Summary
Background
In 1952, Alan Turing suggested that spatial patterns could arise from homogeneous starting conditions by feedback amplification of stochastic fluctuations. One example of such self-organization, called symmetry breaking, involves spontaneous cell polarization in the absence of spatial cues. The conserved GTPase Cdc42p is essential for both guided and spontaneous polarization, and in budding yeast cells Cdc42p concentrates at a single site (the presumptive bud site) at the cortex. Cdc42p concentrates at a random cortical site during symmetry breaking in a manner that requires the scaffold protein Bem1p. The mechanism whereby Bem1p promotes this polarization was unknown.
Results
Here we show that Bem1p promotes symmetry breaking by assembling a complex in which both a Cdc42p-directed guanine nucleotide exchange factor (GEF) and a Cdc42p effector p21-activated kinase (PAK) associate with Bem1p. Analysis of Bem1p mutants indicates that both GEF and PAK must bind to the same molecule of Bem1p, and a protein fusion linking the yeast GEF and PAK bypasses the need for Bem1p. Although mammalian cells lack a Bem1p ortholog, they contain more complex multidomain GEFs that in some cases can directly interact with PAKs, and we show that yeast containing an artificial GEF with similar architecture can break symmetry even without Bem1p.
Conclusions
Yeast symmetry-breaking polarization involves a GEF-PAK complex that binds GTP-Cdc42p via the PAK and promotes local Cdc42p GTP-loading via the GEF. By generating fresh GTP-Cdc42p near pre-existing GTP-Cdc42p, the complex amplifies clusters of GTP-Cdc42p at the cortex. Our findings provide mechanistic insight into an evolutionarily conserved pattern-forming positive-feedback pathway.
Introduction
Polarization is normally oriented toward relevant directional cues, but many cells can polarize spontaneously in a random direction even when deprived of their normal cues. This process, called symmetry breaking, is thought to represent a core polarity program whose direction can be influenced by appropriate cues [1, 2]. The conserved Rho-family GTPase Cdc42p is a master regulator of cell polarity in animal as well as fungal cells, and polarization signals promote GTP-Cdc42p accumulation at the site destined to become the cell’s front [3, 4]. A major unresolved issue is the mechanism whereby GTP-Cdc42p first becomes concentrated at a single, random, cortical site, even under symmetry-breaking conditions when normal cues are unavailable.
In the model eukaryote Saccharomyces cerevisiae, polarity cues are provided by internal bud-site-selection landmarks whose action requires the Ras-related GTPase Rsr1p [4]. Cells lacking Rsr1p can only polarize (and hence bud and proliferate) if they are able to break symmetry: otherwise, the cells enlarge spherically until they eventually burst. Symmetry-breaking polarization of Cdc42p does not require polymerized actin or tubulin but depends on the peripheral membrane scaffold protein Bem1p [5] (Figure 1A). Bem1p associates with multiple proteins [6–18], and given this promiscuity, the mechanism by which it promotes Cdc42p polarization is far from obvious. To address this issue, we combined surgical mutations disrupting specific interactions with a protein-fusion approach to show that a Bem1p-mediated complex between a Cdc42p-directed GEF and a Cdc42p effector PAK suffices to break symmetry.
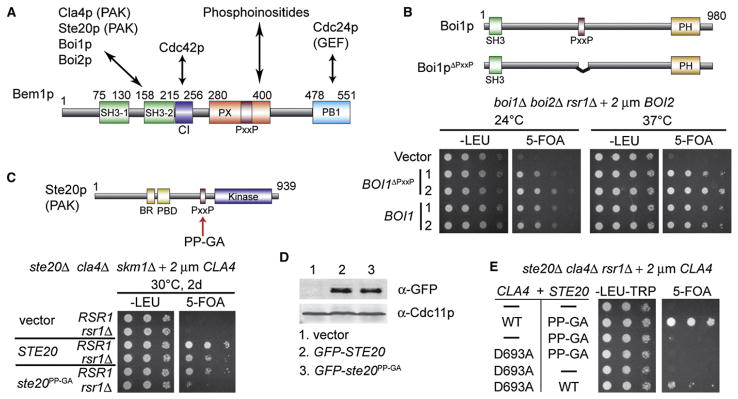
Figure 1. The PAK-Bem1p Interaction Is Essential for Symmetry-Breaking Polarization.
(A) Scheme showing domains of Bem1p and their known direct interactions.
(B) Cells whose only Boi cannot bind Bem1p can polarize and proliferate without Rsr1p. Top: Boi1p domains and ΔPxxP mutation. boi1Δ boi2Δ rsr1Δ cells containing a URA3-marked BOI2 plasmid and plasmids carrying BOI1 or BOI1ΔPxxP were spotted onto permissive –Leu plates or 5-FOA plates, which kill cells that retain the URA3-marked plasmid.
(C) Cells whose only PAK (Ste20pPP-GA) is impaired in its binding to Bem1p cannot grow without Rsr1p. Top: Ste20p domains and PP-GA mutation. ste20Δ cla4Δ skm1Δ rsr1Δ and ste20Δ cla4Δ skm1Δ RSR1 strains containing a URA3-marked CLA4 plasmid and plasmids carrying STE20 or ste20PP-GA were spotted as described above.
(D) GFP-Ste20p and GFP-Ste20pPP-GA are expressed at similar levels. Blots of total cell lysates were probed with anti-GFP and anti-Cdc11p (septin, loading control).
(E) Cells containing both a catalytically active PAK that cannot bind to Bem1p (Ste20pPP-GA) and a kinase-dead PAK that can bind to Bem1p (Cla4pD693A) cannot grow without Rsr1p. ste20Δ cla4Δ rsr1Δ cells containing a URA3-marked CLA4 plasmid and the indicated PAK pairs were spotted as described above.
Results
Bem1p has several protein-protein interaction domains (Figure 1A), among which two (the second SH3 domain and the C-terminal PB1 domain) were shown to be important for symmetry-breaking polarization [5]. We sought to understand which of Bem1p’s many binding partners were necessary and sufficient for this process. The second Bem1p SH3 domain is known to interact with the related scaffold proteins Boi1p and Boi2p as well as the redundant PAKs Cla4p, Ste20p, and (probably) Skm1p [6–8, 11, 16, 19]. To address whether these interactions were important for symmetry breaking, we generated yeast strains in which the endogenous Boi or PAK genes were deleted and replaced with a mutant Boi or PAK impaired for Bem1p SH3 interaction. In addition, we deleted RSR1 so that the cells would only be able to proliferate if they were able to break symmetry. We found that proliferation of rsr1Δ boi1Δ boi2Δ cells was effectively rescued by Boi1pΔPxxP, which lacks the Pro-rich region that mediates Bem1p binding [8] (Figure 1B; also Figure S1 in the Supplemental Data), suggesting that this interaction is dispensable for symmetry breaking.
