Abstract
Intracoronary delivery of endothelial progenitor cells (EPCs) is an emerging concept for the treatment of cardiovascular disease. Enhancement of EPC adhesion to vascular endothelium could improve cell retention within targeted organs. Because extracellular adenosine is elevated at sites of ischemia and stimulates neovascularization, we examined the potential role of adenosine in augmenting EPC retention to cardiac microvascular endothelium. Stimulation of adenosine receptors in murine embryonic EPCs (eEPCs) and cardiac endothelial cells (cECs) rapidly, within minutes, increased eEPC adhesion to cECs under static and flow conditions. Similarly, adhesion of human adult culture-expanded EPCs to human cECs was increased by stimulation of adenosine receptors. Furthermore, adenosine increased eEPC retention in isolated mouse hearts perfused with eEPCs. We determined that eEPCs and cECs preferentially express functional A1 and A2B adenosine receptor subtypes, respectively, and that both subtypes are involved in the regulation of eEPC adhesion to cECs. We documented that the interaction between P-selectin and its ligand (P-selectin glycoprotein ligand-1) plays a role in adenosine-dependent eEPC adhesion to cECs and that stimulation of adenosine receptors in cECs induces rapid cell surface expression of P-selectin. Our results suggest a role for adenosine in vasculogenesis and its potential use to stimulate engraftment in cell-based therapies.
Keywords: adenosine, adenosine receptors, endothelium, adhesion molecules
Intracoronary injection of bone marrow-derived or culture-expanded endothelial progenitor cells (EPCs) is currently tested for the treatment of patients after acute myocardial infarction. Recent double-blinded, placebo-controlled, multicenter clinical trials have shown that this type of therapy is relatively safe without serious adverse effects and may lead to moderate improvement of cardiac output.1 However, the number of donor cells retained in the heart is low, in the range of 3% to 5%,2 limiting the effectiveness of therapy. To overcome this problem, it would be highly desirable to develop methods to improve adhesion and retention of EPCs to cardiac endothelium.
We have previously shown that homing of EPCs to sites of tumor-induced angiogenesis or cardiac ischemia is mediated by active interaction with the vascular wall,3,4 suggesting that preactivation of adhesion molecules in host endothelium and donor-transplanted cells might augment cell retention in target tissues. However, activation of cell adhesion molecules in endothelial cells after ischemic injury or inflammation is likely to be transient and absent by the time of therapeutic intervention. Therefore, there is a need to develop safe ways to activate, locally and acutely, the adhesiveness of vascular beds during cell delivery.
In the present study, we examined the role of adenosine in EPC homing. Adenosine is generated when ATP is catabolized as energy demands increase or oxygen supply decreases in sites of tissue stress, injury, and local hypoxia. Adenosine exerts its actions through interaction with cell surface G protein– coupled adenosine receptors, of which there are 4 subtypes5: A1, A2A, A2B and A3. Once released into the extracellular space, adenosine signals to restore the balance between energy supply and demand.
The concept of adenosine as a retaliatory autacoid, originally proposed by Berne et al, has focused mostly on its acute actions, including vasodilation and negative chronotropic and inotropic effects in the heart.6 Accumulating evidence suggests that adenosine is also important for the long-term restoration of oxygen supply by contributing to neovascularization. Adenosine stimulates blood vessel formation in embryos7 and promotes capillary proliferation in the adult heart and skeletal muscles.8,9 These effects are mediated at least in part by adenosine-stimulated production of growth factors that facilitate new blood vessel formation from preexisting fully differentiated endothelial cells, a process known as angiogenesis.10,11
Neovascularization also occurs in a process known as vasculogenesis. Bone marrow–derived EPCs are critical to this process by differentiating into mature endothelial cells at the site of development of vascular networks. There is evidence that application of an adenosine receptor agonist to experimental excisional wounds stimulates vasculogenesis in the early phase of wound healing.12 However, the role of adenosine in EPC homing to the sites of tissue injury or ischemia has not been studied.
In the present study, we tested the hypothesis that adenosine is involved in the recruitment of EPCs to ischemic or damaged tissues by modulating their interaction with vascular endothelium. We focused our study on regulation of EPC adhesion to cardiac endothelium in view of the potential of cell-based therapies for cardiovascular disease and the possibility that adenosine could be developed as a novel adjunct agent for this purpose.
Materials and Methods
Reagents and Cells
N6-Cyclopentyladenosine (CPA), 5′-N-ethylcarboxamidoadenosine (NECA), 4-[(N-ethyl-5′-carbamoyladenos-2-yl)-aminoethyl]-phenyl-propionic acid (CGS21680), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), and adenosine were purchased from Sigma (St Louis, Mo). Endonorbornan-2-yl-9-methyladenine (N-0861) was a gift from Whitby Research Inc (Richmond, Va), and 5-amino-7-(phenylethyl)-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo-[1,5-c]-pyrimidine (SCH58261) was a gift from Schering Plough (Milan, Italy). 3-Isobutyl-8-pyrrolidinoxanthine (IPDX) was synthesized as previously described.13
Mouse cardiac microvascular endothelial (MCEC-1) cells were generously provided by Dr J. Mason (National Heart and Lung Institute, London, UK). These cells were isolated from mice containing a gene encoding the thermolabile SV40 T antigen and maintained in the presence of interferon-γ at 33°C.14 Six days before experiments, cells were replated and cultured in the absence of interferon-γ at 37°C. Primary cultures of human cardiac microvascular endothelial (HMVEC-c) cells (Cambrex, Walkersville, Md) were maintained according to the recommendations of the supplier up to passages 2 to 5.
Mouse EPCs isolated from E7.5 embryos (eEPCs) have been described previously.15 Human adult culture-expanded EPCs were generated from mononuclear cells obtained from normal peripheral blood leukocytes by culturing in EBM-2 (Cambrex) with supplements according to previously published protocols.16 EPCs were harvested on day 7 and were identified by uptake of 1,1′-dioctadecyl-3,3,3′,3′,-tetramethylindocarbocyanine (DiI)-labeled acetylated LDL and costaining with Ulex europaeus agglutinin-1 lectin, anti–vascular endothelial growth factor receptor 2, and anti–vascular endothelial cadherin.16
Measurement of cAMP Accumulation
cAMP concentrations were determined using a cAMP assay kit (GE Healthcare, Little Chalfont, UK).10
Real-Time RT-PCR
RT-PCR was performed as previously described.17 Primer pairs and 6-carboxy-fluorescein–labeled probes were provided by Applied Biosystems (Foster City, Calif).
Analysis of Cell Adhesion Under Static Conditions
We incubated progenitor cells (5×104 cells per well) prelabeled with calcein–acetoxymethyl ester (Molecular Probes, Eugene, Ore) in 96-well plates precoated with 1% porcine gelatin type A (Sigma) and covered with confluent endothelial monolayers in DMEM at 37°C. At the end of incubation periods indicated under Results, wells were gently washed twice with DMEM and twice with Tyrode’s buffer. Fluorescence of adhered cells was measured at excitation and emission wavelengths of 485 and 535 nm, respectively, and cell adhesion was calculated using a calibration curve constructed for each experiment by measuring fluorescence of predetermined numbers of labeled cells.
