Abstract
OBJECTIVE
Our a priori hypothesis was that depressed patients with diabetes in practices implementing a depression management program would have a decreased risk of mortality compared to depressed patients with diabetes in usual care practices.
RESEARCH DESIGN AND METHODS
Multi-site practice-randomized controlled trial PROSPECT (Prevention of Suicide in Primary Care Elderly: Collaborative Trial) with patient recruitment from 5/99-8/01 and supplemented with a search of the National Death Index. Twenty primary care practices participated from New York City, Philadelphia, and Pittsburgh. In all, 584 participants who were identified though a two-stage, age-stratified (60-74; 75+) depression screening of randomly sampled patients and were classified as depressed with complete information on diabetes status are included in these analyses. Of all the 584 participants, 123 (21.2%) reported a history of diabetes. A depression care manager worked with primary care physicians to provide algorithm-based care. Vital status was assessed at 5 years.
RESULTS
After a median follow-up of 52.0 months, 110 depressed patients had died. Depressed patients with diabetes in the Intervention Condition were less likely to have died during the 5-year follow-up interval than were depressed persons with diabetes in Usual Care after accounting for baseline differences among patients (adjusted hazard ratio 0.49, 95% CI [0.24, 0.98]).
CONCLUSIONS
Older depressed primary care patients with diabetes in practices implementing depression care management were less likely to die over the course of a 5-year interval than were depressed patients with diabetes in usual care practices.
Keywords: aged, depression, diabetes, mortality, primary health care
Diabetes and depression are two of the most common problems seen in primary care settings. Epidemiologic data indicate that diabetes and depression are intimately related. Depression is a risk factor for diabetes (1) and risk of depression is increased by a factor of two in patients with diabetes (2). Depression is not only common in patients with diabetes but also contributes to poor adherence to medication and dietary regimens, poor glycemic control, reduced quality of life, and increased health care expenditures (3). Depression has been specifically linked to prognostic variables in diabetes such as micro- and macrovascular complications (4). Evidence from intervention trials shows that treatment of depression in patients with diabetes improves depression (5-7) but findings regarding improvement in glycemic control have been mixed (5, 8, 9). Although cohort studies document that depression is associated with increased risk of death among persons with diabetes (10-13), no known intervention study has evaluated whether treatment for depression modifies this increased risk of mortality among older primary care patients with diabetes.
We investigated the relationship between diabetes, depression treatment, and all-cause mortality using data from the multi-site, randomized trial, PROSPECT (Prevention of Suicide in Primary Care Elderly: Collaborative Trial), supplemented with a search of the National Death Index. The study intervention was implemented at the practice level and involved a depression care manager working with physicians to provide algorithm-based treatment and on-going care management. Overall, intervention patients had a more favorable course of depression in both degree and speed of symptom reduction compared to usual care patients (14). We took the opportunity afforded by PROSPECT to evaluate the effect of diabetes and of depression care management on all-cause mortality for the following reasons. While multiple medical conditions are of interest in this intervention trial, depression associated with diabetes has been shown to increase the risk of death (10-13). Furthermore, the demonstrated morbidity, mortality, and health services implications of diabetes and depression separately (15-17) support both the understanding of the enormous public health significance of the co-occurrence of diabetes and depression, and the urgency to finding evidence-based solutions to reduce the burden of these conditions. We hypothesized that depressed older adults with diabetes in practices randomized to the intervention condition would be less likely to die over a 5-year follow-up interval compared to depressed older adults with diabetes in usual care.