To find out whether the Bem1p-PAK interaction is important for symmetry breaking, we took advantage of the ste20PP-GA allele described by Pryciak and colleagues; this allele is severely impaired in Bem1p SH3 interaction [16]. A comparable CLA4 mutant is harder to engineer because Cla4p contains several potential SH3-interacting motifs (depicted as purple bars in Figure 2A). Proliferation of rsr1Δ cla4Δ ste20Δ skm1Δ cells was not rescued by Ste20pPP-GA (Figure 1C), suggesting that the interaction of PAKs withBem1pis essential for symmetry breaking. Ste20pPP-GA was expressed at levels similar to those of wild-type Ste20p (Figure 1D). Moreover, Ste20pPP-GA was able to promote proliferation of cla4Δ ste20Δ skm1Δ cells containing Rsr1p (Figure 1C), suggesting that the mutant can perform all essential PAK functions except symmetry breaking.
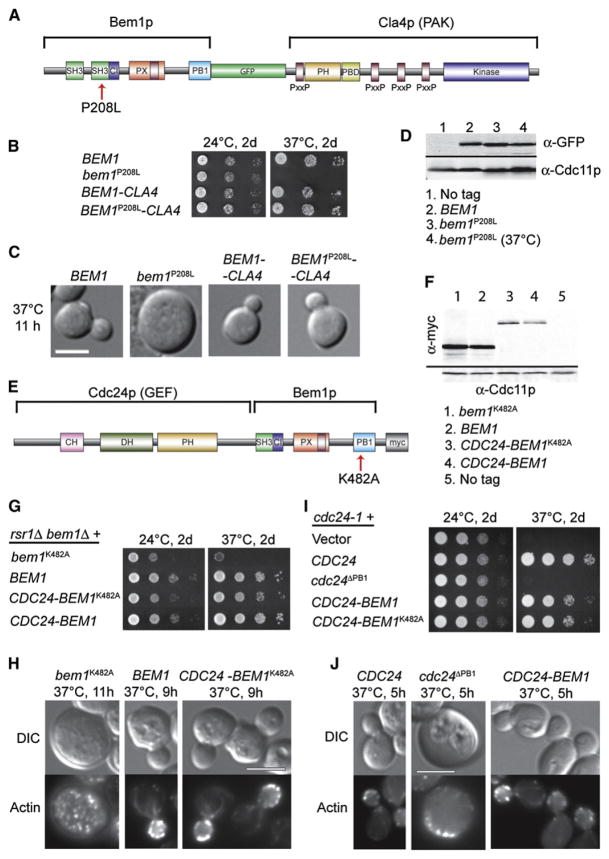
Figure 2. The Bem1p-PAK Fusion Protein Bypasses the Need for SH3-2, and the GEF-Bem1p Fusion Protein Bypasses the Need for PB1.
(A) Schematic representation of BEM1-GFP-CLA4 fusion and P208L mutation. Purple bars indicate PxxP motifs that could interact with the Bem1p SH3-2 domain.
(B) Temperature-sensitive growth of bem1P208L-GFP is rescued by fusion to CLA4. rsr1Δ cells with the indicated BEM1 or fusion alleles were spotted onto plates and incubated at 24°C or 37°C.
(C) DIC images of cells from (B); cells were shifted from 24°C to 37°C for 11 hr. The scale bar represents 5 μm.
(D) Bem1p-GFP and Bem1pP208L-GFP are similarly expressed. Cells were grown at 24°C and (for lane 4) shifted to 37°C for 3 hr. Blots were probed with anti-GFP and anti-Cdc11p (septin, loading control).
(E) Schematic representation of Cdc24p-Bem1p-Myc fusion and K482A mutation. The fusion lacks the C-terminal Bem1p-binding domain of Cdc24p and the N-terminal SH3-1 domain of Bem1p.
(F) Cdc24p-Bem1p fusion proteins are expressed at a lower level than high-copy Bem1p. rsr1Δ bem1Δ strains carrying the indicated Bem1p-Myc (high-copy) or Cdc24p-Bem1p-Myc (low-copy) plasmids were grown at 24°C. Blots were probed with anti-Myc and anti-Cdc11p.
(G) Temperature-sensitive growth of bem1K482A is rescued by fusion to CDC24. Cells as in (F) were spotted onto plates and incubated at 24°C or 37°C.
(H) DIC and actin staining of cells as in (F) but shifted to 37°C for 9–11 hr. The scale bar represents 5 μm.
(I) Temperature-sensitive growth of cdc24-1 is rescued by the CDC24-BEM1 fusion protein but not by cdc24ΔPB1. cdc24-1 cells with the indicated plasmids were spotted onto plates and incubated at 24°C or 37°C.
(J) DIC and actin staining of cells from (I) shifted to 37°C for 5 hr. The scale bar represents 5 μm.
Several prior studies using conditional cla4 mutants had suggested that PAKs were dispensable for polarization [20–22], but surprisingly we found that these mutants retained residual PAK function sufficient for breaking symmetry even under supposedly restrictive conditions (Figure S2). In contrast, the kinase-dead cla4D693A mutant [23] could not rescue proliferation of rsr1Δ cla4Δ ste20Δ skm1Δ cells, even in combination with Ste20pPP-GA (Figure 1E), suggesting that symmetry breaking requires a catalytically active PAK that can also bind to Bem1p.
If PAK interaction is the only essential role of the second Bem1p SH3 domain in symmetry breaking, then we reasoned that it might be possible to bypass the requirement for that domain by making a Bem1p-PAK fusion protein. We constructed a Bem1p-GFP-Cla4p fusion protein that was expressed from the BEM1 promoter (Figure 2A). This fusion protein was polarized prior to bud emergence (Figure S3) and enabled robust proliferation of rsr1Δ bem1Δ mutants (Figure 2B; also Figure S3), indicating that it was functional for symmetry breaking. To find out whether the fusion could bypass the requirement for a functional SH3-2 domain, we inactivated the SH3-2 domain by mutating Pro208 to Leu [5, 7]. rsr1Δ cells containing the mutant Bem1pP208L-GFP as the sole Bem1p exhibited temperature- sensitive growth (Figure 2B) and polarization defects (Figure 2C; also Figure S4), even though Bem1pP208L-GFP was expressed at levels comparable to those of wild-type Bem1p-GFP (Figure 2D). Remarkably, however, rsr1Δ cells containing a Bem1pP208L-GFP-Cla4p fusion protein as their only Bem1p grew well even at 37°C (Figure 2B) and displayed normal cell morphology (Figure 2C; also Figure S4). Thus, a mutation that cripples the Bem1p SH3-2 domain can be rescued by fusion to a PAK, indicating that the Bem1p-PAK interaction is sufficient to account for that domain’s role in symmetry breaking.
