Abstract
Background
Voriconazole pharmacokinetic (PK) and pharmacodynamic (PD) data are lacking in children.
Methods
Records at the Childrens Hospital Los Angeles were reviewed for children with ≥1 serum voriconazole concentration obtained between May 1, 2006 and June 1, 2007. Demographics, dosing histories, serum concentrations, toxicity/survival data and outcomes were obtained. Analysis was with R 2.9.1 and the non-parametric modeling and simulation MM-USCPACK software.
Results
There were 207 voriconazole concentrations obtained from 46 patients (0.8 – 20.5 years). A 2-compartment Michaelis-Menten PK model fit the data best, but only explained 80% of the observed variability. Crude mortality was 13 (28%), and each trough serum voriconazole concentration <1,000 ng/mL was associated with a 2.6-fold increased odds of death (95% CI 1.6 – 4.8, P=0.002). Serum voriconazole concentrations were not associated with hepatotoxicity. Simulations predicted an intravenous dose of 7 mg/kg or oral dose of 200 mg twice daily would achieve a trough >1,000 ng/mL in the majority of patients, but with a very wide range of possible concentrations.
Conclusions
We found a PD association between a voriconazole trough >1,000 ng/mL and survival, and marked PK variability, particularly after enteral dosing, justifying measurement of serum concentrations.
Keywords: Pediatric, Population, Modeling, Pharmacokinetics, Pharmacodynamics, Voriconazole, Fungal
Introduction
Voriconazole is a triazole antifungal drug with activity against yeasts, endemic fungi, and certain molds, notably Aspergillus spp., for which it is currently first-line therapy in adults.[1] In children, voriconazole use has risen steadily since 2002, accounting for about 10% of prescribed systemic antifungal therapy in nearly 63,000 US pediatric inpatients in 2006 [2]. Strikingly, voriconazole has almost completely replaced the use of any amphotericin B formulation for the treatment of aspergillosis. However, to our knowledge there are only two peer-reviewed publications which document voriconazole pharmacokinetics (PK) obtained from prospective clinical trials in children under 12 years of age [3,4] and no studies to measure a pharmacodynamic (PD) link between concentration and outcome in children.
Although a single target trough voriconazole concentration to maximize efficacy has not been defined, several independent studies have demonstrated a relationship between higher voriconazole serum concentrations in adults and improved outcomes [5–8] , such that a target range has emerged, and trough serum voriconazole concentrations are recommended to be more than 500 to 2000 ng/mL and less than 6000 ng/mL [9,10].
Given the paucity of information on voriconazole PK in children, the known inter- and intra-individual variability, and the associations between serum concentrations and outcomes in adults, voriconazole concentrations are frequently measured at our hospital as part of the clinical care of children receiving this drug. Therefore, we reviewed our therapeutic drug management (TDM) experience in hospitalized patients with available voriconazole drug concentrations. Our goal was to refine our voriconazole dosing guidelines by achieving three aims: 1) to analyze associations between voriconazole concentrations and various patient outcomes, including toxicity and survival; 2) to create a population model of voriconazole PK across all ages of childhood and adolescence; and 3) to select the optimal dose(s) based on concentration profiles simulated from the model.
Patients and Methods
Data collection
We reviewed the laboratory and pharmacy records at the Childrens Hospital of Los Angeles (CHLA), a tertiary care, 324-bed pediatric referral hospital with an electronic medical record system. Eligible patients were identified as those who had voriconazole serum concentrations measured during admission to the hospital in a one year period from May 1, 2006 through June 1, 2007. This project was approved by the Institutional Review Board with a waiver of informed consent for anonymous data collection and analysis. Extracted data included demographic information, voriconazole serum concentrations, timing, amount, route, and formulation of voriconazole doses, as well as underlying and infectious diagnoses, concomitant use of drugs known or strongly suspected to influence voriconazole serum concentrations, alanine transferase (ALT) and alkaline phosphatase (ALKP), and mortality. Consistent with definitions used in previous adult and pediatric studies [8], we defined hepatoxicity as a 1.5-fold to 5-fold increase in ALT or ALKP over baseline, depending on the age-specific degree of elevation at baseline.
Voriconazole assay
Serum concentrations of voriconazole were determined by Focus Diagnostics, Inc. (Cypress, CA) using a validated, high-performance liquid chromatography (HPLC) assay. Voriconazole was extracted from serum and concentrations determined using a C18 column, autosampler, column oven, degasser, UV-VIS diode array detector, and Chemstation software. The serum standard curve for voriconazole was linear through a concentration range of approximately 200 to 30,000 ng/mL. The mean recovery from serum was 94%. The validation inter-assay coefficient of variation was less than 5%. No interference was detected with 14 other antimicrobial agents including four additional antifungal agents. Turnaround time ranged from seven to ten days.
Data analysis
Descriptive and analytic statistics and plots were generated using the open-source software R (version 2.9.2, R Foundation, Vienna, Austria, http://cran.r-project.org). For survival and hepatotoxicity analyses, Cox Proportionate Hazards models were constructed to examine associations with measures of voriconazole exposure, all adjusted for time on therapy.
Due to the retrospective nature of the study, we did a population analysis to estimate group and individual voriconazole pharmacokinetic parameters, e.g. clearance and volume of distribution, and the variability in those parameters. Population analysis was essential given the diversity in number and timing of samples from each patient, as well the dose amounts, routes, and other clinical characteristics of the patients. For the population analysis, we used the non-parametric adaptive grid (NPAG) modeling component of the MM-USCPACK software (available by license from the University of Southern California, Los Angeles, CA, http://www.lapk.org) to test the fit of the observed data to various candidate models, including the one used by Karlsson et al [4].
All models included weight as a covariate on volume and clearance terms. In addition, we also explored the influence on model variables of age, sex, creatinine clearance, ALT, and ALKP. We did not include CYP2C19 genotype in the model, which is known to be associated with fast, intermediate and slow phenotypes in voriconazole clearance [11], as this information was not available. However, non-parametric modeling excels at capturing sub-populations like fast and slow metabolizers because no parameter distribution (e.g. normal) is assumed; therefore, the absence of CYP2C19 data were unlikely to affect the model fit except for increasing the variability in parameter estimates. Models were evaluated on the basis of the calculated log-likelihood and inspection of observed vs. predicted plots.