RESEARCH DESIGN AND METHODS
The PROSPECT Study
The PROSPECT Study compared a primary care based intervention with usual care in improving the outcomes of depression. All study procedures were implemented with written informed consent, and the study protocols were approved by the Institutional Review Board of Cornell University, the University of Pittsburgh, and the University of Pennsylvania Schools of Medicine. Details of the study design of the PROSPECT Study are available elsewhere (14). In brief, twenty primary care practices from greater New York City, Philadelphia, and Pittsburgh participated in the study from 5/99 to 8/01, with individual patients followed clinically for 2 years. Practices ranged in size (solo to medium sized), setting (sparsely populated, suburban, and urban), population type (including two serving primarily African-American patients), and affiliation (16 community-based and 4 academic practices). Practices were paired by region (urban versus suburban/sparsely populated), affiliation, size, and population type. Within the 10 pairs, practices were randomly assigned by coin flip to the Intervention Condition or Usual Care (described below). A two-stage sampling design was used to recruit patients. First, an age-stratified (60-74 years, 75 years and older), random sample of patients with an upcoming appointment was obtained. The sampled patients were mailed a letter allowing patients to decline. Second, trained lay interviewers telephoned the patients who did not decline. Patients who gave oral consent were assessed for enrollment using the Centers for Epidemiologic Studies Depression scale (CES-D) (18). All patients with a CES-D score >20 were invited into the study as were those from a 5% random sample of patients with lower scores. Patients with a CES-D score ≤20 and who were not selected randomly were also recruited if they responded positively to supplemental questions about mood, prior depressive episodes, or treatment. A positive response to the supplemental questions triggered a diagnostic assessment.
The intervention, described in detail elsewhere (14), consisted of trained depression care managers offering guideline-concordant recommendations to the primary care physicians and helping patients with treatment adherence. The care managers monitored psychopathology, treatment adherence, response, and side effects and provided follow-up care at predetermined intervals or when clinically necessary. Patients who refused antidepressants were offered interpersonal psychotherapy (IPT) by the depression care managers. In the Intervention Condition (IC), a first-line antidepressant (citalopram, a selective serotonin reuptake inhibitor) and the IPT were provided at no cost. In Usual Care (UC), physicians were informed of patients’ depression diagnoses. Physicians also received informational materials and treatment guidelines for geriatric depression. No specific recommendations were given to these physicians regarding individual patients except for psychiatric emergencies. The types and proportions of treatment received over time by persons in practices randomized to the Intervention Condition or Usual Care have been previously published (14, 19).
Measurement Strategy
Trained research assistants assigned depression diagnoses to patients using the Structured Clinical Interview for Axis I DSM-IV Diagnoses (SCID) (20). Severity of depression was assessed using the 24-item Hamilton Depression Rating Scale (HDRS) (21).
Persons were classified as having a medical comorbidity and as having diabetes by self-report. The questionnaire used was based on the Charlson Comorbidity Index (22). To assess for diabetes, participants were asked “Have you ever been told you have diabetes or high blood sugar?” For the current analysis, patients were considered to have diabetes if they reported having been told they had diabetes or high blood sugar.
We used standard questions to obtain information from the respondents on age, level of educational attainment, gender, marital status, and self-reported ethnicity. Smoking status was based on report of smoking within 6 months of interview. The Philadelphia Multidimensional Assessment Instrument (MAI) assessed instrumental activities of daily living and mobility (23). The Scale for Suicidal Ideation (SSI) measured presence of suicidal ideation (24). The Mini-Mental State Examination (MMSE) is a short standardized mental status examination that has been widely employed for clinical and research purposes (25).
Ascertainment of vital status
Vital status in this investigation was based on follow-up of participants employing the National Center for Health Statistics (NCHS) National Death Index (NDI Plus) (26). Because obtaining vital status requires that we provide personally identifiable information to the NCHS for NDI searches, confidentiality safeguards warrant discussion here. We did not transmit any PROSPECT study data (e.g., information about depression status, physical disorders, or functional status) with identifying data, nor did we transmit identifying data via e-mail. Upon obtaining vital status data, the University of Pennsylvania Data Core sent the data to the sites for verification. Study sites then sent the data file -- stripped of any identifying data -- to the University of Pennsylvania Data Core for final production of the study data linked to vital status for analysis. The time frame for the ascertainment of vital status was the period of five years from overall commencement of the PROSPECT study.
