Abstract
Adjuvants are commonly used in vaccines to augment immune response, but how the inflammatory cytokines elicited by adjuvants directly influence effector and memory CD8 T cell differentiation remains poorly characterized. Here, we used a peptide-pulsed dendritic cell (DC) vaccination model to examine the role of primary cytokines, IL-12 and IFNγ, elicited by CpG-B adjuvant on CD8 T cell priming and memory CD8 T cell development. During DC vaccination, simultaneous exposure to antigen and a heterologous Listeria infection, CpG-B or IL-12 enhanced a portion of the effector CD8 T cells to expand and differentiate to a larger extent. Simultaneously, this also decreased their ability to become long-lived memory CD8 T cells. However, development of memory CD8 T cells and their precursors was largely unaffected by the additional inflammatory cytokines. Moreover, IL-12 production by the antigen-presenting cell (APC) was not required during DC+CpG vaccination or Listeria infection, but rather ‘bystander’ macrophages and DCs appeared to be the physiologically relevant cellular sources of this cytokine. Furthermore, IFNγ induced by CpG was required in vivo for optimal production of IL-12, which in turn, influenced effector CD8 T cell longevity. Together, these findings demonstrate the importance of an interconnected multicellular network between APCs, naïve T cells and bystander cells of the innate immune system that regulate effector and memory CD8 T cell development during vaccination.
Keywords: IL-12, IFNγ, memory T cell, CD8, dendritic cell vaccination
Introduction
Vaccination is the most successful medical intervention against infectious disease[1]. The fundamental goal of vaccines is to generate long-lived memory T and B cells and plasma cells to protect against secondary infection[2]. Some successful Vaccines that have used attenuated live viruses, such as small pox, offer several advantages in that they productively stimulate the innate and the adaptive immune system without causing severe illness due to impaired replication abilities. However, attenuated forms of many pathogens are not available and therefore, a need for devising safe and effective vaccines for several types of infectious diseases exists[3]. Alternative forms of vaccination that deliver antigens via cellular or non-cellular vehicles offer increased safety, but these require accessory adjuvants to activate the innate immune system to enhance costimulation and expression of cytokines that promote activated T cell expansion and differentiation into effector and memory T cells[4, 5]. Details on the cytokines induced by different adjuvants and how they influence effector T cell differentiation and subsequent memory T cell formation require further investigation.
Both the expansion and differentiation of effector CD8 T cells can be influenced by particular inflammatory cytokines present at the time of T cell priming[6–8]. Most notably, IFNα/β, IFNγ and IL-12 have been shown to regulate effector CD8 T cell expansion[6, 9–11]. These cytokines are also critical to enhancing the antiviral and cytolytic activity of activated CD8 T cells[9–11]. However, recent work from several groups showed that IL-12 and IFNγ, also regulate effector CD8 T cell contraction and memory CD8 T cell formation[12–17]. Our prior work suggests that inflammatory signals help to generate short-lived effector CD8 T cells (SLECs), which develop distinctly from memory precursor effector cells (MPECs)[18]. During acute LCMV and Listeria infections the SLECs and MPECs can be distinguished fairly well based on inverse expression patterns of the receptors KLRG1 (Killer cell lectin-like receptor G1) and IL-7R[13, 15, 18, 19]. For the most part, SLECs are KLRG1hi IL-7Rlo and MPECs are KLRG1lo IL-7Rhi. The majority of SLECs die during the contraction phase and do not proliferate well in response to antigen or the cytokines IL-15 and IL-7[13, 18, 20–22]. In contrast, many of MPECs survive and develop into long-lived memory CD8 T cells that can self-renew and respond robustly to secondary infection. Thus, SLECs appear to be a more terminally differentiated effector CD8 T cell population and their formation is promoted by certain inflammatory cytokines including IL-12 and IFNγ[13, 23]. However, the direct role of IL-12 in SLEC formation during CD8 T cell priming in vivo has not been well characterized.
In order to improve vaccine development, many important questions remain to be investigated with regard to how the adaptive immune system incorporates antigenic, costimulatory and inflammatory signals. In particular, how do these signals individually influence whether an effector CD8 T cell becomes short-lived or long-lived? One limitation in previous studies has been the lack of distinction between inflammatory and antigenic and costimulatory signals due to the complex nature of immune responses. In an attempt to simplify this situation, we used a peptide-pulsed DC immunization system to study how antigenic and inflammatory signals are individually or cooperatively involved in effector and memory CD8 differentiation in vivo. In addition, DC vaccination itself is an attractive strategy because it utilizes the optimal antigen presenting cells (APCs) for T cells, and DC vaccination is currently a promising form of immunization for treating cancer. However, the qualitative and quantitative effects of DC vaccination with or without supplementary adjuvants on memory CD8 T cell development remain poorly characterized. Here, we address more precisely how certain adjuvants and the cytokines they produce regulate effector and memory CD8 T cell expansion, differentiation and longevity.