MM-USCPACK was also used to simulate the effect of differing voriconazole dosing strategies on the predicted range of concentration profiles: 10,000 sets of parameter values were randomly selected, with replacement, from the weighted, multivariate distribution of parameter values in the final model, including the full covariance matrix, and predicted concentrations were calculated for each parameter set.
Results
Study Population and Sample Characteristics
From May 1, 2006 until June 1, 2007, there were 207 voriconazole concentrations obtained from 46 CHLA patients, who are summarized in Table 1. As indicated in the table, there was a wide range in doses and serum voriconazole concentrations, both between and within individual patients. Overall, 90% of the doses were administered enterally vs. intravenously.
Table 1.
Characteristics of 46 patients with 207 voriconazole concentrations.
| Patient | Age (y) | Sex | Wt (kg) | Underlying and infectious diagnoses |
EORTC Class [12] |
Died | Dose | No. samples |
Concentration range (ng/mL) |
Total (low) a measured troughs |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19.5 | F | 84.3 | ALL, relapse Pulmonary aspergillosis | Possible | Yes | 200 mg PO | 16 | 600–2900 | 1 (1) |
| 2 | 17.4 | M | 85.7 | ALL, BMT Alternaria spp. sinusitis | Probable | No | 300 mg PO | 1 | 5300 | 1 (0) |
| 3 | 4.5 | M | 18.2 | Liver transplant Pulmonary aspergillosis | Possible | No | 75 mg PO | 2 | 400–500 | 0 (0) |
| 4 | 13.9 | M | 45.8 | ALL, BMT Pneumonia | Possible | No | 200 mg PO | 1 | 1700 | 0 (0) |
| 5 | 15.5 | F | 75.4 | Lymphoma, relapse Pneumonia | Possible | No | 200 mg PO | 1 | 200 | 0 (0) |
| 6 | 8.3 | F | 47.8 | Hemophagocytic lymphocytic histiocytosis Disseminated candidiasis, sinusitis | Possible | Yes | 180 mg IV | 3 | 400–6700 | 1 (1) |
| 7 | 14.5 | M | 65.0 | ALL, relapse Pulmonary aspergillosis | Possible | Yes | 200 mg PO | 1 | 7900 | 0 (0) |
| 8 | 6.3 | M | 20.0 | Aplastic anemia Brain abscesses | Possible | No | 150–200 mg PO | 5 | 1300–2600 | 2 (0) |
| 9 | 0.8 | M | 8.8 | CGD Aspergillus versicolor pneumonia and osteomyelitis | Proven | No | 36–72 mg PO | 18 | BLQ-4400 | 0 (0) |
| 10b | 12.8 | M | 29.0 | CF ABPA | Possible | Yes | 60 mg PO | 1 | 1000 | 0 (0) |
| 11 | 16.5 | F | 47.3 | AML, BMT Candida glabrata esophagitis | Proven | No | 200 mg PO | 1 | 200 | 1 (1) |
| 12 | 20.0 | M | 54.2 | CNS Germ cell tumor Aspergillus fumigatus brain abscess | Proven | No | 350–600 mg PO | 5 | 2300–21300 | 1 (0) |
| 13b | 18.1 | M | 56.1 | Liver transplant Scedosporium apiospermum osteomyelitis | Proven | No | 200 mg PO | 1 | BLQ | 0 (0) |
| 14b | 19.7 | M | 77.3 | None Coccidioidomyces imitis meningitis | Proven | No | NR | 3 | 300–500 | 0 (0) |
| 15 | 7.7 | M | 35.4 | CGD Pulmonary aspergillosis | Possible | No | 300 mg PO | 1 | 3500 | 1 (0) |
| 16 | 5.3 | F | 20.8 | ALL, relapse Pulmonary aspergillosis | Possible | No | 130 mg PO | 1 | 2300 | 1 (0) |
| 17 | 16.0 | F | 58.6 | ALL, relapse Disseminated candidiasis | Possible | No | 200 mg PO | 1 | 6000 | 0 (0) |
| 18 | 9.5 | F | 21.4 | Aplastic anemia, BMT Pulmonary aspergillosis | Possible | Yes | 80–140 mg IV | 3 | 800–2800 | 1 (1) |
| 19 | 20.5 | F | 49.2 | SLE Aspergillus niger pneumonia | Probable | No | 200 mg PO | 1 | 200 | 1 (1) |
| 20 | 11.9 | F | 42.0 | CGD Pulmonary aspergillosis | Proven | Yes | 200–250 mg PO | 21 | BLQ-11900 | 1 (1) |
| 21 | 1.0 | M | 7.9 | CGD Pneumonia | Possible | No | 30 mg PO | 1 | 3000 | 1 (0) |
| 22 | 9.3 | F | 22.3 | AML, relapse Disseminated Candida glabrata | Possible | Yes | 100–150 mg PO | 4 | BLQ-4200 | 0 (0) |
| 23 | 7.5 | M | 21.0 | CF Aspergillus fumigatus pneumonia | Proven | No | 90–270 mg PO | 5 | BLQ-13200 | 2 (0) |
| 24 | 18.2 | M | 50.1 | Lung sarcoma Pulmonary aspergillosis | Proven | No | 200–400 mg NG | 3 | 1900-10500 | 1 (0) |
| 25 | 11.0 | F | 34.0 | ALL, relapse Disseminated candidiasis | Possible | Yes | 150–300 mg PO | 8 | BLQ-7500 | 1 (0) |
| 26 | 19.7 | M | 44.0 | CF, Lung transplant Aspergillus fumigatus sinusitis | Proven | No | 200–400 mg PO | 9 | BLQ-10900 | 1 (0) |
| 27 | 17.0 | F | 58.3 | ALL Pulmonary aspergillosis | Possible | No | 200 mg PO | 2 | 2700–5300 | 0 (0) |
| 28 | 14.0 | M | 47.9 | CF Aspergillus versicolor and terreus pneumonia | Probable | No | 200–300 mg PO | 3 | BLQ-700 | 0 (0) |
| 29 | 12.1 | M | 48.3 | ALL Disseminated candidiasis | Possible | No | 200 mg PO | 5 | 1800–5800 | 1 (0) |
| 30 | 8.8 | M | 20.6 | ALL, relapse Pulmonary aspergillosis | Probable | Yes | 75–200 mg PO | 8 | 300–3300 | 2 (2) |
| 31 | 3.0 | F | 14.4 | ALL Disseminated candidiasis | Proven | Yes | 55–150 mg IV/PO | 3 | BLQ-700 | 0 (0) |