Analytic strategy
Our analysis involved sorting patients into four groups according to whether patients self-reported diabetes at baseline and practice assignment (IC or UC). We carried out survival analysis adjusting for within-practice clustering (27). The Cox proportional hazards model for clustered data was used to explore the effect of variables on survival. Point estimates and associated 95% confidence intervals are provided for the unadjusted and adjusted hazard ratios (as in previous work (14, 28)). Survival curves were prepared using the method of Kaplan and Meier (29) to illustrate the mortality of each group defined by patient diabetes status at baseline and practice randomization assignment to Intervention Condition or Usual Care. We began by exploring potential confounding variables using univariate models with baseline characteristics as predictors of time to death. Our final model included influential covariates identified by their association (p < 0.10) with the outcome of interest, time to death. The final model included terms to adjust for baseline differences in age, gender, education, ethnicity, smoking status, number of medical conditions, number of disabilities, and cognition.
We have been guided by published criteria for performing and reporting subgroup analyses (30, 31). Evaluating our prespecified study hypothesis required a test for effect modification of intervention condition assignment on the risk of death by baseline diabetes status. The formal test for effect modification involved introducing terms representing interaction into the Cox model, in addition to main effects for diabetes status and intervention condition. Consistent with the literature (32), we set α at 0.10 to denote statistical significance for the interaction term in the Cox proportional hazards model. SAS version 9.1 was used to carry out analyses (SAS Institute Inc., Cary, North Carolina).
RESULTS
Study sample
The CONSORT flow diagram for the PROSPECT trial has been published elsewhere (14). In brief, the study screened 9,072 older persons, and 1,888 persons were invited to participate. Out of the 1,888 persons invited to participate, 1,238 (65.8%) agreed to a baseline interview. Our study sample included 599 depressed patients, of whom 396 (66.1%) met DSM-IV criteria for major depression. Fifteen people were excluded due to missing data on baseline diabetes status, leaving a sample size of 584 for this analysis.
Sample characteristics
The mean age of our study sample was 70.3 years with a standard deviation of 7.9 years. The age range was 60 to 94 years. Four hundred and twenty-two (72.3%) of the participants were women. The self-identified ethnic groups of the participants consisted of 407 whites (69.7%), 161 African-Americans (27.6%), and 16 American Indians, Hispanics or Asians (2.7%). Of all 584 participants, 123 (21.2%) reported a history of diabetes. Table 1 compares baseline characteristics between patients in the IC and UC practices, stratified by diabetes status. After 5 years, 110 depressed patients had died. Only one documented suicide occurred during the study in a depressed patient with diabetes in an intervention practice. The median length of follow-up in ascertainment of vital status was 52.0 months (range 0.8 to 67.7 months).
Table 1.
Characteristics of the study sample according to randomization assignment of primary care practice and diabetes status at baseline. Unless noted otherwise, entries represent numbers with percents based on the total number in the corresponding column in parentheses. Test of equality across groups based on regression models. Data gathered from the PROSPECT study
| All Depressed | Diabetes Intervention |
Diabetes Usual Care |
No Diabetes Intervention |
No Diabetes Usual Care |
Test of equality across groups (p-value)1 |
|---|---|---|---|---|---|
| (n =70) | (n =53) | (n=241) | (n=220) | ||
| Sociodemographic characteristics | |||||
| Age, mean in years (s.d.) | 71 (8.5) | 67 (6.8) | 71 (7.6) | 71 (8.1) | 0.0004 |
| Education, mean in years (s.d.) | 12 (3.0) | 12 (3.2) | 13 (3.3) | 13 (3.3) | 0.0006 |