Analysis of Cell Adhesion Under Flow Conditions
Adhesion assays under flow conditions were performed using a parallel plate flow chamber (Glycotech, Rockville, Md) following the instructions of the manufacturer. Endothelial confluent monolayers were perfused for 10 minutes with DMEM containing 10 µmol/L NECA or its vehicle, followed by an EPC suspension (106 cells/mL) in the same medium for another 10 minutes at a constant rate to generate a desired wall shear stress (τ, dynes per centimeter squared) using the formula τ=6Qµ/a2b, where Q is flow rate, µ is medium viscosity, b is channel width, and a is channel height. EPC adhesion was determined by analysis of digitized video recordings using NIH Image software.
Cell-Based P-Selectin Enzyme-Linked Immunoassay
Cell surface P-selectin expression on MCEC-1 cells was analyzed as previously described18 using rat anti-mouse CD62P (Fitzgerald Industries, Concord, Mass) or isotype-matched control antibodies (BD Biosciences, San Jose, Calif) and a secondary goat anti-rat horseradish peroxidase–conjugated antibody (Jackson ImmunoResearch, West Grove, Pa).
Isolated Mouse Heart Model
Twenty eight male 6- to 8-week-old C57Bl/6 mice were used in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Hearts were rapidly removed from mice anesthetized with inhalation of isoflurane. The aorta was cannulated and connected to a Langendorff apparatus. The Langendorff perfusion was performed at a constant rate of 4 mL/min with Krebs–Henseleit buffer equilibrated with a gas mixture of 95% O2 and 5% CO2 at 37°C. Drug effects on coronary flow were measured at a constant pressure of 80 mm Hg. After a 30-minute stabilization period, hearts were perfused with 1.5 mg/L fluorescein isothiocyanate (FITC)-conjugated Helix pomatia lectin (Sigma) for 10 minutes to label endothelial cells followed by a 10 minutes washing period. Hearts were then perfused with eEPCs prelabeled with DiI-C16 (Invitrogen, Carlsbad, Calif) and resuspended in Krebs–Henseleit buffer containing 2% FBS (2500 cells/mL) in the presence or absence of 10 µmol/L adenosine, 3 nmol/L CGS21680, or 100 µmol/L inosine for 10 minutes. After washing for 10 minutes to remove unbound eEPCs, hearts were dissected, and retention of eEPCs was analyzed by taking 10 random images of the left ventricle using epifluorescence microscopy. Area of EPC-emitted fluorescence was measured using NIH ImageJ software and normalized to the area of vascular endothelium stained with FITC–lectin.
Statistical Analysis
All data are presented as means±SEM. The data were analyzed using unpaired 2-tail t test or 1-way ANOVA with Dunnett’s post test.
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Adenosine Receptors in Mouse Embryonic EPCs
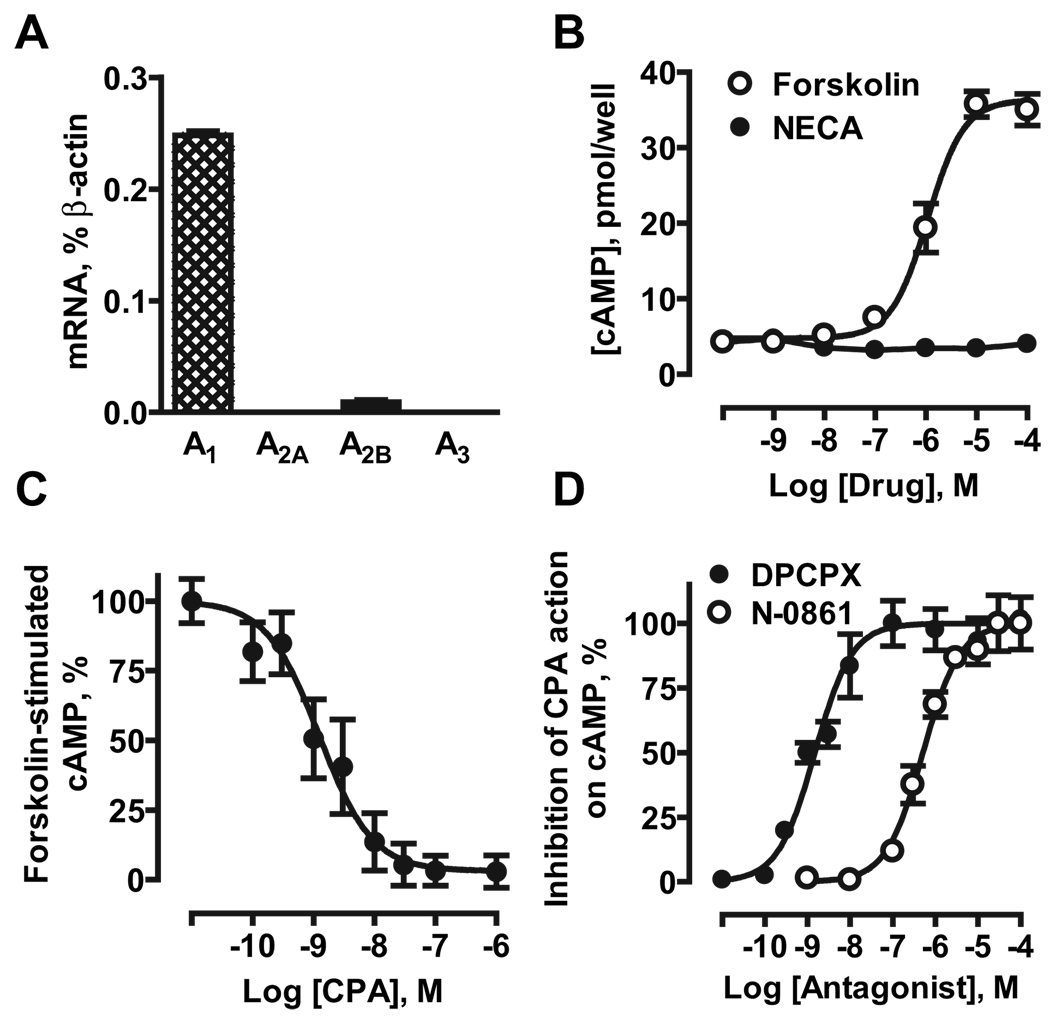
Real-time RT-PCR showed that eEPCs preferentially express mRNA encoding A1 receptors (0.248±0.004% of β-actin; Figure 1A). Very low levels of A2B receptor mRNA were also detected (0.009±0.002% of β-actin), whereas transcripts for A2A and A3 receptors were below detection levels.
Figure 1.
Adenosine receptors in mouse eEPCs. A, Real-time RT-PCR analysis of mRNA encoding adenosine receptor subtypes. B, Effects of forskolin and NECA on cAMP accumulation. C, Effect of the selective A1 receptor agonist CPA on cAMP accumulation induced by 1 µmol/L forskolin. D, Effects of the selective A1 receptor antagonists DPCPX and N-0861 on inhibition of forskolin-induced cAMP accumulation produced by 10 nmol/L CPA. The data are means±SEM (n=3).