| 32 | 14.6 | M | 31.8 | CF ABPA | Possible | No | 150–200 mg PO | 2 | 1900–3200 | 1 (0) |
| 33 | 15.7 | M | 90.0 | ALL Disseminated candidiasis | Possible | No | 200 mg PO | 2 | 600–2800 | 0 (0) |
| 34 | 2.6 | M | 19.4 | ALL high risk, refractory Disseminated Scedosporium apiospermum | Proven | Yes | 50–140 mg PO | 2 | 200–3000 | 2 (1) |
| 35 | 2.9 | M | 14.3 | SCID, BMT Disseminated aspergillosis | Probable | Yes | 55–150 mg IV | 5 | 300–600 | 1 (1) |
| 36 | 7.4 | F | 24.8 | CF ABPA | Possible | No | 150 mg IV | 4 | BLQ-2700 | 0 (0) |
| 37 | 12.7 | M | 37.8 | ALL, relapse Brain abscess | Possible | Yes | 160 mg IV | 2 | BLQ-1000 | 0 (0) |
| 38 | 9.9 | M | 22.1 | CF, liver transplant Pulmonary aspergillosis and Scedosporium apiospermum pneumonia | Probable | No | 95–250 mg PO | 10 | BLQ-18000 | 1 (0) |
| 39 | 9.2 | F | 20.2 | CF Pulmonary aspergillosis | Possible | No | 140 mg PO | 3 | 1100–2600 | 0 (0) |
| 40 | 17.6 | M | 55.5 | SLE Aspergillosis | Possible | No | 200 mg PO | 1 | 200 | 0 (0) |
| 41 | 4.4 | M | 16.8 | Lymphoma Disseminated candidiasis | Possible | No | 110 mg IV | 1 | 1100 | 0 (0) |
| 42b | 4.6 | M | 14.3 | Toxic epidermal necrosis Cutaneous Aspergillus niger | Proven | No | 100 mg IV | 2 | BLQ | 0 (0) |
| 43b | 17.8 | M | 59.8 | CF, lung transplant Aspergillus versicolor pneumonia | Probable | No | 300–400 mg PO | 5 | 900–5900 | 0 (0) |
| 44 | 7.9 | M | 27.2 | CGD Pneumonia | Possible | No | 250 mg PO | 17 | BLQ-12400 | 0 (0) |
| 45b | 14.4 | M | 29.0 | CGD Pneumonia | Possible | No | NR | 4 | 300–2500 | 0 (0) |
| 46 | 1.4 | F | 11.9 | ALL, BMT Pneumonia | Possible | No | 40–120 mg PO | 9 | 500–3600 | 7 (3) |
| Median or percent | 12.0 y | M 65% | 33.3 kg | Proven 26% | Died 28% | IV 150 mg 6.0 mg/kg | 3 | 2,100 ng/mL | ||
| F 35% | Probable 44% | PO/NG 200 mg 5.3 mg/kg | ||||||||
| Possible 59% | ||||||||||
| Range | 0.8– 20.5 | 6.5– 102.2 | IV 55–180 mg 3.4–10.5 mg/kg | 1–21 | BLQ (n=23) - 21,300 ng/mL | |||||
| PO/NG 30–600 mg 2.0–12.9 mg/kg |
Abbreviations: ALL=Acute Lymphoblastic Leukemia; AML=Acute Myelogenous Leukemia; BMT=Bone Marrow Transplant; CGD=Chronic Granulomatous Disease; CF=Cystic Fibrosis; CNS=Central Nervous System; BLQ=Below the Limit of Quantification; NR=Not recorded
Notes:
Total number of samples obtained at least 10 hours after previous dose and subset of these with voriconazole <1,000 ng/mL
These patients were excluded from the population model as all measured concentrations were either BLQ, not after a dose with a verified time (i.e. outpatient), or after an unverified dose amount. The remaining patients may have had some measurements excluded for the same reasons, but had at least one valid sample available for modeling.
Of the 207 samples, 99 (48%) were obtained after outpatient dosing with no verified preceding dose time; these were excluded from population modeling since time after dose was a necessary factor. Twenty-six (13%) of the 207 samples were reported as below the limit of quantification (BLQ) of 200 ng/mL, all of which were obtained after outpatient dosing. Since the mean outpatient dose of 6.4 mg/kg was not significantly different from the mean inpatient enteral dose of 5.8 mg/kg (P=0.14), it is possible that that sub-optimal adherence contributed to these low concentrations.
For the remaining 108 inpatient samples, the median sampling time was 9.0 hours (range 1.3 – 36.0) after the preceding dose, reflecting the clinical practice of monitoring trough serum voriconazole. These samples, from 40 patients, were used for modeling.
Of the medications listed in the voriconazole package insert known to alter voriconazole serum concentrations, only two patients also received phenytoin, which decreases serum voriconazole; none received other medications known to alter serum voriconazole.
Toxicity
Overall, 16 (42%) of 28 patients with baseline and on-therapy ALT or ALKP met the definition for significant hepatoxicity by ALT (10 patients), ALKP (2 patients), or both (4 patients). The median increase in ALT over baseline was 8.3-fold (range 3.7 – 30.5). ALT peaked 63 (5 – 238) days after starting voriconazole and resolved to baseline 49 (11 – 461) days later in 71%, at least two thirds of whom were still receiving voriconazole. The median increase in ALKP over baseline was 6.6-fold (range 5.2 – 12.7). ALKP peaked 69 (33 – 116) days after starting voriconazole and was not documented to resolve to baseline in any patients by the end of the data extraction period. By Cox Proportionate Hazards, the risk of hepatotoxicity was not significantly associated with voriconazole dose, AUC, average concentration, maximum or minimum concentration.
