), which was frameshifted or mutated in marked sequences (*), is not shown. The sequences used to make the synthetic peptides are underlined (two peptides, a 22- and a 15-mer, were synthesized for R8-01). KD values were calculated (kd/ka) from the kinetic parameters obtained from surface plasmon resonance experiments.
| Peptide | Sequence | ka | kd | KDb | χ2 | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M −1s−1 (× 105) | s−1 (× 10−2) | nM | ||||||||||||||||||||||||||||||||||||||||||||
| R8-12 | M | R | L | V | W | I | V | R | S | R | H | F | G | P | R | L | R | M | A | L | L | G | S | D | R | K | M | W | 4.1 | 0.72 | 18 | 1.3 | ||||||||||||||
| R8-14 | M | A | P | R | A | V | W | I | Q | R | A | I | Q | A | M | F | R | L | A | S | R | Q | E | S | K | A | F | N | 7.0 | 1.2 | 18 | 1.7 | ||||||||||||||
| R8-09b* | M | R | Y | V | W | Y | L | R | T | K | H | R | R | S | L | R | L | R | S | A | C | A | R | G | S | S | A | |||||||||||||||||||
| R8-03 | M | G | D | D | M | Y | R | I | R | E | F | L | A | N | Y | R | P | I | W | V | M | R | S | N | L | A | Q | L | ||||||||||||||||||
| R8-01 | M | N | V | S | W | G | S | F | P | S | S | W | L | Q | R | Y | Y | L | A | K | R | R | E | A | D | V | T | L | 6.3 | 1.9 | 31 | 1.5 | ||||||||||||||
| 9.5 | 5.4 | 57 | 0.46 | |||||||||||||||||||||||||||||||||||||||||||
| R8-07 | M | L | K | Y | P | D | T | W | L | A | R | S | L | S | V | F | Y | L | R | K | S | A | R | Q | G | K | S | V | ||||||||||||||||||
| R8-13 | M | E | L | G | Q | F | Q | R | L | S | L | P | Y | Q | W | Y | L | R | T | I | S | Y | V | S | L | R | T | A | ||||||||||||||||||
| R8-07b* | M | S | T | A | G | S | R | A | R | S | T | S | W | G | T | R | S | P | W | T | W | P | T | P | A | R | T | G | ||||||||||||||||||
| R8-04 | M | V | R | I | G | Y | T | S | K | P | G | G | M | N | P | G | N | S | Y | T | M | S | I | I | R | M | L | I | 6.1 | 19.9 | 326 | 0.46 | ||||||||||||||
| R8-08b* | M | S | S | L | S | P | P | W | P | A | S | W | S | P | S | R | P | S | A | P | R | A | A | P | S | T | P | T | ||||||||||||||||||
We synthesized several selected peptides for binding analysis by surface plasmon resonance (SPR). Peptides containing the RWR motif demonstrated high affinity (KD <30 nM) to the Mth ectodomain (Table 1). A scrambled version of the R8-12 synthetic peptide, as well as W7A and R10A mutants of R8-14, exhibited no measurable binding by SPR at concentrations up to 5 μM. Additionally, a fluorescently labeled analog of R8-12 was shown to bind full-length Mth expressed in cell culture (Supp. Fig. 2). Hence, despite targeting the Mth ectodomain, selected peptides recognize the full-length GPCR.
It is not clear why arginine and proline should be interchangeable at the first consensus position of the RWR motif. Proline could provide a conformational anchor, whereas arginine makes favorable electrostatic contacts that net result in two peptides with similar binding affinities to Mth. Analysis of the amino acid types at each position in the aligned peptides also reveals several trends dependent on whether arginine or proline is in the first position (Supp. Fig. 2 and Supp. Table 1).
In vitro competition binding studies suggest that the selected peptides share the same binding site. Synthetic, unlabeled peptide R8-01 (10 μM) competed with radiolabeled, full-length R8-01 and R8-04 for binding to immobilized Mth ectodomain, resulting in 96% and 94% reductions in binding, respectively, compared to the amount bound without competitor. N-Stunted, a 30-mer synthetic peptide previously shown to activate the Mth receptor14, also competed for binding to the Mth ectodomain: at 30 μM, N-Stunted reduced binding of radiolabeled R8-01 by 79% compared to binding without competitor. These results suggest that the natural ligand binding site is an interaction “hot spot”15 and at least partially reconstituted by the Mth ectodomain. Alternatively, allosteric competition may occur through Mth conformational changes upon ligand binding.