We measured cAMP accumulation as a way to determine whether expression of mRNA translates into functional presence of adenosine receptors in eEPCs; A2A and A2B receptors stimulate adenylate cyclase via coupling to Gs proteins, whereas A1 and A3 receptors inhibit this enzyme via coupling to Gi proteins.5 The affinity to adenosine receptor subtypes of the agonists and antagonists used are summarized in the Table.
Table.
Affinity of Agonists and Antagonists to Adenosine Receptor Subtypes
| Subtypes |
|||
|---|---|---|---|
| Compounds | A1 | A2A | A2B |
| NECA | 6.3–30 | 4.2–20 | 330–449 |
| CPA | 0.59–4 | 148–2000 | 21 000–34 000 |
| CGS21680 | 290–36 300 | 3.6–27 | 361 000 |
| DPCPX | 0.3–3.9 | 129–598 | 50–86 |
| SCH58261 | 120–854 | 0.6–2.3 | >100–1868 |
| IPDX | 20 000–24 000 | 36 000 | 603–625 |
| N-0861 | 511–575 | 39 350–56 200 | |
The range of estimated binding or inhibition constants is given in nmol/L. An expanded table with detailed information and references is available in the online data supplement.
Forskolin increased cAMP levels in eEPCs from 4.3±0.7 to 35±2 pmol per well, with an EC50 of 1.1 µmol/L, whereas the nonselective adenosine receptor agonist NECA did not elevate cAMP (Figure 1B). This is contrary to what would be expected for activation of A2B receptors. However, the selective A1 agonist CPA inhibited forskolin-stimulated cAMP accumulation with an EC50 of 1.3 nmol/L (Figure 1C), corresponding to its reported affinity at A1 receptors.5 Furthermore, DPCPX and N-0861 antagonized the action of 10 nmol/L CPA on forskolin-stimulated cAMP accumulation with EC50 values of 1.5 and 511 nmol/L, respectively (Figure 1D), corresponding to their affinities at A1 receptors (Table). Thus, we conclude that A1 receptors are functionally present in eEPCs.
A1 receptor transcripts were also detected (2.1±1.4% of β-actin) in human adult culture-expanded EPCs along with mRNA encoding other adenosine receptors (3.4±2.1%, 1.0±0.3%, and 0.3±0.1% of β-actin for A2A, A2B, and A3 subtypes, respectively; n=4).
Adenosine Receptors in Cardiac Microvascular Endothelial Cells
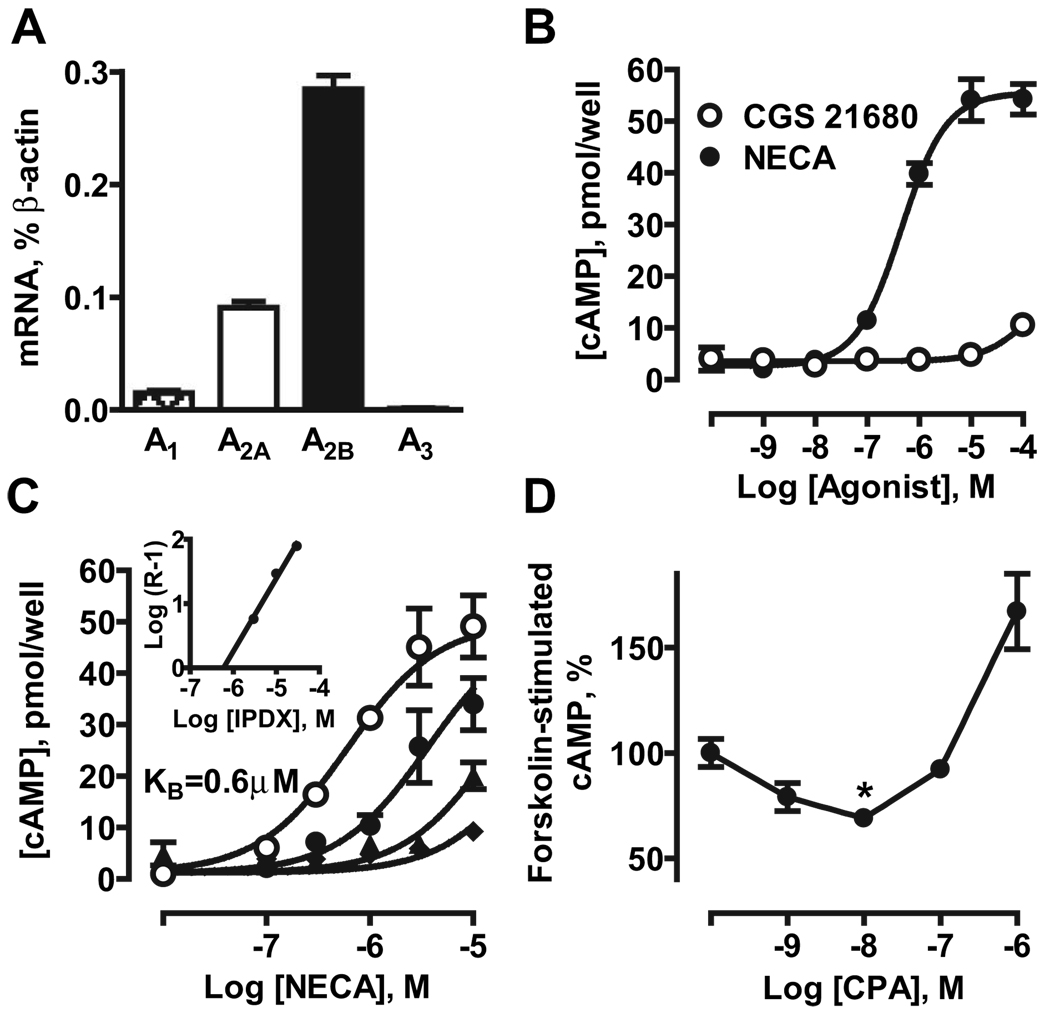
Real-time RT-PCR analysis of MCEC-1 cells revealed preferential expression of mRNA encoding A2B receptors (0.284±0.012% of β-actin), with lower expression of A1 and A2A receptors (0.016±0.002 and 0.091±0.005% of β-actin, respectively) and no detectable levels of A3 receptor transcripts (Figure 2A).
Figure 2.
Adenosine receptors in MCEC-1 cells. A, Real-time RT-PCR analysis of mRNA encoding adenosine receptor subtypes. B, cAMP accumulation induced by the nonselective agonist NECA and the A2A selective agonist CGS21680. C, Effect of the selective A2B antagonist IPDX on NECA-induced cAMP accumulation. Concentration–response curves for NECA were repeated in the absence (open circles) and presence of 3 µmol/L (closed circles), 10 µmol/L (triangles), and 30 µmol/L (diamonds) IPDX. Inset, Schild analysis indicated simple competitive antagonism at A2B receptors (slope of 1.1) with a KB value of 603 nmol/L. D, Effect of the selective A1 receptor agonist CPA on cAMP accumulation induced by 1 µmol/L forskolin. The data are means±SEM (n=3).