Survival
Of the 46 patients, 12 (26%) had proven invasive fungal disease [12] with a positive fungal culture from a normally sterile site and radiographic and/or clinical evidence of disease at that site. An additional 7 (15%) patients had probable disease [12] with at least one positive fungal culture from respiratory secretions (e.g. deep endotracheal suction, or bronchoaveolar lavage) and/or galactomannan test, and clinical/radiographic evidence of localized disease. Recovered organisms included Aspergillus spp (fumigatus, versicolor), Alternaria spp, Scedosporium apiospermum, and Candida spp, (albicans, glabrata).
As shown in Table 1, crude mortality in this population was 28%. Seventy-five percent of those who died had at least one low voriconazole trough, vs. only 20% of those who survived. Adjusted for annualized observation time and peak fold-increase in ALT over age-specific ULN, each voriconazole trough concentration <1,000 ng/mL increased the relative risk of death by 6.3-fold (95% CI 1.6 – 24.0, P=0.008, Cox Proportional Hazards).
Modeling: Non-Parametric Population Analysis
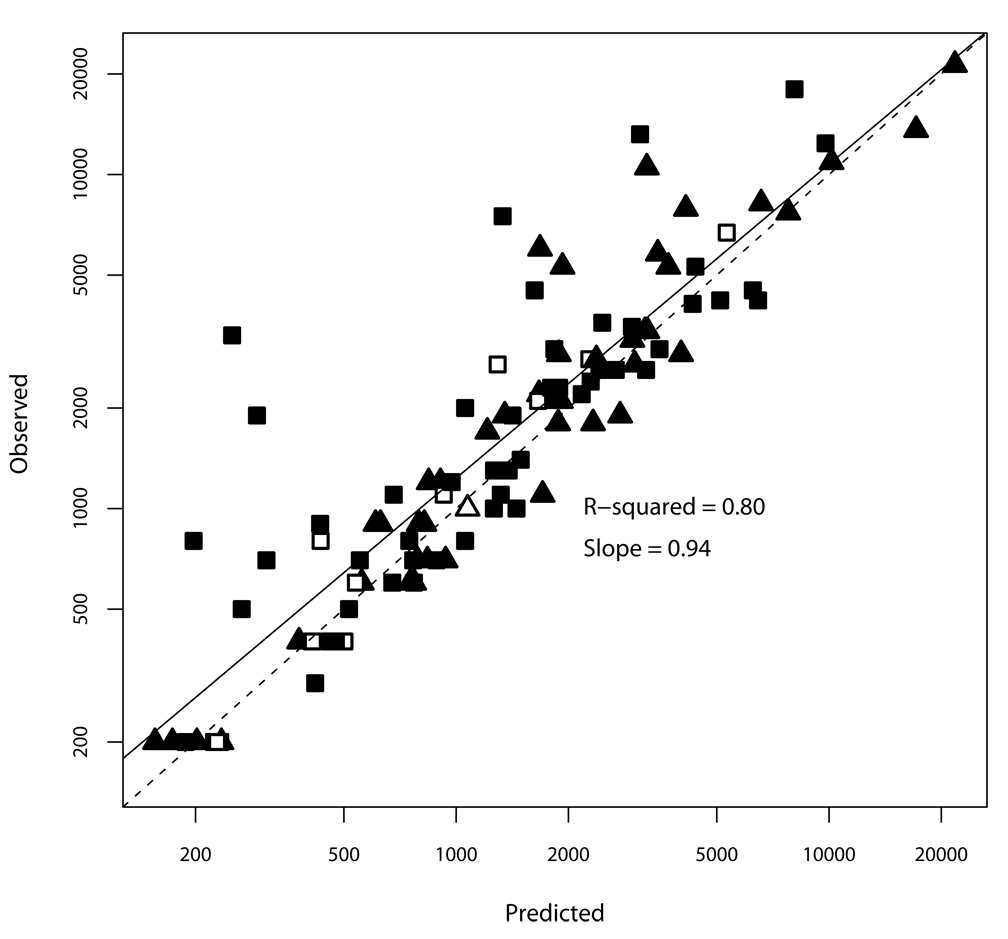
The final population model was a two-compartment model with absorption after a delay and Michaelis-Menten elimination, which implies a constant amount of drug cleared per unit of time. As shown in Figure 1, overall, the model explained 80% of the observed variability in individual voriconazole concentrations, with no difference in accuracy according to dosing route, despite the low number of samples obtained after intravenous dosing. The model under predicted observed concentrations by an average of 36% at the lower limit of the assay (200 ng/mL) and only 3% at the highest concentrations. The five most-underpredicted concentrations occurred in three children aged <12 years, with three of them from a single 9 year old with unexpectedly high trough concentrations.
Figure 1.
Observed vs. individual model-predicted concentrations. Solid line is the linear regression through the points; dashed line is the line of unity. Triangles are CHLA patients aged ≥12 years; squares are aged <12 years. Filled symbols are concentrations obtained after enteral dosing; open are after intravenous dosing.