| Women | 50 (71) | 37 (70) | 167 (69) | 168 (76) | 0.3944 |
| Ethnic minority | 23 (33) | 27 (51) | 58 (24) | 69 (31) | 0.0802 |
| Married | 22 (31) | 21 (40) | 91 (38) | 81 (37) | 0.5074 |
| Medical conditions | |||||
| Current smoker | 9 (13) | 3 (6) | 26 (11) | 12 (5) | 0.2472 |
| Medical conditions, mean (s.d.) | 5 (2.9) | 5 (2.4) | 2 (1.9) | 2 (1.9) | <0.0001 |
|
Number of disabilities, mean (s.d.) MAI score |
3 (2.3) | 3 (2.2) | 2 (1.9) | 2 (1.8) | 0.0040 |
| Baseline depression and cognitive status | |||||
|
Depression severity, mean (s.d.) HDRS score |
18 (5.3) | 19 (5.6) | 18 (6.3) | 17 (5.8) | 0.1789 |
| Suicidal ideation (SSI score > 0) | 23 (33) | 12 (23) | 67 (28) | 44 (20) | 0.0997 |
|
Cognitive function, mean (s.d.) MMSE score |
27 (4.2) | 27 (2.6) | 28 (2.4) | 27 (2.5) | 0.0848 |
CI, confidence interval; MAI, Multidimensional Assessment Instrument; HDRS, Hamilton Depression Rating Scale; MMSE, Mini-Mental State Examination; s.d., standard deviation. The range of scores for the MMSE is 0 to 30 (inclusion criteria limited range to 18-30), with high scores indicating less severe cognitive impairment; HDRS range, 0 to 76, with high scores indicating greater depressive symptoms; and SSI range, 0 to 38, with high scores indicating greater suicidal ideation.
Univariate logistic or linear regression model with random effects.
Mortality risk according to diabetes status
Depressed patients with diabetes in the Intervention Condition experienced a mortality rate of 68.2/1000 person years (95% CI [41.0, 106.5]) whereas depressed patients with diabetes in Usual Care experienced a mortality rate of 103.4/1000 person years (95% CI [63.2, 159.7]). Persons without diabetes experienced similar mortality rates in the Intervention Condition and in Usual Care (mortality rate 36.0/1000 person years (95% CI [25.3, 49.6]) versus mortality rate 38.2/1000 person years (95% CI [26.4, 53.3])).
Table 2 provides unadjusted and adjusted hazard ratio estimates according to diabetes status. In the univariate model, depressed patients with diabetes in the Intervention Condition were less likely to have died during the 5-year follow-up interval than were depressed patients with diabetes in Usual Care (unadjusted hazard ratio 0.66) but the 95% confidence bounds included the null (95% CI [0.36, 1.21]). The final model accounted for baseline imbalances in age, gender, education, ethnicity, smoking status, number of medical conditions, number of disabilities, and cognition among patients. Depressed patients with diabetes in the Intervention Condition were significantly less likely to have died during the 5-year follow-up interval than were depressed persons with diabetes in Usual Care (adjusted hazard ratio 0.49, 95% CI [0.24, 0.98]). In contrast, depressed patients without diabetes in the Intervention Condition were not at decreased risk compared to those without diabetes in Usual Care (adjusted hazard ratio 0.79, 95% CI [0.42, 1.47]). The interaction of randomization group by diabetes status was statistically significant (p-value = 0.04). We have provided Kaplan-Meier curves according to diabetes status for the Intervention Condition versus Usual Care (Figure 1).
Table 2.
Relationship of practice random assignment, patient baseline diabetes status, and mortality during a 5-year follow-up interval. Hazard ratio estimates with 95% confidence intervals shown in brackets. Data gathered from the PROSPECT study
| Practice randomization assignment |
Patient baseline diabetes status |
Unadjusted hazard ratio (Intervention vs. Usual Care) stratified by diabetes status |
Adjusted hazard ratio (Intervention vs. Usual Care)* stratified by diabetes status |
|---|---|---|---|
| Intervention | Diabetes | 0.66 [0.36, 1.21] |
0.49 [0.24, 0.98] |
| Usual care | Diabetes | ||
| Intervention | No Diabetes | 0.94 [0.58, 1.52] |
0.79 [0.42, 1.47] |
| Usual care | No Diabetes | ||
adjusted for baseline age, gender, education, ethnicity, smoking status, number of medical conditions, number of disabilities, and cognition.