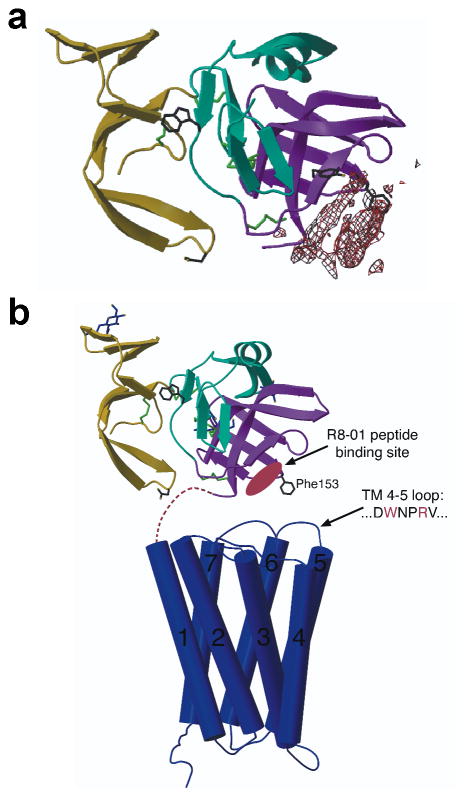
We determined the crystal structure of the Mth ectodomain in complex with an RWR motif peptide to identify the binding site. Electron density putatively corresponding to the R8-01 15-mer peptide (Table 1) places the binding site near the C terminus of the ectodomain (Fig. 2a). In the context of the full-length receptor, this suggests that the peptides bind at an interface between the Mth ectodomain and extracellular loops (Fig. 2b). These results contradict the hypothesis that the single exposed Trp residue in the Mth ectodomain is the binding site for the natural ligand9. Further fluorescence studies with Mth, R8-01, and R8-04 confirm that Trp120 is not required for peptide binding to Mth (Supp. Fig. 3).
Fig. 2.
Structure of the Mth ectodomain in complex with the R8-01 15-mer peptide. (a) Electron density reveals the putative peptide binding site on the Mth ectodomain (shown as a ribbon diagram) from an averaged 3.5 Å FO – FC map contoured at 9 σ. Trp120 (a previously proposed natural ligand binding site9), Tyr130 (at the R8-01 binding site), and Asp46 and Phe153 (suggested to interact with the extracellular face of the TM domain9) are shown as stick models. (b) Scaled model depicting the full-length structure of Mth (adapted from 9). The TM domain is depicted by the structure of rhodopsin30 as a representative GPCR.
The second extracellular loop (EL2) of Mth contains a WxxR peptide sequence (a partial RWR motif), which may interact with the ectodomain in a similar fashion as the RWR motif peptides and form a distinct surface for agonist binding (Fig. 2b). However, at concentrations of up to 150 μM, a synthetic EL2 peptide did not compete with radiolabeled R8-01. This may not preclude an in vivo interaction since the affinity may be enhanced in the full-length receptor where the EL2 sequence and the ectodomain are co-localized. Additionally, a high affinity, optimized interaction may be undesirable for the modulation of natural signaling. Indeed, the bias of in vitro selection for high affinity ligands may favor the recovery of antagonists rather than agonists, which are evolved for function rather than binding.
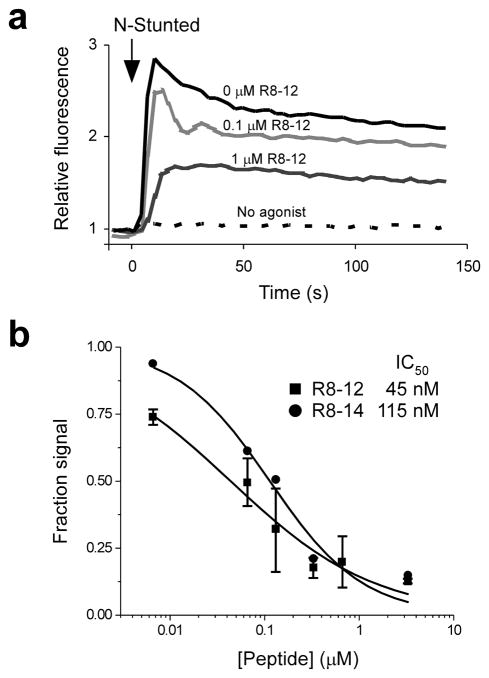
The Stunted peptide was previously identified as an agonist for Mth using a cell-based, fluorescence reporter system for calcium mobilization, a common consequence of GPCR activation14. We used the same technique here to determine if the selected peptides antagonize Mth signaling. R8-12 and R8-14 were strong antagonists of N-Stunted–induced Mth signaling, with 50% inhibitory concentration (IC50) values of 45 ± 10 and 115 ± 25 nM, respectively (Fig. 3). The W7A and R10A mutants of R8-14, as well as the R8-12 scrambled peptide, failed to antagonize Mth at concentrations up to 10 μM. No signaling was induced by any of the peptides in non-transfected, control HEK cells. RWR motif peptides likely antagonize Mth by blocking interaction with agonist. However, it is also possible that the peptides desensitize the receptor (e.g., by triggering internalization) or block GPCR oligomerization, which may be required for proper signaling.