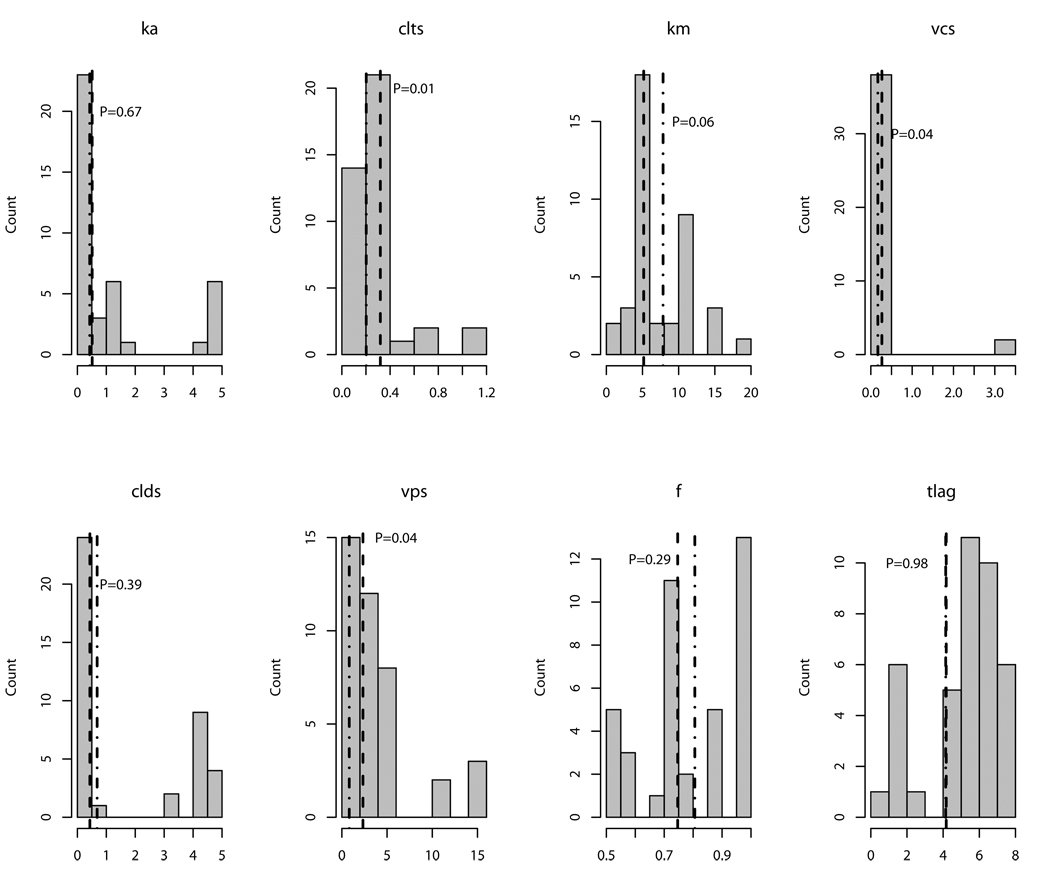
Estimates for the population model variables are detailed in Table 2. Because non-parametric analysis does not assume any distribution in the values of model variables (such as a normal or Gaussian distribution), means and standard deviations can obscure information. Therefore, Figure 2 shows the distributions of values in the patients for each variable in the model. Volumes and clearances were indexed to weight in kilograms, and there was a modestly significant additional effect of age, as shown in Table 2 and Figure 2. Sex, race, ethnicity, ALT and ALKP were not significantly associated with mean values of any of the variables in the model. Inclusion of all of these covariates only accounted for 10 % to 40% of the variability, suggesting that unmeasured covariates, such as CYP219C genotype, disease state, gut motility or others were important.
Table 2.
Geometric mean (GM) and relative standard error (GRSE%) voriconazole PK parameter estimates in CHLA patients stratified by age. Published values in children 2–12 years of age [4] are provided for comparison.
| CHLA ≥12 y (n=18) | CHLA <12 y (n=22) | Published 2–12 y | ||||
|---|---|---|---|---|---|---|
| GM | GRSE% | GM | GRSE% | Mean | RSE% | |
| Ka (h−1) | 0.43 | 212 | 0.51 | 164 | 0.85 | 40 |
| CL (L/kg/h) | 0.20 | 170 | 0.32* | 125 | 0.58 | 19 |
| Vc (L/kg) | 0.17 | 188 | 0.27* | 188 | 0.81 | 14 |
| Q (L/kg/h) | 0.68 | 191 | 0.43 | 246 | 0.61 | 13 |
| Vp (L/kg) | 0.83 | 127 | 2.34* | 42 | 2.17 | 11 |
| Km (mg/L) | 7.84 | 5 | 5.16 | 9 | 3.03 | 45 |
| F (%) | 81 | 37 | 75 | 35 | 45 | 14 |
| Tlag (h) | 4.14 | 11 | 4.17 | 13 | - | - |
Abbreviations: Ka=Rate constant of absorption; CL=Clearance; Vc=Volume of the central compartment; Q=Intercompartmental clearance; Vp=Volume of the peripheral compartment; Km=Michaelis-Menten constant; F=Bioavailability; Tlag=lag (delay) prior to absorption;
P≤0.05 for comparison between CHLA patients <12 y and ≥12 y by Student’s T-test.
Figure 2.
Distribution of individual values for non-parametric model variables in the study population. Vertical dashed line is the geometric mean for patients 12 years of age and under, while dashed-dotted line is for patients over 12 years of age. P-values are for the difference between the geometric means according to age. Variable names are the same as for Table 2.