Previous studies established that the Bem1p PB1 domain interacts with the C-terminal PB1 domain in the GEF Cdc24p [10, 19]; this interaction is necessary for proper cell polarization [19, 24, 25]. Nevertheless, it remained possible that the Bem1p and Cdc24p PB1 domains might also interact with other proteins important for symmetry breaking. To find out whether Bem1p-Cdc24p interaction is sufficient to account for the role of these domains, we constructed a Cdc24p-Bem1p-Myc fusion protein expressed from the CDC24 promoter (Figure 2E). This fusion protein lacked the Cdc24p PB1 domain (and for ease of construction, we also deleted the Bem1p N terminus containing the nonessential SH3-1 domain). This fusion protein was more highly expressed than endogenous Bem1p (not shown) but was less abundant than Bem1p expressed from a high-copy plasmid (Figure 2F). The fusion protein was polarized prior to bud emergence and enabled proliferation of rsr1Δ bem1Δ mutants (Figure 2G; also Figure S5), indicating that it retained Bem1p function. The fusion protein also promoted robust growth (Figure 2I) and actin polarization (Figure 2J) of cdc24-1 cells at 37°C, indicating that it retained Cdc24p function despite the lack of a Cdc24p PB1 domain (in contrast to unfused Cdc24pΔPB1, which was nonfunctional).
To find out whether Cdc24p-Bem1p fusion could bypass the requirement for a functional Bem1p PB1 domain, we inactivated the Bem1p PB1 by mutating Lys482 to Ala [5, 19]. Bem1pK482A was unable to rescue growth of bem1Δ rsr1Δ cells, but when expressed from a high-copy plasmid it was able to provide a partial, temperature-sensitive rescue (Figure 2G). At 37°C, these cells were large and contained a depolarized actin cytoskeleton, indicative of a polarity defect (Figure 2H). Because elevated expression could compensate to some degree for the K482A functional defect, and because the single-copy Cdc24p-Bem1p fusion protein was expressed at a higher level than single-copy Bem1p, in what follows we compared single-copy Cdc24p-Bem1p to high-copy Bem1p. Despite being expressed at a considerably lower level than the high-copy Bem1pK482A (Figure 2F), the Cdc24p-Bem1pK482A fusion was able to promote robust growth (Figure 2G) and normal actin organization and cell morphology (Figure 2H) of bem1Δ rsr1Δ cells even at 37°C. Cdc24p-Bem1pK482A was also able to rescue the growth and polarity defects of cdc24-1 cells at 37°C (Figures 2I and 2J; also Figure S6). Thus, fusing Cdc24p to Bem1p renders both the Cdc24p and the Bem1p PB1 domains dispensable for cell polarization, suggesting that binding of Cdc24p to Bem1p is sufficient to account for the role of those domains.
The data presented thus far indicate that symmetry-breaking polarization involves interaction of a PAK with the Bem1p SH3-2 domain and interaction of the Cdc24p GEF with the Bem1p PB1 domain. Although the GEF and PAK do not stably interact on their own, Bem1p can bridge formation of a GEF-Bem1p-PAK complex, indicating that both proteins can bind to the same molecule of Bem1p [11]. However, it is unclear whether the function of Bem1p in symmetry breaking requires the formation of such three-way complexes or whether it suffices for some Bem1p molecules to bind the PAK and others to bind the GEF. To address this issue, we tested whether Bem1pP208L-GFP (which can bind the GEF but not the PAK) and Bem1pK482A-Myc (which can bind the PAK but not the GEF) could in combination rescue a bem1Δ rsr1Δ strain (Figure 3A). As shown in Figure 3B, these mutants failed to show intragenic complementation, even though the mutant proteins were expressed to the same level as complementing wild-type Bem1p (Figure 3C). At 37°C the cells were large, displayed depolarized actin (Figure 3D), and were frequently multinucleate or lysed, just like cells containing only Bem1pP208L or Bem1pK482A. This finding suggests that the GEF and PAK must bind to the same molecule of Bem1p in order to promote polarization.
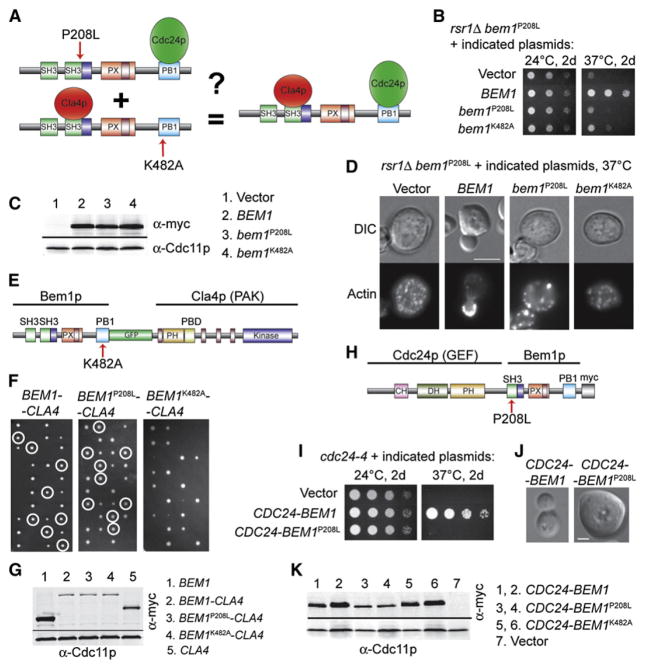
Figure 3. GEF and PAK Must Bind to the Same Molecule of Bem1p.
(A) Schematic representation of the experiment: Can two copies of Bem1p defective in separate interactions (left) function comparably to one wild-type Bem1p (right)?
(B) Cells expressing Bem1pP208L and Bem1pK482A cannot grow at 37°C. bem1P208L-GFP cells with high-copy plasmids expressing the indicated BEM1 alleles were spotted onto plates and incubated at 24°C or 37°C.