Fig. 3.
RWR motif peptides are antagonists of Mth signaling. (a) Mth activation by Stunted results in intracellular calcium mobilization and enhanced fluorescence. The N-Stunted agonist peptide (20 μM final) was added to HEK-Mth cells pre-incubated with and without the indicated concentration of R8-12 peptide. The dashed line indicates a control where only buffer (without Stunted agonist) was added. (b) Concentration dependence of the inhibition of Mth signaling by the R8-12 and R8-14 peptides. The fluorescence values at a time point ~13 sec after the addition of N-Stunted agonist (10 μM final) are expressed as a fraction of the fluorescence observed in the absence of antagonists (± s.d. when more than one trial was performed).
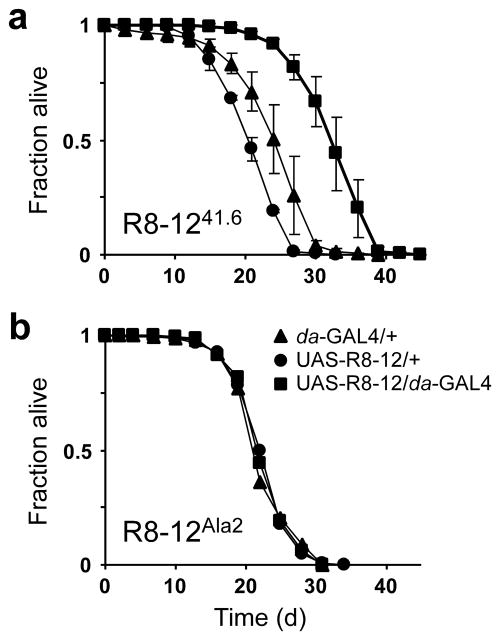
Since down-regulation of mth increases lifespan3, we speculated that the in vivo application of a Mth antagonist would affect Drosophila longevity. We generated transgenic flies that constitutively express the R8-12 peptide using the UAS/GAL4 system16. Expression of the R8-12 peptide extended the mean and maximal adult lifespans at 29 °C by 38% and 26%, respectively (Fig. 4a). Two other independent UAS–R8-12 insertion lines showed similar lifespan increases (Supp Fig. 4a). F1 heterozygous lines, each carrying one of the two P-elements (da-GAL4 or UAS–R8-12), show similar lifespans to parental controls, suggesting that the longevity seen with peptide expression is not due to heterosis (Supp. Fig. 4b). Additionally, expression of a mutant R8-12 peptide, where critical residues of the RWR motif were mutated to Ala, resulted in no extension of lifespan (Fig. 4b). Male survival curves at 25 °C also show lifespan extension with R8-12 expression (Supp. Fig. 4c). These results suggest that the expressed R8-12 peptide antagonizes Mth in vivo. However, because the role of other Mth-like receptors in longevity is unknown, and because other Mth-like extracellular domains share significant sequence similarity to the Mth ectodomain (up to 60% identity), the action of the R8-12 peptide may be through other or multiple proteins. Studying the effects of R8-12 expression on other phenotypes observed in the mth mutant may reveal the relationships between aging, synaptic transmission, and fertility 3,17–19.
Fig. 4.