Simulation scenarios
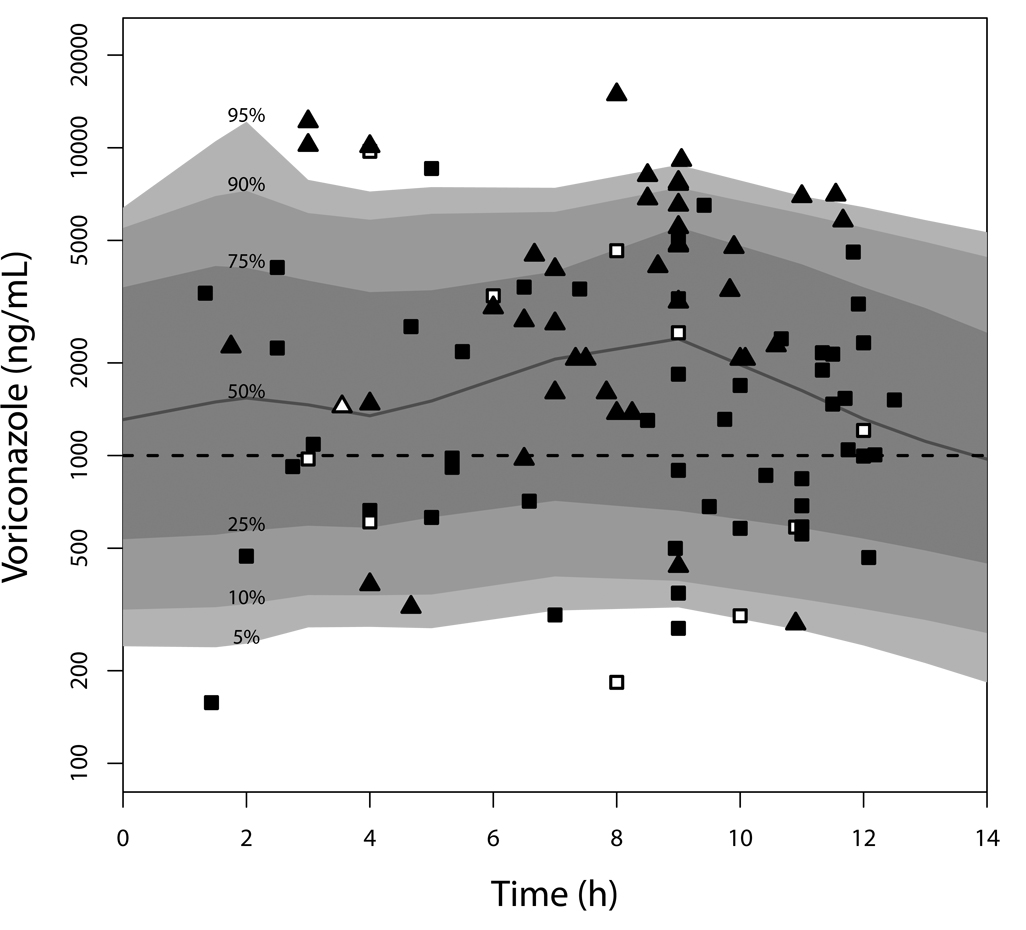
Figure 3 shows the distribution of 10,000 model-simulated steady-state concentration-time curves after dosing 5.8 mg/kg every 12 hours, with 10% administered intravenously (the median dose and percentage of intravenous doses in the CHLA population). The median simulated 12-hour trough concentration was 1,315 ng/mL, and the 5th and 95th percentiles were 242 and 6,411 ng/mL, respectively. Included in Figure 3 are the observed voriconazole concentrations in the CHLA patients, stratified symbolically by age >12 years and dosing route. These observed concentrations have been normalized to the median dose of 5.8 mg/kg and condensed to a single dosing interval of 12 hours to enable comparison with the simulated concentrations. Based on the observed vs. predicted voriconazole concentrations in Figure 1 and Figure 3, the model was judged a good representation of the data, and appropriate for further simulations.
Figure 3.
Simulated voriconazole concentration-time plot. Shaded bands are the indicated percentiles of 10,000 concentration-time profiles simulated from the final nonparametric model. Triangles are measured concentrations obtained in CHLA patients aged ≥12 years; squares are concentrations from CHLA patients aged <12 years. Filled symbols are concentrations obtained after enteral dosing; open are after intravenous dosing. The horizontal dashed line is the target minimum concentration of 1,000 ng/mL. Concentrations have been normalized to the median population dose, which was the same dose used for the simulations; hence, two measured concentrations appear to be at or below the assay limit of quantification (200 ng/mL).
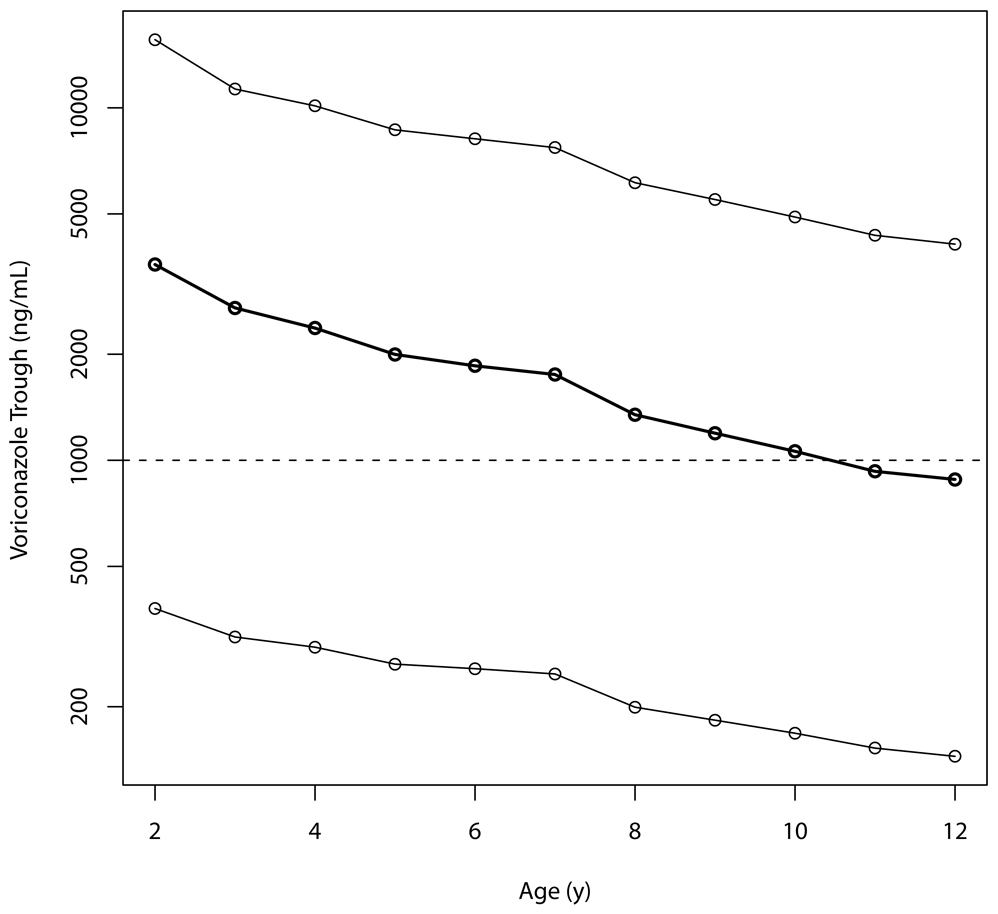
With a regimen of 7 mg/kg IV every 12 hours, as approved for use in Europe [13], the median simulated steady-state trough concentration was 1,287 ng/mL, with the 5th and 95th percentiles of 176 and 3,406 ng/mL; 66% of patients with this dose would be expected to be above a trough concentration of 1,000 ng/mL. Figure 4 shows the simulated range of steady-state voriconazole trough concentrations after the recommended European enteral dose of 200 mg twice daily at all ages from two to 12 years. The simulated median trough concentration ranges from 3,600 ng/mL at age two years to 880 ng/mL at 12 years, although there is significant variability around these median values. It must be noted that 200 mg as a fixed dose can result in doses in excess of 15 mg/kg in the youngest children. The highest enteral dose in our population was 12.9 mg/kg so the model may not be generalizable to higher doses.