(C) Bem1p-Myc proteins are expressed at similar levels. Blots were probed with anti-Myc and anti-Cdc11p (loading control).
(D) Cells expressing Bem1pP208L and Bem1pK482A fail to polarize at 37°C. The indicated cells were shifted to 37°C for 10 hr prior to fixation and actin staining. The scale bar represents 5 μm.
(E) Schematic representation of a Bem1pK482A-GFP-Cla4p fusion protein unable to bind Cdc24p.
(F) The Bem1pK482A-GFP-Cla4p fusion protein is nonfunctional. Diploid rsr1Δ/rsr1Δ bem1Δ/BEM1 strains carrying the indicated fusion allele at the CLA4 locus were sporulated, and tetrads dissected (four spores left-right). Circles indicate viable bem1Δ rsr1Δ colonies containing Bem1p-Cla4p fusion proteins, as scored by replica plating for appropriate markers (note the absence of circled colonies in the third panel, indicating inability of the fusion protein to rescue). In the absence of a Bem1p-Cla4p fusion protein, there would be two viable spores (rsr1Δ BEM1) and two inviable spores (rsr1Δ bem1Δ) in every tetrad. If the Bem1p-Cla4p fusion protein is capable of symmetry breaking, the rsr1Δ bem1Δ spores that inherit it will be viable, thereby allowing tetrads with three or four viable spores. This is observed for the wild-type and P208L fusion proteins but not for the K482A fusion protein, indicating that Bem1pK482A-GFP-Cla4p is unable to break symmetry.
(G) Bem1p-Cla4p fusion proteins are similarly expressed. Because the fusion proteins were not recognized well by our anti-GFP (see Supplemental Data), equivalent fusion proteins were tagged with Myc and compared to Cla4p-Myc and Bem1p-Myc controls. Blots are as in (C).
(H) Schematic of Cdc24p-Bem1pP208L-Myc fusion protein unable to bind PAKs.
(I)Cdc24p-Bem1pP208L-MycFusion cannot replaceCdc24p. cdc24-4 cells with the indicated plasmids werespotted onto platesand incubated at 24°Cor 37°C.
(J) cdc24-1 CDC24-BEM1P208L arrest as large unbudded cells at 37°C. Cells were shifted to 37°C for 5 hr. The scale bar represents 2 μm.
(K) Cdc24p-Bem1p-Myc fusion proteins are similarly expressed. cdc24-1 cells with the indicated plasmids were grown at 24°C (1, 3, and 5) or shifted to 37°C for 4 hr (2, 4, and 6). Blots are as in (C).
Consistent with the hypothesis that both GEF and PAK must assemble in the same complex, we found that a Bem1pK482A-Cla4p fusion protein (impaired in GEF binding: Figure 3E) was unable to rescue a bem1Δ rsr1Δ strain (Figures 3F and 3G; we were unable to recover viable segregants at any temperature, so tetrads are shown instead of spot assays). In the converse experiment, a Cdc24p-Bem1pP208L fusion protein (impaired in PAK binding: Figure 3H) was unable to effectively rescue either a bem1Δ rsr1Δ strain (data not shown) or temperature-sensitive cdc24 strains (Figures 3I–3K; also Figure S6). Thus, the Bem1p-PAK fusion protein needs to bind the GEF in order to function, and the GEF-Bem1p fusion protein needs to bind a PAK in order to function. The fact that the P208L mutation is tolerated in the Bem1p-PAK fusion protein (Figures 2B and 2C) but not the GEF-Bem1p fusion protein (Figures 3I and 3J), whereas the K482A mutation shows the reverse behavior (Figures 2G–2I versus Figure 3F), demonstrates specificity in the effects of the mutations and in the nature of the compensatory effect provided by each fusion protein. In aggregate, these data strongly argue that the role of Bem1p in symmetry-breaking polarization involves assembly of a three-way GEF-Bem1p-PAK complex.
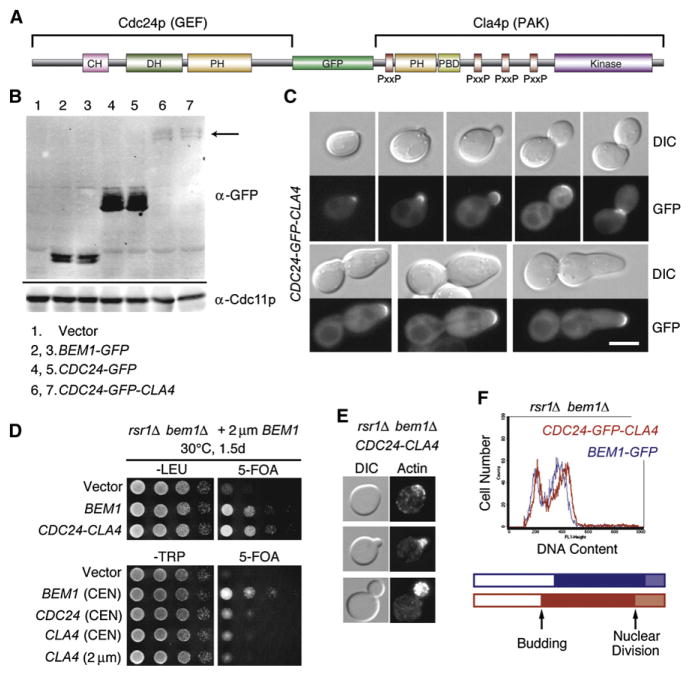
If bringing together the Cdc42p GEF and PAK is the sole essential role of Bem1p in symmetry breaking, then a direct GEF-PAK fusion protein should be able to promote spontaneous polarization in the complete absence of Bem1p. To test this prediction, we made a Cdc24p-GFP-Cla4p fusion protein (Figure 4A) that appeared to be expressed at significantly reduced levels in comparison to endogenous Bem1p (Figure 4B). This fusion protein complemented both a cdc24-1 temperature-sensitive strain (Figure 4C; also Figure S7A) and a strain containing temperature-sensitive cla4 as the only PAK (Figure S7B), indicating that it retains both GEF and PAK function. However, a minority of cdc24-1 cells complemented by the GEF-PAK fusion protein developed elongated buds, suggestive of a defect in depolarization afterbud formation (Figure 4C). The fusion protein was localized to polarization sites, although unlike Cdc24p, it did not concentrate in G1 cell nuclei (Figure 4C). Strikingly, the GEF-PAK fusion protein complemented the lethality of bem1Δ rsr1Δ strains (Figure 4D). Additional copies of the GEF or PAK alone did not complement bem1Δ rsr1Δ strains (Figure 4D), indicating that physical linkage between the GEF and the PAK, rather than an excess of either protein, is required for complementation. The bem1Δ rsr1Δ strains expressing the GEF-PAK fusion protein displayed normal actin organization (Figure 4E), proliferated at wild-type rates, and exhibited a normal cell-cycle profile (Figure 4F), showing that a GEF-PAK fusion protein can fully replace Bem1p to promote symmetry breaking.