Figure 1.
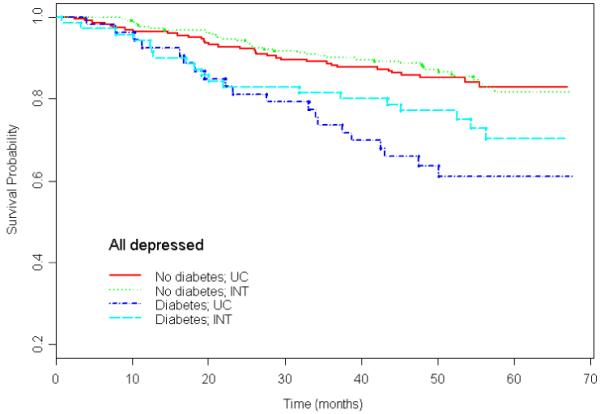
Survival curves for patients with diabetes (n = 53) and patients without diabetes (n = 220) in practices randomized to Usual Care and patients with diabetes (n = 70) and patients without diabetes (n = 241) patients randomized to practices in the Intervention Condition. Data gathered from the PROSPECT study.
CONCLUSIONS
Depressed older adults with diabetes who were in practices randomized to the Intervention Condition were less likely to have died at the end of the 5-year follow-up interval than were depressed persons with diabetes in Usual Care after adjustment for baseline differences. Depressed patients without diabetes in the Intervention Condition were not at decreased risk compared to depressed patients without diabetes in Usual Care. The intervention attenuates the influence of diabetes on mortality risk among older adults with depression. We believe these findings support the integration of depression evaluation and treatment with diabetes management in primary care.
Before discussing our findings, the results must first be considered in the context of potential study limitations. First, we obtained our results from primary care sites in greater New York City, Philadelphia, and Pittsburgh, whose patients may not be representative of other primary care practices in the United States. However, the participating sites were diverse practices of varying size located in urban, suburban, and sparsely populated areas. Second, diabetes mellitus was based on self-report alone; however, relying on medical records may also be incomplete because many persons receive health care from providers in multiple systems. Studies have shown that self-reported data on diabetes as well as other chronic diseases is reliable (33). Third, the question regarding diabetes might have included patients with impaired glucose tolerance who did not have diabetes. However, misclassification of some persons with impaired glucose tolerance as having diabetes would lead to a conservative bias towards the null (i.e., that there was no intervention effect for patients with diabetes on mortality). Fourth, the mortality reduction among depressed patients with diabetes randomized to the intervention may be due to factors other than the specific effects of a depression management program. For example, we have only a limited ability to address whether those patients with diabetes in the intervention practices were seen more frequently for reasons other than depression by their physicians; and similarly, we do not have information on specific diabetes outcomes such as hemoglobin A1c. Fifth, misclassification of vital status was also a potential limitation. However, overall sensitivity of the NDI for ascertainment of vital status has generally been well over 90% in most studies (34).
We selected patients with diabetes as a subgroup from the larger intervention trial (14, 35) realizing that we must proceed with caution about the inferences we make. Statisticians are wary of subgroup analyses, but clinicians must make decisions about individual patients (30, 36, 37). At the same time, large-scale intervention studies carried out in primary care practice are limited, so we need to make the most of the data we have from intervention studies. Guided by published criteria for performing and reporting subgroup analyses (30, 31), we have identified a group, older persons with diabetes, for whom risk of death has been reported to be increased (10-13). In addition, the link between diabetes, depression, and the outcome (mortality) may have common pathophysiologic mechanisms (38, 39). Finally, uncertainty persists about the influence of treatment of depression on outcomes for diabetes and other medical co-morbidity (8, 9). Consistent with recommendations regarding subgroup analyses, we reported the statistical significance of the interaction between intervention assignment and the condition of interest on the outcome (32, 40) and adjusted our estimates for potential imbalances in covariates across treatment groups (41).