Figure 4.
Predicted voriconazole trough concentrations based on 1000 simulated patients at each age, at the licensed European dose of 200 mg enterally every 12 hours. Lines are (top to bottom) the 95th, median, and 5th percentiles of simulated concentrations. The target trough concentration is the horizontal reference line at 1,000 ng/mL.
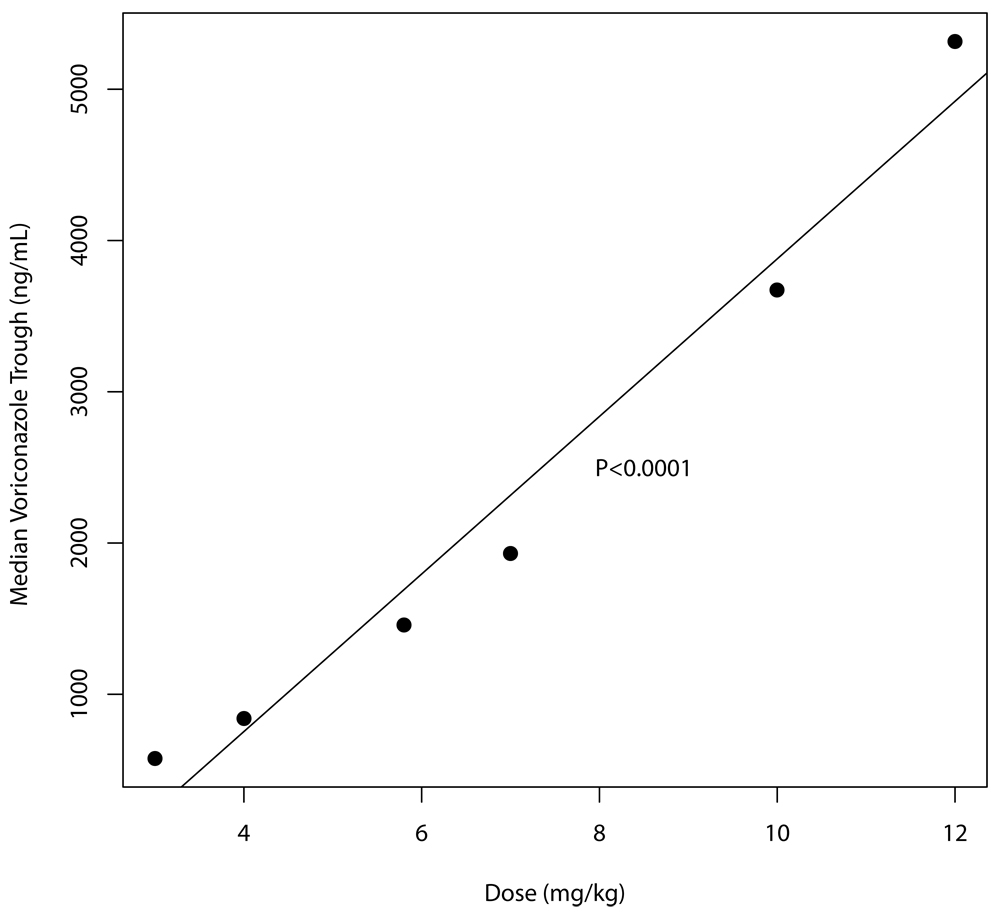
Finally, the relationship between the median simulated voriconazole trough concentration and enteral dose is shown in Figure 5. There is a constant increase of about 520 ng/mL for every increase in dose of 1 mg/kg, which is consistent with the Michaelis-Menten kinetics of the drug. For example, a 50% increase in dose from 4 to 6 mg/kg does not increase the median trough by 50%, but from 750 to 1800 ng/mL – an increase of 240%.
Figure 5.
Median simulated trough voriconazole concentration for a given enteral dose, showing the regression line with associated P-value.
Discussion
The data from the present report indicate that monitoring serum concentrations is important in children. There was a statistically significant association between crude mortality and voriconazole 12-hour trough concentrations below 1,000 ng/mL, even in this relatively small population. This breakpoint is consistent with several other investigations [14,15,5–7].
There was also a great deal of between-patient and inter-occasion PK variability, as noted by Karlsson [4]. Failure to account for inter-occasion variability increases model error and introduces biases in some parameter estimates [16]. Because 98% of the samples used for our model were obtained during different dosing intervals, in some cases over a period of months, and often after different dose amounts or routes, we were unable to model the changes in parameter values within a single individual over time. This likely contributed to the large variability in estimates of pharmacokinetic parameters (Table 2). Despite this, simulation of 10,000 concentration profiles closely matched the observed distribution of concentrations in the CHLA patients (Figure 3).
Significant features of our model that differ from that of Karlsson [4], as shown in Table 2, are the delay in voriconazole absorption after an oral dose and the higher bioavailability. These differences may be due to our relative lack of samples early in the dosing interval as well as within-individual, inter-occasion variability. Our oral bioavailability was approximately 80%, compared to the 96% reported for adults in the package insert and 45% reported by Karlsson [4]. Although not known to be pH dependent, absorption of voriconazole is reduced by food [17], which may explain some of the differences. Since the calculation of bioavailability depends on ratios of achievable concentrations after enteral and IV dosing, the small number of samples obtained after IV dosing in our population may have caused bias. However, our estimate was closer to that in the package insert, and we are unaware of any drug which has as large a discrepancy in bioavailability as reported by Karlsson [4], that could be attributed to age alone.