Figure 4. An Artificial GEF-PAK Fusion Protein Bypasses the Need for Bem1p in Symmetry-Breaking Polarization.
(A) Schematic representation of GEF-GFP-PAK fusion construct. The GEF Cdc24p lacks its C-terminal Bem1p-binding domain, whereas the PAK Cla4p is the full length.
(B) The GEF-PAK fusion protein (arrow) appears to be expressed at significantly lower levels than Bem1p. Blots of cell extracts from strains expressing the indicated GFP-tagged proteins were probed with anti-GFP and anti-Cdc11p (loading control).
(C) The GEF-PAK fusion protein is localized to polarization sites but not nuclei. DIC and GFP images of cdc24-1 cells expressing Cdc24p-GFP-Cla4p and grown at 37°C. The bottom row illustrates a minority phenotype (hyperpolarized buds) seen in ~11% of cells.
(D) The GEF-PAK fusion protein rescues rsr1Δ bem1Δ lethality. Top: rsr1Δ bem1Δ cells containing a URA3–marked BEM1 plasmid and the indicated control or CDC24-CLA4 plasmids were spotted onto permissive –Leu plates or 5-FOA plates, which kill cells that retain the URA3-marked plasmids, and incubated at 30°C. Bottom: similar experiment showing that additional plasmid copies of CDC24 or CLA4 do not rescue.
(E) DIC and actin staining of rsr1Δ bem1Δ CDC24-CLA4 cells grown at 30°C.
(F) The GEF-PAK fusion protein promotes robust growth and cell-cycle progression in the absence of Bem1p. FACS profiles for BEM1-GFP rsr1Δ(blue, doubling time 119 min) and CDC24-CLA4 bem1Δ rsr1Δ(red, doubling time 121 min) grown at 30°C. The proportion of unbudded cells, budded cells with a single nucleus, and budded cells after nuclear division was determined from a score of DAPI-stained cells (n > 500) and is shown as a cell-cycle timeline representing when an “average cell” in the population buds and undergoes nuclear division.
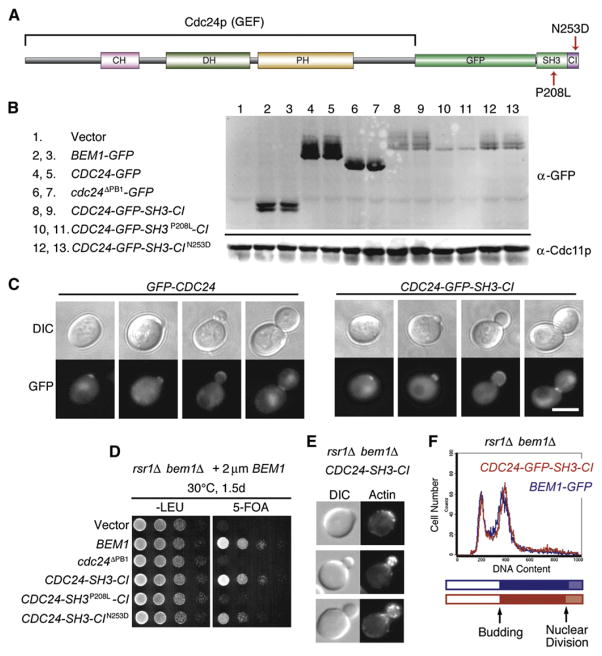
Proteins with a domain organization similar to that of Bem1p are present in many fungi [26] but are difficult to discern in more distant organisms: Although the domains themselves (SH3, PX, PB1) are abundantly represented in animal genomes, they have been extensively shuffled and combined in complex ways. Nevertheless, it is noteworthy that about a third of mammalian Cdc24p-related GEFs contain one or more SH3 domains in the same polypeptide [27] and that many such SH3 domains (e.g., in the PIX GEFs [28]) interact with PAKs, potentially generating complexes similar to the yeast GEF-Bem1p-PAK complex discussed above. To find out whether a GEF with an architecture similar to that of these mammalian GEFs would be able to promote symmetry breaking in yeast, we generated a construct in which the C-terminal Bem1p-binding PB1 domain of the GEF Cdc24p was replaced with GFP fused to the PAK-binding SH3 domain of Bem1p (Figure 5A). A protein containing only the Bem1p SH3-2 domain was not stably expressed (not shown), but one containing a slightly larger piece (Bem1p residues 140–257, which includes the adjacent “CI” domain that binds GTP-Cdc42p [12]) was expressed at somewhat lower levels than the wild-type GEF (Figure 5B). This artificial GEF-SH3-CI complemented the temperature-sensitive cdc24-1 mutant (Figure S7C) and was localized to polarization sites and nuclei in a manner similar to that of wild-type Cdc24p (Figure 5C). The GEF-SH3-CI also successfully complemented the lethality of bem1Δ rsr1Δ strains (Figure 5D), indicating that a GEF with this domain architecture can bypass the need for Bem1p in yeast.
Figure 5. A GEF-SH3-CI Protein Able to Bind PAK Directly Bypasses the Need for Bem1p in Symmetry-Breaking Polarization.
(A) Schematic representation of the Cdc24p- SH3-CI construct.
(B) Cdc24p-SH3-CI is expressed at lower levels than Bem1p or Cdc24p. Blots of cell extracts from strains expressing the indicated GFP-tagged proteins were probed with anti-GFP and anti-Cdc11p (loading control).
(C) Cdc24p-SH3-CI is localized to polarization sites and nuclei. DIC and GFP images of cdc24-1 cells expressing GFP-Cdc24p or Cdc24p-GFP-SH3- CI and grown at 37°C.
(D) Cdc24p-SH3-CI rescues rsr1Δ bem1Δ lethality. rsr1Δ bem1Δ cells containing a URA3-marked BEM1 plasmid and the indicated control or Cdc24p-SH3-CI plasmids were spotted onto permissive –Leu plates or 5-FOA plates, which kill cells that retain the URA3-marked plasmids, and were incubated at 30°C (similar results were obtained at 37°C).
(E) DIC and actin staining of CDC24-SH3-CI bem1Δ rsr1Δ cells grown at 30°C.