Despite some limitations, our study warrants attention because older depressed primary care patients with diabetes in practices implementing depression care management were significantly less likely to die over the course of a 5-year interval than were depressed patients with diabetes in usual care practices. To our knowledge, this is the first study to report on the relationship between diabetes and mortality in a depression intervention trial. A formal test of the interaction between intervention assignment and diabetes on the outcome of interest, all-cause mortality, was significant (32, 40). This suggests that persons with diabetes were more likely to benefit from the intervention than were persons without diabetes. Adjustment of the hazard ratio for imbalance in the distribution of baseline covariates assessed at baseline can be expected to yield estimates of the hazard closer to the true estimate of the treatment effect (40, 41). Because our sample was derived from primary health care, the public health significance of these findings is high.
The combination of clinical evaluation and monitoring, pharmacotherapy, and in some cases, interpersonal psychotherapy in the PROSPECT intervention appears to be effective in depressed patients with diabetes in reducing all-cause mortality risk. We realize that our study does not examine potential mechanisms underlying the relationship between the PROSPECT intervention and a decreased mortality risk among depressed patients with diabetes. Both physiologic factors such as increased inflammation (38, 39) and poor glucose regulation (3, 4) and behavioral processes such as poor adherence (3) may link depression with increased mortality in patients with diabetes. The potential mediators between treatment assignment and outcomes for patients with diabetes deserve further study.
Our results add to the literature on clinical trial outcomes from treatment of depression in patients with diabetes. Specifically, the collaborative care model for depression, of which PROSPECT is one example, has been found to improve depression care and depression outcomes in patients with diabetes. The Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) trial found that depressed older adults with diabetes in a depression care management intervention had better depression outcomes at one year when compared to depressed older adults with diabetes in usual care although hemoglobin A1c levels were unaffected by the intervention (9). The authors point out that because patients had good glycemic control at baseline, power to detect small but clinically important improvements in glycemic control was limited. The Pathways Study randomized 329 patients with diabetes and comorbid major depression or dysthymia to depression care management or usual care and found that although depression outcomes were improved, no differences in hemoglobin A1c levels were observed (8). However, these authors also point out that the patients in the Pathways Study had good glycemic control at baseline.
In summary, our investigation adds new evidence to the literature on depression and diabetes, by examining whether the PROSPECT intervention influenced survival among depressed older primary care patients with diabetes. Specifically, these results indicate that a depression care management intervention can significantly reduce all-cause mortality among depressed patients with diabetes. These results should propel the development and dissemination of models of care that better integrate depression management for people with diabetes.
ACKNOWLEDGEMENT
Funding: PROSPECT was a collaborative research study funded by the National Institute of Mental Health. The mortality follow-up of PROSPECT participants was funded by the National Institute of Mental Health (R01 MH065539). Participation of Dr. Bogner, Dr. Post, and Dr. Bruce was also supported by NIMH awards: K23 MH67671, K23 MH01879, and K02 MH01634. Dr. Bogner is a Robert Wood Johnson Foundation Generalist Physician Faculty Scholar (2004-2008).