Expression of the Mth antagonist R8-12 peptide extends fly lifespan. (a) Male lifespan for the UAS–R8-12 transgene insertion line (41.6). (▲) Heterozygous control for the daughterless-GAL4 driver (da-GAL4/+). (●) Heterozygous control for the UAS–R8-12 peptide construct in the absence of the driver (UAS–R8-12/+). (■) The combination in which the da-GAL4 driver, by activating UAS promoter elements, drives expression of the R8-12 peptide (UAS–R8-12/da-GAL4). Data are shown ± s.d. (b) Male lifespan for a mutant UAS–R8-12 transgene (Ala2). Flies were maintained at 29 °C, and approximately 90 to 120 flies were used for each trial.
In vitro selection has emerged as a powerful alternative to screening approaches for the identification of modulators of G protein signaling20,21. Our work provides a clear strategy for designing functional ligands for class B GPCRs, a protein family associated with a number of human diseases2. New expression and presentation platforms for TM proteins will be needed to broaden the utility of mRNA display selection for developing novel ligands. Selections targeting full-length GPCRs expressed in cells remain an unattractive option due to the large background of cell-surface proteins. The recent successes in assembling pure GPCRs in paramagnetic proteoliposomes22 or in a reconstituted bilayer on a biosensor surface23,24 may be favorable alternatives for presenting a TM protein for selection. The method we describe for the identification of GPCR effectors may provide new tools applicable to aging and other fields.
Methods
mRNA display library preparation
The initial DNA pool was generated by PCR [0.1 M initial template, 5 total cycles of PCR amplification using Herculase DNA polymerase (Stratagene)] of the 142.1 template (5′-TTA AAT AGC GGA TGC ACG CAG ACC GCC ACT AGT (SNN)27 CAT TGT AAT TGT AAA TAG TAA TTG TCC C; N = A, C, G, or T; S = C or G) with the primers 47T7FP (5′-GGA TTC TAA TAC GAC TCA CTA TAG GGA CAA TTA CTA TTT ACA ATT AC) and 21.2 (5′-TTA AAT AGC GGA TGC ACG CAG). This library encoded a T7 promoter for transcription, a 5′-UTR sequence, and an ORF for the peptide, M-X27-TSGGLRASAI. The template DNA was transcribed and purified as described previously25. Cross-linking of a puromycin-psoralen linker to the mRNA was performed as described using oligo 28A.1 (5′-[Ps]-UAG CGG AUG C-dA16-[S9]2-dCdC-[Pu]; where unlabeled bases are 2′-OMe RNA, Ps = psoralen C6, S9 = spacer phosphoramidite 9, and Pu = puromycin-CPG, Glen Research)26.
In vitro translation with the mRNA-28A.1 library was performed in Red Nova Lysate (Novagen) as per the manufacturer’s instructions with optimized conditions (100 mM KOAc, 0.5 mM MgOAc, 0.4 μM mRNA-28A.1, and ~25 μM overall L-methionine; 10 ml total reaction volume) and 35S-methionine (0.5 mCi ml−1 final). RNA-peptide fusions were salt-treated and purified as described previously25. Fusions were reverse-transcribed (oligo 21.2) and desalted into Mth buffer [50 mM HEPES-KOH at pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% (w/v) BSA, 1 μg ml−1 yeast tRNA and 0.05% Tween 20] by gel filtration (NAP-25, GE Healthcare). Based on the 35S-methionine incorporated into the RNA-peptide fusions and an average of 1.3 methionine residues per peptide, the initial complexity of the library was approximately 1.5 × 1013.
In vitro selection
RNA:cDNA-peptide fusions were incubated with ~0.1 ml of Mth-agarose at 4 °C for 1 h, then filtered and washed with 4 × 1 ml Mth buffer followed by 2 × 1 ml Mth buffer without BSA or tRNA. Bound fusions were eluted with 2 × 100 μl of 0.15% SDS. After removal of the SDS (SDS-OUT, Pierce), fusions were isopropanol-precipitated (50 μg ml−1 linear acrylamide, 1/40 volume of 3 M NaOAc at pH 5.2, and 1 volume of isopropanol). The reduced salt used for isopropanol precipitation was necessary to prevent inhibition of subsequent PCR, due to the high salt introduced by the SDS-OUT reagent. Precipitated cDNA was PCR-amplified to produce a new dsDNA pool.