(F) Cdc24p-SH3-CI promotes robust growth and cell-cycle progression in the absence of Bem1p. FACS profiles for BEM1-GFP rsr1Δ (blue, doubling time 119 min) and CDC24-SH3-CI bem1Δ rsr1Δ (red, doubling time 121 min) grown at 30°C. Cell-cycle timelines were derived as in Figure 4F.
Mutation of the SH3 domain (P208L) reduced the expression level and phosphorylation of GEF-SH3-CI (Figure 5B). This mutant GEF failed to localize to polarization sites (Figure S8) and failed to rescue either cdc24-1 (Figure S7C) or bem1Δ rsr1Δ cells (Figure 5D), indicating that the SH3 domain is critical for this protein’s function. In contrast, mutation of the Cdc42p-binding CI domain (N253D) [12] did not affect expression, phosphorylation (Figure 5B), localization (Figure S8), or cdc24-1 rescue (Figure S7). However, this mutation did slightly reduce the efficiency with which the GEF-SH3-CI protein rescued bem1Δ rsr1Δ proliferation (Figure 5D), suggesting that in this context, the GEF-Cdc42p-GTP interaction can assist the GEF-PAK interaction in breaking symmetry.
Discussion
Combined with previous studies, our data suggest that, in symmetry-breaking polarization of Cdc42p, PAK binding is necessary and sufficient to account for the role of the Bem1p SH3-2 domain and that GEF binding is necessary and sufficient to account for the role of the Bem1p PB1 domain. Moreover, both interactions must occur with a single molecule of Bem1p. The fact that each individual interaction can be effectively replaced by a protein fusion implies that physical linkage per se, rather than specific steric or allosteric effects of binding, is most important. The lipid-binding PX domain is also important in the context of endogenous Bem1p [5] but is dispensable when GEF-PAK interaction is facilitated (GEF-SH3-CI fusion protein) or enforced (GEF-PAK fusion protein) artificially. This is presumably because the GEF and PAK each have their own domains that promote plasma-membrane association. Thus, although Bem1p can bind to many other proteins, our findings suggest that its key role in symmetry breaking is to link the GEF to a PAK in a single complex.
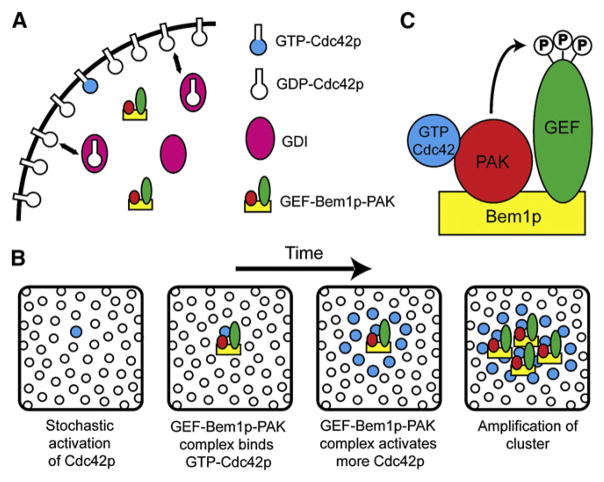
How does the GEF-PAK complex contribute to symmetry breaking? A simple model is illustrated in Figure 6. Interaction of the complex with GTP-Cdc42p through the PAK would both activate the PAK (see below) and recruit the GEF to the immediate vicinity of pre-existing GTP-Cdc42p and lead to auto-amplifying growth of GTP-Cdc42p clusters. Thus, a small GTP-Cdc42p cluster arising at a random cortical site through stochastic fluctuations could grow by sequential rounds of GEF-PAK recruitment from the cytosol into a dominant polarization site. Mathematical modeling suggests that such mechanisms can robustly generate a polarized cluster of GTP-Cdc42p from homogeneous starting conditions [29, 30]. Such models incorporate previous findings that Cdc42p can exchange rapidly between membrane and cytosolic pools through the action of GDI proteins that mask the Cdc42p prenyl moiety [31] and that, whereas cytosolic Cdc42p diffuses rapidly, diffusion of membrane-bound Cdc42p is very slow [32]. A key feature of this amplification mechanism is that GEF action is targeted to pre-existing cortical GTP-Cdc42p via its association with the PAK, which explains why cells in which the GEF and PAK bind to different molecules of Bem1p are unable to break symmetry. In addition, this mechanism for polarization explains the experimental observation that a GTP-locked Cdc42p mutant is normally unable to promote polarization [5]: The amplification mechanism would fail because the mutant does not interact with the GEF. GTP hydrolysis by Cdc42p is also presumably important for ensuring that any GTP-Cdc42p that diffuses away from the cluster is inactivated.
Figure 6. Model for Feedback Amplification of GTP-Cdc42p Clusters by the GEF-Bem1p-PAK Complex.
(A) Cdc42p is associated with the plasma membrane (black line) via prenylation but can be extracted into the cytosol by guanine nucleotide dissociation inhibitor (GDI, shown in pink). The GEF-Bem1p-PAK complex is cytosolic but can associate with the membrane either nonspecifically or via PAK binding to GTP-Cdc42p.
(B) The panels represent sequential snapshots of a small patch of plasma membrane as one looks down from the cell interior. The GEF-PAK complex links an active GEF to pre-existing GTP-Cdc42p at the membrane via the PAK. By enhancing local GTP-loading of Cdc42p in the immediate vicinity of pre-existing GTP-Cdc42p, this complex would amplify a small stochastically generated cluster of GTP-Cdc42p into a larger cluster.
(C) In the GEF-Bem1p-PAK complex, the PAK Cla4p phosphorylates the GEF Cdc24p at multiple sites. Such phosphorylation may enhance GEF activity to promote generation of sufficient GTP-Cdc42p to break symmetry.
In the above model, the central role for the PAK is to link GTP-Cdc42p to Bem1p. However, Boi1p is also thought to bind GTP-Cdc42p [8], as is Bem1p itself [11], so it would seem that alternative complexes lacking PAKs should also be able to participate in analogous amplification loops. However, mutations that impaired Bem1p-PAK interaction severely impacted symmetry breaking, whereas mutations that impaired Bem1p-Boi1p interaction (this work) or the direct Bem1p-Cdc42p interaction [12] had little effect. We did observe a mild defect when the direct GTP-Cdc42p interaction was impaired in the context of the GEF-SH3-CI construct, suggesting that this interaction can assist the GEF-PAK interaction to promote polarization. Nevertheless, the finding that a PAK, rather than other GTP-Cdc42p interactors, is a critical component of polarity complexes suggests that PAKs do more than simply tether the complex to pre-existing GTP-Cdc42p.