NECA stimulated cAMP accumulation with an EC50 of 449 nmol/L, corresponding to its affinity at A2B receptors,5 whereas the A2A agonist CGS21680 had no effect when used at selective concentrations (Figure 2C). The selective A2B antagonist IPDX progressively shifted concentration–response curves of NECA-stimulated cAMP accumulation to the right (Figure 2C). Schild plot analysis (inset) determined that IPDX inhibits this A2B-mediated process with a dissociation constant of 603 nmol/L, a value similar to that found in human cells.13 Functional, albeit low, expression of A1 receptors in MCEC-1 cells was also detected; the A1 agonist CPA inhibited forskolin-stimulated adenylate cyclase at selective (low nanomolar) concentrations (Table). Inhibition was reversed with increasing concentrations of CPA (>100 nmol/L) presumably because of stimulation of A2B receptors (Figure 2D). Taken together, our data suggest that A2B is the predominant receptor subtype regulating adenylate cyclase in MCEC-1 cells.
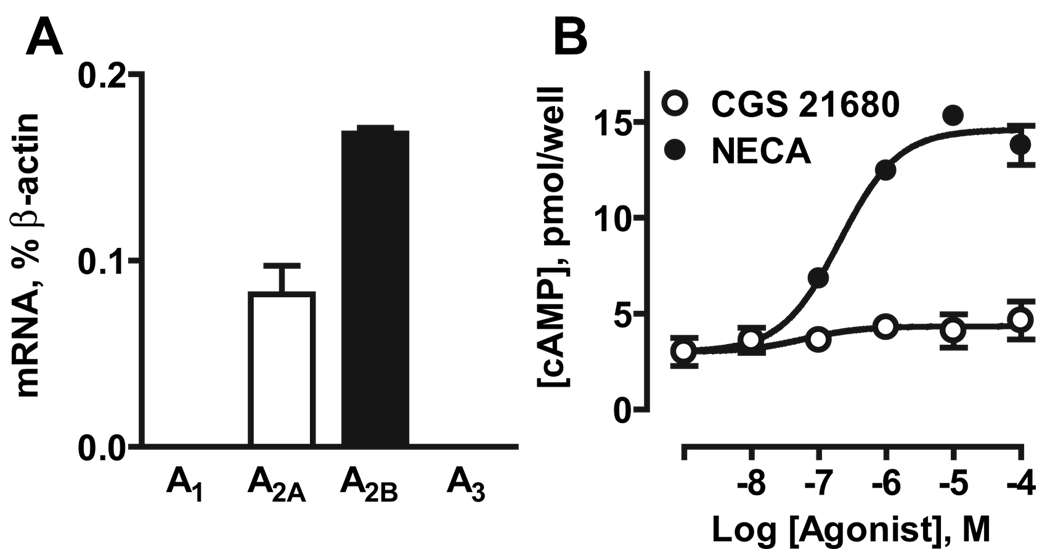
HMVEC-c cells preferentially expressed mRNA encoding A2B receptors (0.168±0.003% of β-actin), lower levels of A2A receptor transcripts (0.082±0.015% of β-actin), and no detectable levels of A1 or A3 receptor mRNA (Figure 3A). Similarly to MCEC-1 cells, A2B receptor was the predominant subtype regulating adenylate cyclase in HMVEC-c cells; the nonselective agonist NECA stimulated cAMP accumulation 5.1±0.1-fold, whereas the selective A2A agonist CGS21680 had no significant effect (Figure 3B).
Figure 3.
Adenosine receptors in HMVEC-c cells. A, Real-time RT-PCR analysis of mRNA encoding adenosine receptor subtypes. B, cAMP accumulation induced by the nonselective agonist NECA and the A2A selective agonist CGS21680. The data are means±SEM (n=3).
Role of Adenosine Receptors in EPC Adhesion to Cardiac Microvascular Endothelial Cells
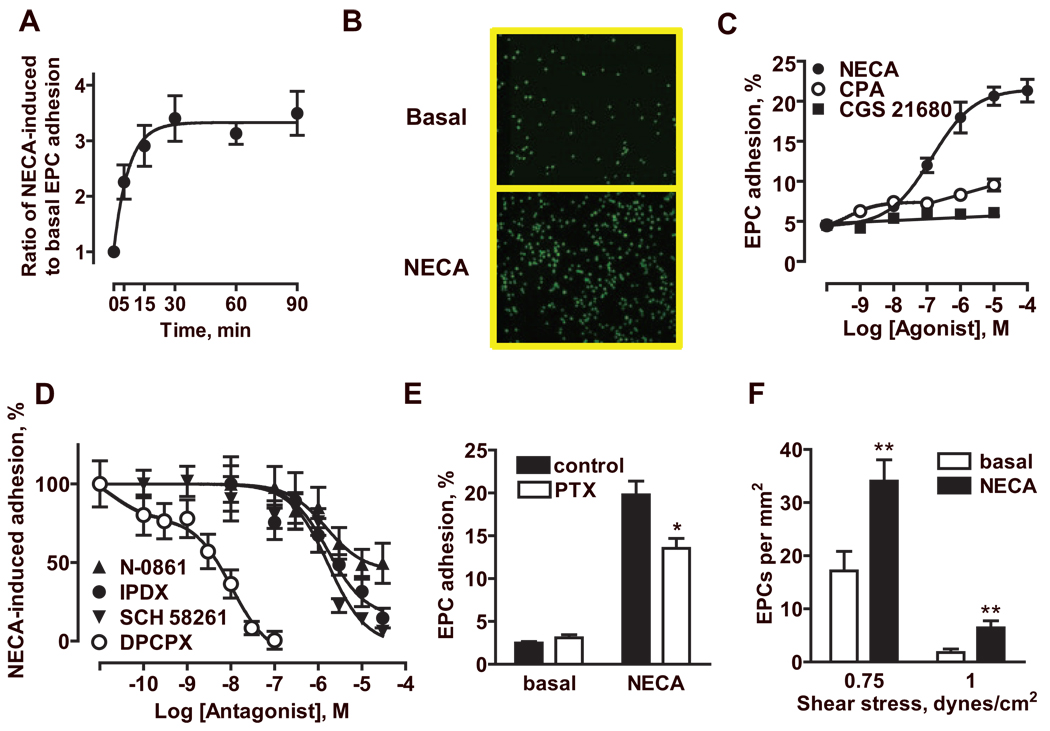
Adhesion of fluorescently labeled mouse eEPCs to MCEC-1 cells was rapidly stimulated by 1 µmol/L NECA (Figure 4A and 4B), with a half-maximal effect observed at 5 minutes. Adhesion in the continuous presence of NECA was greater compared with adhesion in the absence of NECA after individual pretreatment of MCEC-1 cells and/or eEPCs with NECA (Figure I in the online data supplement). From data in Figure 4A, we selected 30 minutes as the incubation time that produced maximal increase in adhesion, and performed a pharmacological analysis of the adenosine receptor subtypes involved in this action. NECA increased eEPC adhesion from 4.5±0.3% to 21.3±1.4% in a concentration-dependent manner with an EC50 of 139 nmol/L. Selective stimulation of A1 receptors with 10 nmol/L CPA only slightly increased eEPC adhesion to 7.4±0.6%, whereas stimulation of A2A receptors with CGS21680 had virtually no effect (Figure 4C). Based on these results, we selected 1 µmol/L NECA, a concentration producing submaximal increase in eEPC adhesion to MCEC-1 cells, to analyze the effects of adenosine receptor-specific antagonists. As seen in Figure 4D, DPCPX, N-0861, and IPDX inhibited NECA-induced eEPC adhesion with IC50 alues of 4 nmol/L, 1.5 µmol/L, and 1.6 µmol/L, consistent with their respective potency at A1 and A2B receptors (Table). Of note, the selective A2A antagonist SCH58261 inhibited NECA-induced eEPC adhesion with an IC50 value of 1.7 µmol/L that was consistent with its potency at A1 and A2B receptors, whereas it had no effect at lower concentrations that selectively block A2A receptors (Table). Taken together, these data suggest that A1 and A2B, but not A2A, receptors are involved in stimulation of eEPC adhesion to MCEC-1 cells by adenosine.