Further rounds of selection were performed as described for the initial round except that in vitro translation reactions were smaller (~0.3 ml), less immobilized Mth was used for the selective step (~20 μl), and in rounds 5 through 8, bound fusions were eluted by competition with non-biotinylated Mth (0.5 mg ml−1) in Mth buffer without BSA or tRNA. Additionally, rounds 5 through 8 included a preclearing step where the precipitated RNA:cDNA-peptide fusions were passed through columns containing NeutrAvidin-agarose and/or protein G-sepharose to remove peptides with high non-specific binding for the immobilization matrix.
Kinetics determination by surface plasmon resonance (SPR)
SPR measurements were performed at 25 °C on a BIAcore 2000 instrument equipped with research-grade SA (streptavidin) sensor chips. Biotinylated Mth was immobilized to a surface density of 450 to 700 response units (RU). HBS-EP [10 mM HEPES at pH 7.4, 150 mM NaCl, 3 mM EDTA, and 0.005% polysorbate 20 (Tween 20)] was used as the running buffer for all experiments. To collect kinetics data, a concentration series of each peptide was injected for at least 60 s at a flow rate of >45 μl min−1. Raw data was processed with Scrubber and globally fit with CLAMP using a 1:1 bimolecular interaction model27. Sensorgrams for the R8-04 peptide are shown in Supp. Fig. 5.
Cell-based GPCR signaling assay
Calcium response assays were performed with HEK 293 cells stably expressing Mth-B essentially as described14. Fluorescence spectra were divided by a baseline average, calculated from the region of data prior to the addition of N-Stunted. Further details are provided in the Supplementary Methods online.
Lifespan assays
P-element transformants with the full-length R8-12 peptide transgene were generated in a w1118 background by standard techniques28. R8-12Ala2 is a W5A and R8A mutant of the full-length R8-12 sequence. UAS-controlled R8-12 lines (UAS–R8-12) were crossed with da-GAL4, which had previously been outcrossed with w1118. F1 heterozygous adults (0–4 days old) were transferred to new bottles, aged for 2 days, and males were separated under CO2 anesthesia (30 males per vial). After allowing the flies to recover overnight at 25 °C, flies (3–7 days old) were incubated in a light- and humidity-controlled environment at the indicated temperature. The UAS–R8-12 and da-GAL4 lines were each crossed with w1118 to produce F1 heterozygous animals for controls. Flies were transferred to fresh food vials [(0.45% agar, 5% dextrose, 2.5% sucrose, 8.3% corn meal, and 1.5% dried yeast, all by weight/volume) with phosphoric and propionic acids supplemented to prevent mold, as previously described29] every 3–4 days and scored for survival.
Other methods
Additional methods are provided in the Supplementary Methods online.
Supplementary Material
Acknowledgments
We thank A.M. Giannetti for technical expertise on the Biacore; D.G. Myszka (University of Utah) for the SPR analysis software, Scrubber and CLAMP; M.I. Simon for use of the Flexstation automated fluorescence plate reader; T. Brummel (Sam Houston State University, TX) and D. Walker for their technical expertise on the lifespan experiments; T.T. Takahashi and G.B. Carvalho for comments on the manuscript; and S. Cvejic and X.-Y. Huang (Cornell University Weill Medical College, NY) for providing the HEK-Mth cell lines and details on their protocols. We are grateful to P.M. Snow (deceased, 2004) for his expertise in protein purification. This work was supported by grants from the NIH (R01 GM60416 to R.W.R. and R01 AG016630 to S.B.) and the Beckman Foundation (R.W.R.). W.W.J. was supported in part by a DOD National Defense Science and Engineering Graduate Fellowship, a Scholarship for Research in the Biology of Aging sponsored by the Glenn Foundation for Medical Research and the American Federation for Aging Research, and a John Douglas French Alzheimer’s Foundation Postdoctoral Fellowship. A.P.W., Jr., was supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund.
Footnotes
Competing financial interests statement. The authors declare no competing financial interests.