In addition to localizing the process of Cdc42p GTP loading, the PAK in the complex phosphorylates the GEF Cdc24p at multiple sites both in vitro and in vivo [11, 33]. Conflicting models have been proposed for the role of PAK-mediated GEF phosphorylation, arguing for stimulatory [11] or inhibitory [33] effects. Our finding that PAK catalytic activity is important for symmetry breaking is most easily accommodated by the hypothesis that PAK-mediated phosphorylation stimulates GEF activity, and the GEF in the complex thus becomes more effective at amplifying GTP-Cdc42p clusters. However, as yet it is not known whether the GEF is the relevant PAK substrate.
An alternative hypothesis on the role of GEF phosphorylation by PAKs proposes that such phosphorylation dissociates the GEF from Bem1p and thus terminates polarized growth in budded cells [33]. This model predicts that permanent linkage of Bem1p to the GEF would result in an inability to terminate polarized growth, whereas permanent linkage of Bem1p to a PAK would lead to rapid dissociation of any GEF from the complex and preclude polarized growth. Our observations do not support this hypothesis: Cells with GEF-Bem1p fusion proteins did not display elongated buds, and cells with Bem1p-PAK fusion proteins polarized normally.
GEF-PAK complexes are found in a much broader array of organisms than are Bem1p orthologs, and they have been implicated in polarization of mammalian cells responding to chemoattractant gradients [34]. We suggest that complexes with the basic architecture described here represent an evolutionarily conserved symmetry-breaking positive-feedback mechanism.
Experimental Procedures
Yeast Strains and Plasmids
Yeast strains and plasmids used in this study are listed in Tables S1 and S2. Standard media and methods were used for plasmid and yeast genetic manipulations. All constructs involving PCR were confirmed by sequencing, and all plasmid integrations and gene replacements via homologous recombination were confirmed by appropriate PCR tests using the genomic DNA of transformed yeast strains as a template. Details on all mutants and constructs are provided in the Supplemental Data.
Spot Assays
For analysis of cell viability, overnight liquid cultures were diluted to a final cell count of 5 × 107 cells/ml. Next, 10-fold serial dilutions were performed, and 2 μl of each dilution was spotted on an appropriate medium as indicated in the figure legends. The highest spotted amount of cells was either 105 or 104 cells. Spot assays were cropped and joined with Photoshop so that empty space was removed, but all assays in an individual panel are from the same plate.
Microscopy
Actin and DNA staining were performed as described [35, 36], with rhodamin-phalloidin (Molecular Probes, Eugene, OR) and 4,6-diamidino-2-phenyl-indole (Sigma, St. Louis, MO), respectively. For visualization of Sec4p by indirect immunofluorescence, cells were fixed as described [37], and mouse Sec4 antibody (generously provided by Patrick Brennwald, UNC Chapel Hill) was used at a 1:100 dilution. For visualization of Cdc11p, cells were fixed for 3 hr in 3.6% formaldehyde, and Cdc11 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:200 dilution. Typically, cells were incubated with the primary antibody at room temperature for 1 hr, then with secondary antibody at room temperature for 45 min; anti-mouse (Sec4) or anti-rabbit (Cdc11) Cy3-conjugated secondary antibodies (Molecular Probes) at 1:200 dilution were used. Cells were examined with a Zeiss Axioscope (Carl Zeiss, Thornwood, NY) equipped with an ORCA cooled charge-coupled-device camera (Hamamatsu, Bridgewater, NJ) and interfaced with MetaMorph software (Universal Imaging, Silver Spring, MD). Images were processed with Photoshop (Adobe systems, San Jose, CA).
Immunoblotting
Lysis of yeast, SDS-PAGE, and immunoblotting were performed as described previously [11]. The monoclonal mouse GFP (Roche Diagnostics, Indianapolis, IN) antibody was used at a 1:1000 dilution. The Myc 9E10 mouse monoclonal antibody (Santa Cruz Biotechnology) was used at a 1:250 dilution. The Cdc11 polyclonal antibody (Santa Cruz Biotechnology) was used at a 1:10000 dilution.
Analysis of Growth Rate and Cell Cycle
For measurement of the population doubling time, cultures were diluted to 1.5 × 106–2 × 106 cells/ml in YEPD or synthetic dextrose medium lacking leucine and grown at 30°C. Aliquots (1 ml) were fixed with 3.7% formaldehyde at 30 min intervals and sonicated, and the absorbance was measured at 600 nm with a Beckman DU640B Spectrophotometer (Beckman Coulter, Fullerton, CA).
For determination of the proportion of cells that had undergone budding or nuclear division, 1 × 107 cells from an exponentially growing culture were fixed with 3.7% formaldehyde. DNA was stained with 3 μg/ml DAPI in PBS, and cells were resuspended in mounting medium (90% glycerol, 9.2 mM p-phenylenediamine, and 3 μM DAPI). At least 500 cells were scored for each sample.
FACS analysis was performed as previously described [38]. Cells were fixed overnight in 70% ethanol, washed with H2O, and incubated in 2 mg/ml RNaseA (Sigma) in 50 mM Tris-HCl (pH 8.0) overnight at 37°C. After treatment with 5 mg/ml pepsin (Sigma) in 0.45% HCl (vol/vol) for 15 min, DNA was stained with Sytox Green (Invitrogen) in 50 mM Tris-HCl (pH 7.5). DNA content of 10,000 cells was measured with a Becton Dickinson FACScan and analyzed with CellQuest software (Becton Dickinson Biosciences, San Jose, CA).
Supplementary Material
Acknowledgments
We thank Alan Bender, Erfei Bi, Peter Pryciak, Doug Johnson, Robert Arkowitz, and Eric Weiss for providing strains or plasmids and Pat Brennwald for Sec4p antibody. Thanks to Dan Kiehart, Sally Kornbluth, Steve Haase, Robin Wharton, Dennis Thiele, and John York for comments on the manuscript. Thanks also to anonymous reviewers for excellent suggestions. A.H. was supported by a National Science Foundation predoctoral fellowship. This work was supported by National Institutes of Health grant GM62300 to D.J.L.
Footnotes
Supplemental Data
Supplemental Data include Supplemental Experimental Procedures, eight figures, and two tables and are available with this article online at: http://www.current-biology.com/S0960-9822(08)01295-5.