Figure 4.
Adenosine receptor-mediated eEPC adhesion to MCEC-1 cells. A, Time course of the effect of 1 µmol/L NECA on eEPC adhesion to MCEC-1 cells. The data are presented as increases over basal adhesion in the absence of NECA at each time point. The data are means±SEM (n=12). B, Representative micrographs showing adhesion of fluorescently labeled eEPCs (green) to MCEC-1 monolayers in the absence (Basal) or presence of 1 µmol/L NECA. C, Effects of the nonselective adenosine agonist NECA, the selective A1 agonist CPA, and the selective A2A agonist CGS21680 on eEPC adhesion to MCEC-1 cells. The data are means±SEM (n=12 for NECA and CGS21680; n=24 for CPA). D, Effects of the selective A1 antagonists DPCPX and N-0861, the selective A2A antagonist SCH58261, and the selective A2B antagonist IPDX on NECA-induced eEPC adhesion to MCEC-1 cells. The data are presented as percentages of an increase in adhesion induced by 1 µmol/L NECA. The data are means±SEM (n=12). E, Effect of pretreatment of eEPCs with pertussis toxin (PTX) compared with untreated cells (control) on their adhesion to MCEC-1 cells in the absence (basal) or presence of 10 µmol/L NECA. The data are means±SEM (n=6). *P<0.05 (t test) compared with control. F, Effect of NECA (10 µmol/L) on eEPC adhesion to MCEC-1 cells under defined flow conditions. The data are means±SEM (n=7). **P<0.01 (t test) compared with corresponding basal values.
We also used a complementary approach to evaluate the contribution of A1 receptors by preincubating eEPCs with 100 nmol/L pertussis toxin for 12 hours to uncouple the receptor to Gi proteins.5 In ancillary studies, we documented that this treatment completely abrogated the ability of CPA to inhibit forskolin-stimulated adenylate cyclase, thus confirming the functional uncoupling of A1 receptors. Pertussis toxin treatment significantly attenuated but did not completely block the stimulation of adhesion induced by 10 µmol/L NECA (Figure 4E). In contrast, this treatment had no effect on TNF-α–induced eEPC adhesion (supplemental Figure II). Thus, we conclude that stimulation of A2B receptors on MCEC-1 cells is essential for EPC adhesion to endothelium but that stimulation of A1 receptors on eEPCs can additionally increase their adherence. An increase in eEPC adhesion induced by stimulation of adenosine receptors can eventually lead to increased numbers of cells transmigrating endothelial layer, and our ancillary studies indicate this possibility (supplemental Figure III).
Next, we evaluated the adhesion of these cells under laminar flow conditions by perfusing eEPCs over MCEC-1 monolayers at 2 different levels at the low end of physiologically relevant range of wall shear stress values,19 0.75 and 1 dyne/cm2 for 10 minutes. As expected, an increase in shear stress reduced adhesion of eEPCs to endothelial cells. However, stimulation of adenosine receptors with 10 µmol/L NECA significantly increased eEPC adhesion at both levels of shear stress (Figure 4F). On stopping and resuming flow, the adhered eEPCs withstood further increase in laminar flow applied in increments of 1 dyne/cm2 and started to detach only when shear stress exceeded 10 dyne/cm2.
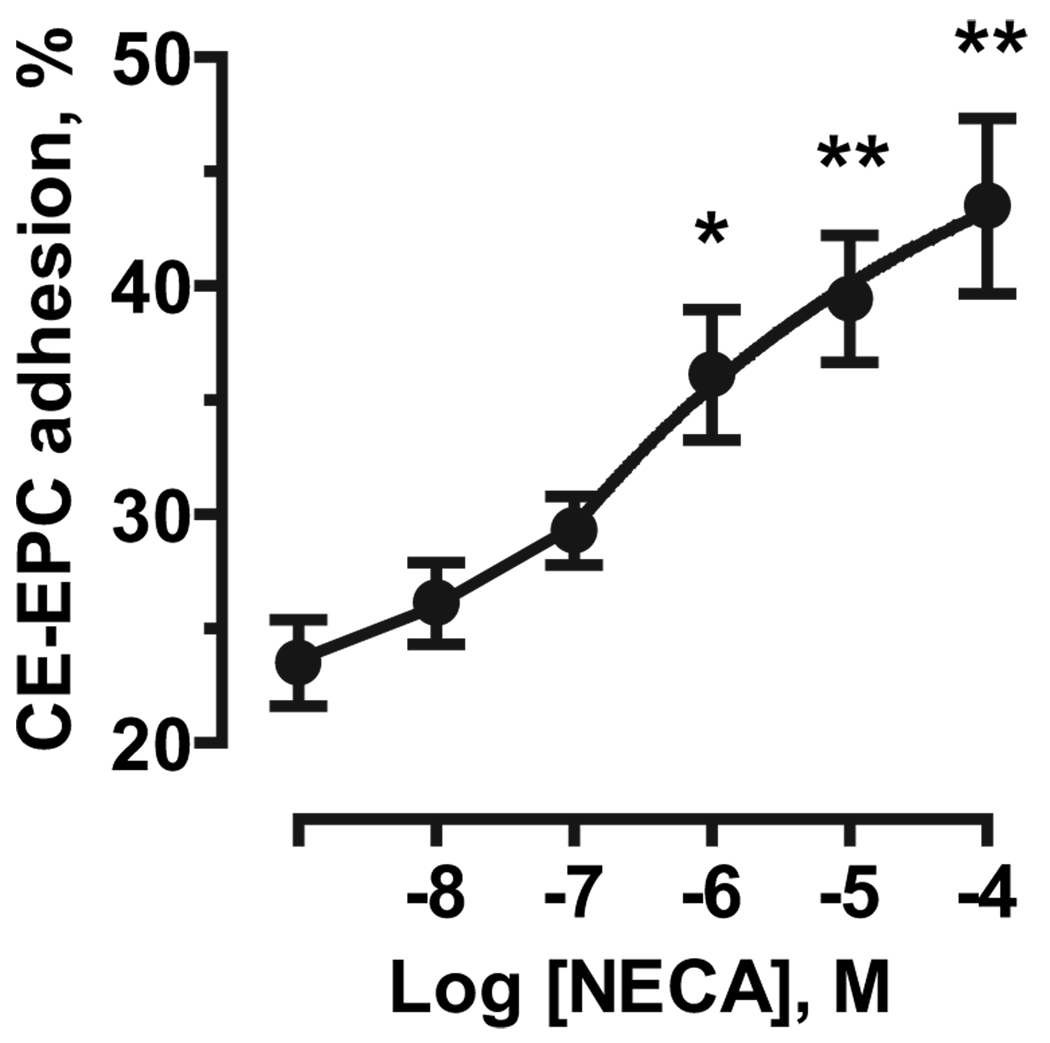
We then measured the effect of adenosine receptor stimulation on the adhesion of human adult culture–expanded endothelial progenitor cells (CE-EPCs) to HMVEC-c cells. As seen in Figure 5, NECA stimulated CE-EPC adhesion to HMVEC-c cells in a concentration-dependent manner. These results indicate that adenosine receptors can regulate not only adhesion of mouse embryonic EPCs but also homing of adult human progenitor cells to cardiac microvascular endothelial cells.