Based on our simulations an IV dose of 7 mg/kg every 12 hours in children up to age 12, as approved in Europe, is likely to achieve a voriconazole trough concentration of >1000 ng/mL in about two thirds of children, and appears to be a good starting dose. The recommended European enteral dose of 200 mg every 12 hours, regardless of age or weight, was chosen to best match the voriconazole exposure (AUC) in children to that in adults who also receive 200 mg every 12 hours. The somewhat surprising dose equivalence, which can result in doses in excess of 15 mg/kg in the youngest children, is due to the single report of voriconazole’s markedly decreased bioavailability and increased clearance in children relative to adults [4].
Despite the higher bioavailability in our data, simulation predicts that a fixed dose of 200 mg will result in reasonable trough concentrations close to 1,000 ng/mL in many children. However, clinicians should be aware that concentrations may be markedly above or below this target due to the variability in absorption. Furthermore, there is no actual reported experience of which we are aware of children receiving an enteral dose as great as 20 mg/kg. The largest enteral dose in the CHLA patients was 12.9 mg/kg twice daily (with associated trough concentrations of 13,200 ng/mL and 4,200 ng/mL on different occasions), and there is one report of a 4-year old child who tolerated 13.3 mg/kg twice daily, based on measured serum concentrations [18]. The tolerability and absorption of higher doses are not reported in the peer-reviewed literature, although a prospective trial to study this dose is underway.
Given the uncertainty associated with the PK of enteral voriconazole in children, it may be appropriate to prolong IV therapy until there is clear evidence of sustained improvement. These doses should be considered as initial, with measurement of serum trough concentrations at a minimum, followed by rational dosage adjustment as necessary. Our data suggest that for every increase in dose of 1 mg/kg, serum concentrations will rise approximately 520 ng/mL. However, measured concentrations outside the desired range should prompt a careful examination of other factors such as fasting status, concomitant medication use, or organ function.
We did not find any significant association between voriconazole concentrations and ALT or ALKP. Half of the CHLA children started voriconazole therapy with elevated ALT and ALKP, and further elevations after starting voriconazole were common, regardless of baseline values. However, most patients returned to baseline ALT or below by 2–4 weeks after peak values, without interrupting voriconazole therapy.
The obvious limitation of this study is the retrospective design. We did not control which children had concentrations measured or any other factor associated with voriconazole therapy. However, population modeling is the analytic technique of choice under these circumstances, as the individual doses, sample times, sample numbers, serum concentrations, and patient characteristics are all included in the modeling process. It is reassuring that our findings do not differ from the published prospective study [4] in terms of recommendations for initial dosing.
In conclusion, we wish to emphasize two major points for the provider who uses voriconazole therapy in children. First, we found a significant survival benefit when voriconazole trough concentrations were above 1,000 ng/mL. This is the first such demonstration in children, and it is consistent with evidence in adults. Second, this study confirms that voriconazole pharmacokinetics in children are highly variable, particularly with the enteral formulations. While 7 mg/kg IV or 200 mg enterally every 12 hours may be reasonable starting doses, we suggest, as has been done for adults, that voriconazole concentrations be measured and the dose adjusted to maintain a trough of at least 1,000 ng/mL. This strategy is especially important prior to the prospective validation of these doses that is currently underway.
Acknowledgements
The authors sincerely thank Rosa Cruz for data abstraction. MN is on the Speaker’s Bureau for Virco, Inc, and Merck, Inc.. and has attended an advisory meeting for Virco. None of the other authors has a conflict. MN is supported by NIH-NIAID 1 K23 AI076106-01. MN and RJ are supported by NIH-NBIB, R01 EB005803-01A1.
Footnotes
Main Point: In a retrospective study of 46 children, voriconazole troughs >1,000 ng/mL were significantly associated with survival. Modeling and simulation suggest the optimal starting dose is 7 mg/kg IV or 200 mg enterally twice daily, but individual monitoring is strongly recommended.
Presentations: Data from this manuscript has been submitted in abstract form to the Population Approach Group Europe (PAGE) meeting to be held June 23–26, 2009. Decision on the abstract is pending.
References
- 1.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 2.Prasad PA, Coffin SE, Leckerman KH, Walsh TJ, Zaoutis TE. Pediatric antifungal utilization: new drugs, new trends. Pediatr Infect Dis J. 2008;27(12):1083–1088. doi: 10.1097/INF.0b013e31817eeee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48(6):2166–2172. doi: 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson MO, Lutsar I, Milligan PA. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob Agents Chemother. 2009;53(3):935–944. doi: 10.1128/AAC.00751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–571. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 6.Pascual A, Calandra T, Bolay S, et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 7.Smith J, Safdar N, Knasinski V, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50(4):1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46(2):235–243. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 9.Smith J, Andes D. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther Drug Monit. 2008;30(2):167–172. doi: 10.1097/FTD.0b013e318167d0e0. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. J Antimicrob Chemother. 2008;61(1):17–25. doi: 10.1093/jac/dkm389. [DOI] [PubMed] [Google Scholar]
- 11.Weiss J, Ten Hoevel MM, Burhenne J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2):196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed January 13, 2009];EPARs for authorised medicinal products for human use - Vfend. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/vfend/vfend.htm.
- 14.Andes D, marchillo K, Stamstad T, Conklin R. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob.Agents Chemother. 2003;47(10):3165–3169. doi: 10.1128/AAC.47.10.3165-3169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Diekema DJ, Rex JH, et al. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J Clin Microbiol. 2006;44(3):819–826. doi: 10.1128/JCM.44.3.819-826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21(6):735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 17.Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br J Clin Pharmacol. 2003;56(s1):17–23. doi: 10.1046/j.1365-2125.2003.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Destino L, Sutton DA, Helon AL, et al. Severe osteomyelitis caused by Myceliophthora thermophila after a pitchfork injury. Ann Clin Microbiol Antimicrob. 2006;5:21. doi: 10.1186/1476-0711-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]