References
- 1.Strader CD, Fong TM, Tota MR, Underwood D, Dixon RAF. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 2.Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2:REVIEWS3013. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 4.Roberts RW, Szostak JW. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc Natl Acad Sci USA. 1997;94:12297–12302. doi: 10.1073/pnas.94.23.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi TT, Austin RJ, Roberts RW. mRNA display: ligand discovery, interaction analysis and beyond. Trends Biochem Sci. 2003;28:159–165. doi: 10.1016/S0968-0004(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 6.Howard AD, et al. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 7.Milligan G. Strategies to identify ligands for orphan G-protein-coupled receptors. Biochem Soc Trans. 2002;30:789–793. doi: 10.1042/bst0300789. [DOI] [PubMed] [Google Scholar]
- 8.Szekeres PG. Functional assays for identifying ligands at orphan G protein-coupled receptors. Receptor Channel. 2002;8:297–308. [PubMed] [Google Scholar]
- 9.West AP, Jr, Llamas LL, Snow PM, Benzer S, Bjorkman PJ. Crystal structure of the ectodomain of Methuselah, a Drosophila G protein-coupled receptor associated with extended lifespan. Proc Natl Acad Sci USA. 2001;98:3744–3749. doi: 10.1073/pnas.051625298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grauschopf U, et al. The N-terminal fragment of human parathyroid hormone receptor 1 constitutes a hormone binding domain and reveals a distinct disulfide pattern. Biochemistry. 2000;39:8878–8887. doi: 10.1021/bi0001426. [DOI] [PubMed] [Google Scholar]
- 11.Wilmen A, Göke B, Göke R. The isolated N-terminal extracellular domain of the glucagon-like peptide-1 (GLP)-1 receptor has intrinsic binding activity. FEBS Lett. 1996;398:43–47. doi: 10.1016/s0014-5793(96)01214-8. [DOI] [PubMed] [Google Scholar]
- 12.Schatz PJ. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Bio-Technol. 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- 13.Ja WW, Olsen BN, Roberts RW. Epitope mapping using mRNA display and a unidirectional nested deletion library. Protein Eng Des Sel. 2005;18:309–319. doi: 10.1093/protein/gzi038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat Cell Biol. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- 15.Clackson T, Wells JA. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 16.Phelps CB, Brand AH. Ectopic gene expression in Drosophila using GAL4 system. Methods. 1998;14:367–379. doi: 10.1006/meth.1998.0592. [DOI] [PubMed] [Google Scholar]
- 17.Baldal EA, Baktawar W, Brakefield PM, Zwaan BJ. Methuselah life history in a variety of conditions, implications for the use of mutants in longevity research. Exp Gerontol. 2006;41:1126–1135. doi: 10.1016/j.exger.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Exp Gerontol. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Song W, et al. Presynaptic regulation of neurotransmission in Drosophila by the G protein-coupled receptor Methuselah. Neuron. 2002;36:105–119. doi: 10.1016/s0896-6273(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 20.Ja WW, Roberts RW. G-protein-directed ligand discovery with peptide combinatorial libraries. Trends Biochem Sci. 2005;30:318–324. doi: 10.1016/j.tibs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Ja WW, Wiser O, Austin RJ, Jan LY, Roberts RW. Turning G proteins on and off using peptide ligands. ACS Chem Biol. 2006;1:570–574. doi: 10.1021/cb600345k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol. 2000;18:649–654. doi: 10.1038/76501. [DOI] [PubMed] [Google Scholar]
- 23.Bieri C, Ernst OP, Heyse S, Hofmann KP, Vogel H. Micropatterned immobilization of a G protein-coupled receptor and direct detection of G protein activation. Nat Biotechnol. 1999;17:1105–1108. doi: 10.1038/15090. [DOI] [PubMed] [Google Scholar]
- 24.Stenlund P, Babcock GJ, Sodroski J, Myszka DG. Capture and reconstitution of G protein-coupled receptors on a biosensor surface. Anal Biochem. 2003;316:243–250. doi: 10.1016/s0003-2697(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 25.Ja WW, Roberts RW. In vitro selection of state-specific peptide modulators of G protein signaling using mRNA display. Biochemistry. 2004;43:9265–9275. doi: 10.1021/bi0498398. [DOI] [PubMed] [Google Scholar]
- 26.Kurz M, Gu K, Lohse PA. Psoralen photo-crosslinked mRNA-puromycin conjugates: a novel template for the rapid and facile preparation of mRNA-protein fusions. Nucleic Acids Res. 2000;28:e83. doi: 10.1093/nar/28.18.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myszka DG, Morton TA. CLAMP©: a biosensor kinetic data analysis program. Trends Biochem Sci. 1998;23:149–150. doi: 10.1016/s0968-0004(98)01183-9. [DOI] [PubMed] [Google Scholar]
- 28.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 29.Lewis EB. A new standard food medium. Drosophila Inf Serv. 1960;34:117–118. [Google Scholar]
- 30.Palczewski K, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.