Figure 5.
Adenosine receptor–mediated stimulation of adhesion of adult human EPCs to HMVEC-c cells. Effect of increasing concentrations of NECA on adhesion of human peripheral blood culture–expanded EPCs (CE-EPCs) to HMVEC-c cells. The data are mean±SEM of 3 separate cell preparations. *P<0.05, **P<0.01 (1-way ANOVA with Dunnett’s post test).
Adenosine Promotes EPC Retention in Isolated Mouse Hearts
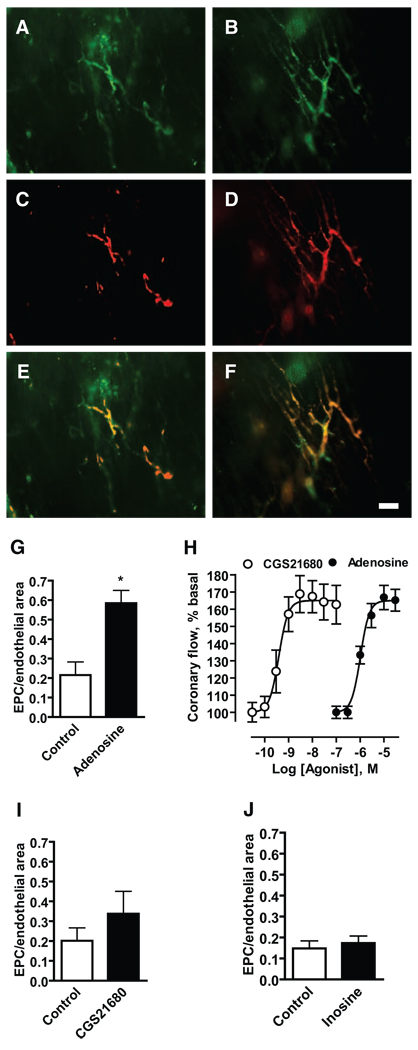
To determine whether the observed adenosine-dependent increase in EPC adhesion to cardiac microvascular endothelial cells translates into increased retention of circulating EPCs in the coronary vasculature, we used a conventional Langendorff retrograde perfusion system. Endothelial cells in coronary vessels were marked with FITC-conjugated Helix pomatia lectin (green; Figure 6A and 6B). Mouse eEPCs were labeled with DiI-C16 to allow their detection at the surface of the left ventricle using epifluorescence microscopy (red; Figure 6C and 6D). Figure 6 shows representative images obtained from hearts perfused with eEPC suspension in the absence (A, C, and E) or presence of 10 µmol/L adenosine (B, D, and F). We found that adenosine significantly increased the relative area of vascular network occupied by eEPCs (Figure 6G).
Figure 6.
Adenosine promotes retention of eEPCs in isolated hearts. Retention of eEPCs in mouse hearts was studied using a conventional Langendorff retrograde perfusion system. A through F, Representative fluorescent micrographs of perfused vessels (green) (A and B), retained eEPCs (red) (C and D), and their overlay (E and F) were obtained from hearts perfused with eEPC suspension in the absence (A, C, and E) or presence (B, D, and F) of 10 µmol/L adenosine. (Scale bar=50 µm.) G, I, and J, Retention of eEPCs in hearts perfused in the absence (Control) or presence of 10 µmol/L adenosine, 3 nmol/L CGS21680, and 100 µmol/L inosine was estimated by measuring the area of EPC-emitted fluorescence and by normalizing to the area of endothelial staining in 10 random images of the left ventricle taken for each heart. The data are means±SEM (n=3). *P<0.05 (t test). H, Concentration–response curves of CGS21680 and adenosine effects on coronary flow (mL/min per gram). The data are expressed as percentages from baseline and represent means±SEM (n=5).
A2A receptors are known to participate in adenosine-induced coronary vasodilation.5 Perfusion of hearts with the selective A2A agonist CGS21680 (3 nmol/L) produced comparable vasodilation as 10 µmol/L adenosine (Figure 6H) but had a considerably less effect on eEPC retention (Figure 6I), indicating that vasodilation per se cannot explain this phenomenon. In rodents, adenosine can also trigger the release of vasoactive compounds from mast cells via A3 receptors.20 However, stimulation of A3 receptors with 100 µmol/L inosine20 had no effect on eEPC retention in perfused hearts (Figure 6J).
Role of P-Selectin Glycoprotein Ligand-1 and P-Selectin in the Mechanism of Adenosine-Dependent EPC Adhesion to Cardiac Microvascular Endothelium
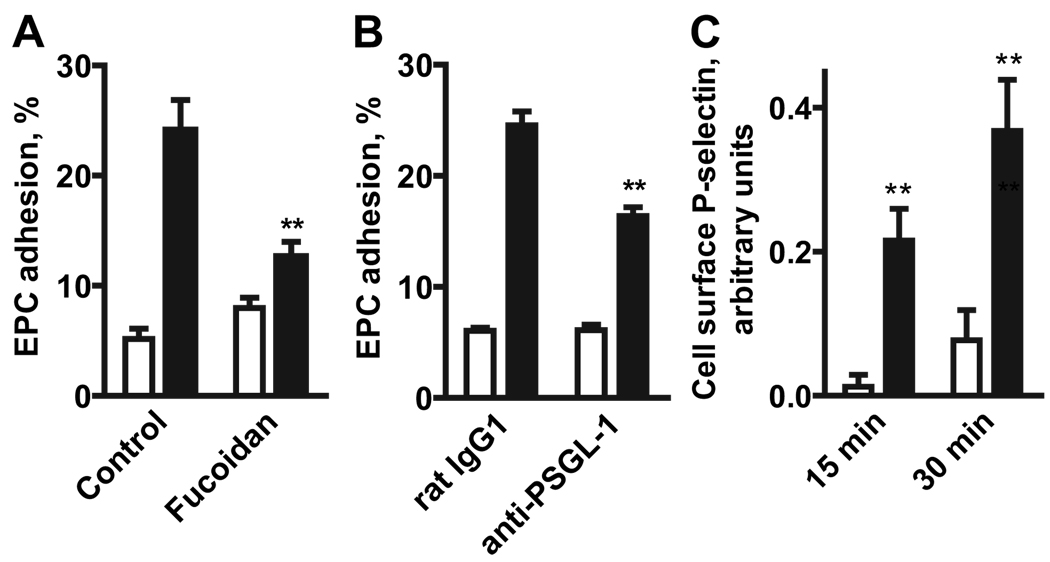
Because the P-selectin glycoprotein ligand (PSGL)-1 has been previously implicated in eEPC adhesion to the vascular wall,3 we investigated its potential role in adenosine receptor-stimulated eEPC adhesion to MCEC-1 cells. Fucoidan, a polysaccharide known to block PSGL-1 interaction with P-selectin,21 inhibited NECA-dependent stimulation of eEPC adhesion (Figure 7A). Furthermore, NECA-induced eEPC adhesion to MCEC-1 cells was partially blocked if mouse eEPCs (106 cells/mL) were preincubated with 10 µg/mL a blocking monoclonal anti-PSGL-1 antibody (clone 2PH1, Fitzgerald Industries) but was not affected by preincubation with a control isotype-matched antibody (Figure 7B). These data suggest that interaction between PSGL-1 and P-selectin plays a role in adenosine-induced eEPC adhesion. Therefore, we next tested whether stimulation of adenosine receptors on MCEC-1 cells could acutely increase P-selectin expression on the cell surface. Indeed, our results show that stimulation of MCEC-1 cells with 10 µmol/L NECA for 15 or 30 minutes significantly increased P-selectin expression on the surface of endothelial cells (Figure 7C).