References
- 1.Turing A. The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedlich-Soldner R, Li R. Spontaneous cell polarization: Undermining determinism. Nat Cell Biol. 2003;5:267–270. doi: 10.1038/ncb0403-267. [DOI] [PubMed] [Google Scholar]
- 3.Etienne-Manneville S. Cdc42—The centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 4.Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 6.Leeuw T, Fourest-Lieuvin A, Wu C, Chenevert J, Clark K, Whiteway M, Thomas DY, Leberer E. Pheromone response in yeast: Association of Bem1p with proteins of the MAP kinase cascade and actin. Science. 1995;270:1210–1213. doi: 10.1126/science.270.5239.1210. [DOI] [PubMed] [Google Scholar]
- 7.Matsui Y, Matsui R, Akada R, Toh-e A. Yeast src homology region 3 domain-binding proteins involved in bud formation. J Cell Biol. 1996;133:865–878. doi: 10.1083/jcb.133.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender L, Shuen Lo H, Lee H, Kokojan V, Peterson J, Bender A. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J Cell Biol. 1996;133:879–894. doi: 10.1083/jcb.133.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons DM, Mahanty SK, Choi KY, Manandhar M, Elion EA. The SH3-domain protein Bem1 coordinates mitogen-activated protein kinase cascade activation with cell cycle control in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4095–4106. doi: 10.1128/mcb.16.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson J, Zheng Y, Bender L, Myers A, Cerione R, Bender A. Interactions between the bud emergence proteins Bem1p and Bem2p and Rho-type GTPases in yeast. J Cell Biol. 1994;127:1395–1406. doi: 10.1083/jcb.127.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose I, Irazoqui JE, Moskow JJ, Bardes ES, Zyla TR, Lew DJ. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J Biol Chem. 2001;276:7176–7186. doi: 10.1074/jbc.M010546200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi Y, Ota K, Ito T. A novel Cdc42-interacting domain of the yeast polarity establishment protein Bem1. Implications for modulation of mating pheromone signaling. J Biol Chem. 2007;282:29–38. doi: 10.1074/jbc.M609308200. [DOI] [PubMed] [Google Scholar]
- 13.McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, 3rd, Gygi SP, Kellogg DR. Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol. 2007;9:506–515. doi: 10.1038/ncb1568. [DOI] [PubMed] [Google Scholar]
- 14.Park HO, Bi E, Pringle JR, Herskowitz I. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc Natl Acad Sci USA. 1997;94:4463–4468. doi: 10.1073/pnas.94.9.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XD, Sperber LM, Kane SA, Tong Z, Tong AH, Boone C, Bi E. Sequential and distinct roles of the cadherin domain-containing protein Axl2p in cell polarization in yeast cell cycle. Mol Biol Cell. 2007;18:2542–2560. doi: 10.1091/mbc.E06-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winters MJ, Pryciak PM. Interaction with the SH3 domain protein Bem1 regulates signaling by the Saccharomyces cerevisiae p21-activated kinase Ste20. Mol Cell Biol. 2005;25:2177–2190. doi: 10.1128/MCB.25.6.2177-2190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.France YE, Boyd C, Coleman J, Novick PJ. The polarity- establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. J Cell Sci. 2006;119:876–888. doi: 10.1242/jcs.02849. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Wickner W. Bem1p is a positive regulator of the homotypic fusion of yeast vacuoles. J Biol Chem. 2006;281:27158–27166. doi: 10.1074/jbc.M605592200. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Matsui Y, Ago T, Ota K, Sumimoto H. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. EMBO J. 2001;20:3938–3946. doi: 10.1093/emboj/20.15.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvrckova F, De Virgilio C, Manser E, Pringle JR, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 21.Weiss EL, Bishop AC, Shokat KM, Drubin DG. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat Cell Biol. 2000;2:677–685. doi: 10.1038/35036300. [DOI] [PubMed] [Google Scholar]
- 22.Goehring AS, Mitchell DA, Tong AH, Keniry ME, Boone C, Sprague GF., Jr Synthetic Lethal Analysis Implicates Ste20p, a p21-activated Protein Kinase, in Polarisome Activation. Mol Biol Cell. 2003;14:1501–1516. doi: 10.1091/mbc.E02-06-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjandra H, Compton J, Kellogg D. Control of mitotic events by the Cdc42 GTPase, the Clb2 cyclin and a member of the PAK kinase family. Curr Biol. 1998;8:991–1000. doi: 10.1016/s0960-9822(07)00419-8. [DOI] [PubMed] [Google Scholar]
- 24.Butty AC, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 2002;21:1565–1576. doi: 10.1093/emboj/21.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada Y, Wiget P, Gulli MP, Bi E, Peter M. The nucleotide exchange factor Cdc24p may be regulated by auto-inhibition. EMBO J. 2004;23:1051–1062. doi: 10.1038/sj.emboj.7600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo M, Shirouzu M, Yokoyama S. The Cdc42 binding and scaffolding activities of the fission yeast adaptor protein Scd2. J Biol Chem. 2003;278:843–852. doi: 10.1074/jbc.M209714200. [DOI] [PubMed] [Google Scholar]
- 27.Schiller MR, Chakrabarti K, King GF, Schiller NI, Eipper BA, Maciejewski MW. Regulation of RhoGEF activity by intramolecular and intermolecular SH3 domain interactions. J Biol Chem. 2006;281:18774–18786. doi: 10.1074/jbc.M512482200. [DOI] [PubMed] [Google Scholar]
- 28.Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: Key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem. 2002;277:5644–5650. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- 29.Otsuji M, Ishihara S, Co C, Kaibuchi K, Mochizuki A, Kuroda S. A mass conserved reaction-diffusion system captures properties of cell polarity. PLoS Comput Biol. 2007;3:e108. doi: 10.1371/journal.pcbi.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goryachev AB, Pokhilko AV. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 2008;582:1437–1443. doi: 10.1016/j.febslet.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Tiedje C, Sakwa I, Just U, Hofken T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol Biol Cell. 2008;19:2885–2896. doi: 10.1091/mbc.E07-11-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulli MP, Jaquenoud M, Shimada Y, Niederhauser G, Wiget P, Peter M. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol Cell. 2000;6:1155–1167. doi: 10.1016/s1097-2765(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 35.Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pringle JR, Adams AEM, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- 37.Redding K, Holcomb C, Fuller RS. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991;113:527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haase SB, Reed SI. Improved flow cytometric analysis of the budding yeast cell cycle. Cell Cycle. 2002;1:132–136. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.