Footnotes
Trial registration: ClinicalTrials.gov identifier: NCT00000367
REFERENCES
- 1.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19(10):1097–102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 2.Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53(4):903–6. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 3.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19(2):113–22. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59(3):241–50. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129(8):613–21. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):618–23. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 8.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–9. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 9.Williams JW, Katon W, Lin EHB, Noel PH, Worchel J, Cornell J, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Annals of Internal Medicine. 2004;140(12):1015–1024. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. 2005;161(7):652–60. doi: 10.1093/aje/kwi089. [DOI] [PubMed] [Google Scholar]
- 11.Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28(11):2668–72. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 12.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–8. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 13.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28(6):1339–45. doi: 10.2337/diacare.28.6.1339. [DOI] [PubMed] [Google Scholar]
- 14.Bruce ML, Ten Have TR, Reynolds CF, 3rd, Katz II, Schulberg HC, Mulsant BH, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. Jama. 2004;291(9):1081–91. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 15.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray C, editors. Global Burden of Disease and Risk Factors. Oxford University Press and The World Bank; Washington, D.C.: 2006. [PubMed] [Google Scholar]
- 16.Gallo JJ, Bogner HR, Morales KH, Post EP, Ten Have T, Bruce ML. Depression, cardiovascular disease, diabetes, and two-year mortality among older, primary-care patients. American Journal of Geriatric Psychiatry. 2005;13:748–755. doi: 10.1176/appi.ajgp.13.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter GM, Bell RM, Dubois RW, Goldberg GA, Keeler EB, McAlearney JS, et al. A clinically detailed risk information system for cost. Health Care Financ Rev. 2000;21(3):65–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 19.Schulberg HC, Post EP, Raue PJ, Have TT, Miller M, Bruce ML. Treating late-life depression with interpersonal psychotherapy in the primary care sector. Int J Geriatr Psychiatry. 2007;22(2):106–14. doi: 10.1002/gps.1700. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) American Association Press, Inc.; Washington, D.C.: 1995. [Google Scholar]
- 21.Hamilton M. A rating scale for depression. Journal of Neurology and Neurosurgical Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. J Gerontol. 1982;37(1):91–9. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- 24.Beck A, Brown G, Steer R. Psychometric characteristics of the scale for suicide. Ideation with psychiatric outpatients. Behavior Research and Therapy. 1997;35:1039–1046. doi: 10.1016/s0005-7967(97)00073-9. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Doody MM, Hayes HM, Bilgrad R. Comparability of National Death Index Plus and standard procedures for determining causes of death in epidemiologic studies. Annals of Epidemiology. 2001;11:46–50. doi: 10.1016/s1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Wei L, Amato D. Survival Analysis: State of the Art. Kluwer Academic Publishers; Norwell, MA: 1992. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. [Google Scholar]
- 28.Bogner HR, Ford DE, Gallo JJ. The role of cardiovascular disease in the identification and management of depression by primary care physicians. Am J Geriatr Psychiatry. 2006;14(1):71–8. doi: 10.1097/01.JGP.0000192479.82189.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 30.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365(9454):176–86. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 31.Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Ann Intern Med. 1992;116(1):78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- 32.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7(4):267–75. doi: 10.1016/0197-2456(86)90034-6. [DOI] [PubMed] [Google Scholar]
- 33.Bowlin SJ, Morrill BD, Nafziger AN, Lewis C, Pearson TA. Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the behavioral risk factor survey. J Clin Epidemiol. 1996;49(5):511–7. doi: 10.1016/0895-4356(96)00010-8. [DOI] [PubMed] [Google Scholar]
- 34.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40(9):808–13. doi: 10.1097/00043764-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Gallo JJ, Bogner HR, Morales KH, Post EP, Lin JY, Bruce ML. The effect on mortality of a practice-based depression intervention program for older adults in primary care: A cluster randomized trial. Annals of Internal Medicine. 2007;146(10):689–98. doi: 10.7326/0003-4819-146-10-200705150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82(4):661–87. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senn S. Individual response to treatment: is it a valid assumption? Bmj. 2004;329(7472):966–8. doi: 10.1136/bmj.329.7472.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–29. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 39.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54(3):248–61. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 40.Lu M, Lyden PD, Brott TG, Hamilton S, Broderick JP, Grotta JC. Beyond subgroup analysis: improving the clinical interpretation of treatment effects in stroke research. J Neurosci Methods. 2005;143(2):209–16. doi: 10.1016/j.jneumeth.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Buyse M. Analysis of clinical trial outcomes: some comments on subgroup analysis. Controlled Clinical Trials. 1989;10:187S–94S. doi: 10.1016/0197-2456(89)90057-3. [DOI] [PubMed] [Google Scholar]