Figure 7.
Interactions between PSGL-1 and P-selectin contribute to NECA-induced adhesion of eEPCs to MCEC-1 cells. A, Effect of fucoidan on eEPC adhesion to MCEC-1 cells in the absence (open bars) or in the presence of 10 µmol/L NECA (closed bars). The data are means±SEM (n=12). **P<0.01 (t test) compared with corresponding control values. B, Effect of blocking anti–PSGL-1 monoclonal antibody on eEPC adhesion to MCEC-1 cells in the absence (open bars) or presence (closed bars) of 10 µmol/L NECA. EPCs were preincubated for 15 minutes with a PSGL-1 blocking or control (rat IgG1) antibodies and then assayed for adhesion to MCEC-1 cells. The data are means±SEM (n=18). **P<0.01 (t test) compared with corresponding control values. C, Effect of NECA on cell surface P-selectin expression in MCEC-1 cells. Cells were incubated in the absence (open bars) or presence (closed bars) of 10 µmol/L NECA for 15 or 30 minutes at 37°C. Cell surface P-selectin expression was measured by an enzyme-linked immunoassay and presented in arbitrary units calculated from optical density of samples by subtracting corresponding values for nonspecific binding. The data are means±SEM (n=6) **P<0.01 (t test) compared with values obtained in the absence of NECA.
Discussion
Because extracellular adenosine is elevated at sites of ischemia5 and stimulates neovascularization,8,9 we examined the potential role of adenosine in augmenting EPC retention to cardiac microvascular endothelium. We chose mouse embryonic EPCs and cardiac microvascular endothelial cells MCEC-1 cells as a model to study the role of adenosine receptors in promoting adhesion. Our choice of these cells was determined by their robust growth properties in culture, and hence the availability of considerable quantities required for a systematic pharmacological analysis of adenosine receptors and their functions. eEPCs express early endothelial markers, differentiate to mature endothelial cells, form vascular tubes in vitro, and build blood vessels after transplantation during embryogenesis.15 In addition, the homing of eEPCs to hypoxic tumors, their participation in tumor vessel formation,3 as well as their stimulation of angiogenesis in chronic and acute ischemia4 render these cells a relevant model system to study the biology of EPCs. Likewise, the conditionally immortalized MCEC-1 cells, containing a gene encoding the thermolabile SV40 T antigen, assume a phenotype virtually identical to that of primary cardiac microvascular endothelial cells when cultured at 37°C.14
Our study demonstrated that mouse eEPCs preferentially express functional high-affinity A1 adenosine receptors, whereas MCEC-1 cells preferentially express functional low-affinity A2B receptors. We verified that primary cultured HMVEC-c cells also preferentially express functional A2B receptors, thus validating the use of MCEC-1 cells as a relevant cell model to study adenosine actions on cardiac microvascular endothelium.
Adenosine has been shown previously to modulate adhesion of other cells to vascular endothelium. Studies in neutrophils suggested differential roles of adenosine receptor subtypes in regulating their adhesion to endothelial cells. Stimulation of A1 receptors promoted neutrophil adhesion to endothelial cells, whereas stimulation of A2A receptors inhibited their adhesion22; opposite roles of A1 and A2A receptors in neutrophil adherence to cardiac vascular endothelium were also demonstrated in the guinea pig isolated heart.23 Endothelial A2A receptors have been shown to inhibit the expression of E-selectin and VCAM-1 stimulated by proinflammatory cytokines and endotoxin in human umbilical cord vein endothelial cells.24 However, we found no functional presence of A2A receptors in MCEC-1 or HMVEC-c cells. Based on these observations, we reasoned that in contrast to the inhibitory action of A2A receptors in neutrophil adhesion, A1 and A2B receptors might promote EPC adhesion to microvascular endothelium.
In the present study, we found that mouse eEPCs expressing A1 receptors increased their adherence to MCEC-1 cells in the presence of the nonselective adenosine receptor agonist NECA under static and flow conditions. However, activation of A1 receptors on eEPCs per se was not sufficient for efficient stimulation of cell adhesion. NECA was as efficacious as adenosine (4.7±0.3 versus 4.3±0.3-fold stimulation) in promoting EPC adhesion to MCEC-1 cells, but the effect of the selective A1 agonist CPA was considerably lower than the effect of NECA. Furthermore, uncoupling of A1 receptors from intracellular signaling pathways with pertussis toxin in eEPCs attenuated, but did not block completely, the effect of NECA on their adhesion to MCEC-1 cells. Therefore, our data suggest that A2B receptors expressed on MCEC-1 cells are essential for stimulation of cell adhesion and A1 receptors expressed on eEPCs can additionally contribute to the increased interactions between these cells. It is possible that engagement of the high-affinity A1 receptors is especially important for circulating cells moving toward a gradient of adenosine concentrations generated by hypoxia, whereas the low affinity A2B receptors are important for regulation of adhesive properties of endothelium located in the vicinity of the ischemic loci where concentrations of adenosine are the highest.
Because adenosine-dependent stimulation of EPC adhesion to endothelial cells is a rapid process, it is likely that adenosine regulates translocation of preexisting adhesion molecules to the endothelial surface. In this regard, the adhesion molecule P-selectin is known to be stored in endothelial Weibel–Palade bodies, and G protein-coupled receptors or substances that increase cAMP, intracellular Ca2+, or protein kinase C activity can induce exocytosis of the content of Weibel–Palade bodies, thereby increasing P-selectin surface expression within minutes of stimulation.18,25 We have shown previously that A2B receptors are linked to these pathways in microvascular endothelial cells,10 and in this study, we found that their stimulation with NECA (but not A1 or A2A receptors with CPA or CGS21680) increased P-selectin surface expression on MCEC-1 cells, suggesting that this mechanism may be relevant to the rapid increase of EPC adhesion to cardiac microvascular endothelial cells induced by adenosine. Our observation is in agreement with the rapid recruitment of circulating EPCs observed in a mouse model of myocardial ischemia,26 and adenosine is known to be released into the coronary circulation within minutes from the onset of ischemia.27
Mouse eEPCs express a wide range of adhesion molecules on their surface that potentially can interact with their counterparts on endothelial cells.3 In particular, the P-selectin ligand PSGL-1 has been suggested to play an important role in adhesion of these cells to the vascular wall.3 In this study, we found that the P-selectin inhibitor fucoidan and a blocking antibody against PSGL-1 attenuated the NECA-induced adhesion of eEPCs to MCEC-1 cells. This inhibition, however, was partial suggesting that multiple adhesion molecules may be involved in this process.
To evaluate whether our proof-of-concept studies in a murine progenitor cell model can be applied to human progenitor cells, key experiments were performed using human adult culture–expanded EPCs. Indeed, we found that stimulation of adenosine receptors increased adhesion of human EPCs to cardiac microvascular endothelial cells. These results, obtained in murine and human cells, may have important implications not only for our understanding of molecular mechanisms of neovascularization but also for a novel therapeutic use of adenosine. There is growing interest in cell-based therapeutic approaches to improve vascularization of ischemic organs, including the heart. One of the major problems for the therapeutic use of EPCs is that a majority of injected cells may pass through the targeted organ (eg, heart) and accumulate in other organs such as spleen, liver, and kidney.2 In this study, we demonstrated that adenosine promotes EPC retention in vasculature of isolated hearts, suggesting its potential use for improvement of cell delivery. Adenosine can be given directly into the coronary circulation, and its extremely short half-life in the bloodstream provides the unique advantage of increasing EPC retention locally. Intracoronary adenosine has been administered in humans without significant adverse events.28,29 Our data suggest that adenosine could further improve delivery of progenitor cells by increasing their adhesion to cardiac endothelium, a particularly appealing prospect because of the clinical availability of adenosine. Future research may validate the utility of this approach.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants R01 HL76306 and R01 HL083958, American Heart Association Southeastern Affiliate Grant-in-Aid 0755221B, the Vanderbilt University Discovery program, and funds from the Departments of Medicine and Cardiac Surgery of Vanderbilt University.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
Dr. Hatzopoulos received an honorarium from Momenta Pharmaceuticals for a lecture on EPCs.
References
- 1.Bartunek J, Vanderheyden M, Wijns W, Timmermans F, Vandekerkhove B, Villa A, Sanchez PL, Arnold R, San Roman JA, Heyndrickx G, Fernandez-Aviles F. Bone-marrow-derived cells for cardiac stem cell therapy: safe or still under scrutiny? Clin Pract Cardiovasc Med. 2007;4:S100–S105. doi: 10.1038/ncpcardio0744. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 3.Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos AK. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos AK. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Berne RM, Knabb RM, Ely SW, Rubio R. Adenosine in the local regulation of blood flow: a brief overview. Fed Proc. 1983;42:3136–3142. [PubMed] [Google Scholar]
- 7.Adair TH, Montani JP, Strick DM, Guyton AC. Vascular development in chick embryos: a possible role for adenosine. Am J Physiol. 1989;256:H240–H246. doi: 10.1152/ajpheart.1989.256.1.H240. [DOI] [PubMed] [Google Scholar]
- 8.Tornling G. Capillary neoformation in the heart and skeletal muscle during dipyridamole–treatment and exercise. Acta Pathol Microbiol Immunol Scand [A] 1982;278:1–63. [PubMed] [Google Scholar]
- 9.Ziada AM, Hudlicka O, Tyler KR, Wright AJ. The effect of long-term vasodilatation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res. 1984;18:724–732. doi: 10.1093/cvr/18.12.724. [DOI] [PubMed] [Google Scholar]
- 10.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 11.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003;92:485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 12.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A2A receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feoktistov I, Garland E, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I. Inhibition of human mast cell activation with the novel selective adenosine A2B receptor antagonist 3-isobutyl-8- pyrrolidinoxanthine (IPDX) Biochem Pharmacol. 2001;62:1163–1173. doi: 10.1016/s0006-2952(01)00765-1. [DOI] [PubMed] [Google Scholar]
- 14.Lidington EA, Rao RM, Marelli-Berg FM, Jat PS, Haskard DO, Mason JC. Conditional immortalization of growth factor-responsive cardiac endothelial cells from H-2Kb-tsA58 mice. Am J Physiol. 2002;282:C67–C74. doi: 10.1152/ajpcell.2002.282.1.C67. [DOI] [PubMed] [Google Scholar]
- 15.Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 16.Teleron AA, Carlson B, Young PP. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion. 2005;45:21–25. doi: 10.1111/j.1537-2995.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 17.Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J Pharmacol Exp Ther. 2007;382:565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- 18.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2005;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DA, Smith CW, McIntire LV. Effects of fluid shear stress on leukocyte adhesion to endothelial cells. In: Granger DN, Schonbein GW, editors. Physiology and Pathophysiology of Leukocyte Adhesion. New York: Oxford University Press; 1995. pp. 148–168. [Google Scholar]
- 20.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handa K, Nudelman ED, Stroud MR, Shiozawa T, Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Lea and sialosyl-Lex, and sulfated glycans modulate this binding. Biochem Biophys Res Commun. 1991;181:1223–1230. doi: 10.1016/0006-291x(91)92069-v. [DOI] [PubMed] [Google Scholar]
- 22.Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;92:2201–2206. [PubMed] [Google Scholar]
- 23.Zahler S, Becker BF, Raschke P, Gerlach E. Stimulation of endothelial adenosine A1 receptors enhances adhesion of neutrophils in the intact guinea pig coronary system. Cardiovasc Res. 1994;28:1366–1372. doi: 10.1093/cvr/28.9.1366. [DOI] [PubMed] [Google Scholar]
- 24.Bouma MG, van den Wildenberg FA, Buurman WA. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol. 1996;270:C522–C529. doi: 10.1152/ajpcell.1996.270.2.C522. [DOI] [PubMed] [Google Scholar]
- 25.Vischer UM, Wollheim CB. Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood. 1998;91:118–127. [PubMed] [Google Scholar]
- 26.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 27.Chlopicki S, Jakubowski A, Niezabitowski P, Lomnicka M, Gryglewski RJ. Kinetics of purine release from ischemic isolated guinea pig heart. Exp Clin Cardiol. 1998;3:59–64. [Google Scholar]
- 28.Leesar MA, Stoddard M, Ahmed M, Broadbent J, Bolli R. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation. 1997;95:2500–2507. doi: 10.1161/01.cir.95.11.2500. [DOI] [PubMed] [Google Scholar]
- 29.Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–2159. doi: 10.1161/01.cir.101.18.2154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.