Materials and Methods
Mice
Thy1.1+ P14 TCR tg mice have been described previously[18]. To make “P14 chimeric mice”, ~1×104 Thy1.1+ P14 CD8 T cells were transferred into naïve Thy1.2+ C57BL/6 (B6) mice. B6 mice were purchased from Natl. Cancer Institute (NCI, Charles River) and IFNγ−/− (B6.129S7-Ifngtm1Ts/J), IFNγR1−/− (B6.129S7-Ifngr1tm1Agt/J), IL-12p35−/− (B6;129S-Il12atm1Jm/J), IL-12p40−/− (B6.129S1-Il12btm1Jm/J), IL-12Rβ2−/− (B6.129S1-Il12rb2tm1Jm/J), Itgax-DTR/GFP mice were purchased from Jackson Laboratories (Bar Harbor, ME). P14 IFNγR1−/− Thy1.1+CD8 T cells were a kind gift of Dr. Lindsay Whitton (The Scripps Research Institute, La Jolla, CA.)[11]. Kb−/− Db−/− mice were purchased from Taconic (Hudson, NY). All animal experiments were performed under approved Institutional Animal Care and Use Committee protocols.
Antibodies and surface and intracellular staining
Lymphocyte isolation, GP33–41 peptide stimulations and surface/intracellular staining was performed as described. All antibodies were purchased from E-biosciences (San Diego, CA) except anti-GranzymeB-PE (Caltag, Burlingame, CA) and IL-12p40-PE (Becton-Dickinson (BD) Biosciences, San Jose, CA). Anti-KLRG1 (2F1) hybridoma was a generous gift from Dr. Raulet (University of California, Berkley, CA) and was conjugated to alexa-647 (Invitrogen, Eugene, OR). Mice were immunized with CpG-B 1826 (10μg) or infected with LM (2×104 cfu) for 6h or 24h respectively. Splenocytes were harvested and cultured in vitro for 6h and BFA (BD) was added for the last 4h. Splenocytes were stained with different surface lineage makers, fixed, permeabilized and then stained intracellularly for IL-12p40 and IFNγ. All flow cytometry was analyzed on a FACSCalibur (BD) with FloJo software (Treestar, San Carlos, CA).
Generation of bone marrow-derived dendritic cells and DC immunization
Bone marrow-derived CD11c+ DCs were generated after 5 days of culture with GM-CSF and matured with LPS (50 ng/ml) overnight as described previously[24]. Matured DCs were pulsed with GP33–41 peptide (200ng/ml) for 2h, washed with PBS and 1×106 DC-33 cells were injected i.v. Simultaneously, DC immunized mice were injected i.p. with either CpG-B 1826 ODNs[12] (50μg), recombinant IL-12 (1μg) (R&D Systems Inc., Minneapolis, MN), IFNγ (30ng) (R&D Systems) or infected i.v. with 1×104 Lm strain XFL235 (kind gift of Dr. Hao Shen, University of Pennsylvania)[25].
ELISA
IL-12p70 and IL-12p40 were measured in mice sera using the OptEIA ELISA sets according to manufacturer’s instructions (BD).
Construction of bone marrow chimeras and LM infection
Kb−/− Db−/− recipient mice were irradiated (1,000 cGy) and reconstituted, in various combinations, with ~7×106 bone marrow cells (depleted of T and NK cells) from WT, IL-12p35−/− or Kb−/− Db−/− mice. Six weeks later, the level of engraftment was checked. Small numbers of purified P14 CD8 T cells were adoptively transferred into the mice, and infected with 1×107 actA-deficient recombinant Lm-33 (ΔActA Lm-33; strain XFL703, gift of Dr. H. Shen).
RESULTS
The impact of the inflammatory milieu on effector CD8+ T cell differentiation during DC immunization
To study the differential effects of antigenic stimulation and inflammation on effector and memory CD8+ T cell differentiation we designed an experimental system using DC immunization, in which exposure to inflammatory signals could be varied while keeping antigenic stimulation relatively constant. To this end, ‘P14 chimeric mice’ were generated in which a small number (~1×104) of naive Thy1.1+ P14 CD8+ T cells, which recognize the DbGP33–41 LCMV epitope, were transferred into wild type (WT) C57BL/6 mice. These mice were immunized with either LPS-matured bone marrow derived DCs coated with LCMV GP33–41 peptide alone (referred to as DC-33) or with DC-33 plus the TLR9 ligand CpG-B (referred to as CpG) or a heterologous Listeria monocytogenes (Lm) infection. The Lm infection serves as a source of inflammation, but does not provide antigen to the P14 CD8 T cells because it does not express GP33–41.
No expansion of P14 CD8 T cells was observed without DC-33 immunization (data not shown), but DC-33 immunization alone induced robust clonal expansion 7 days later. The responding CD8+ T cells differentiated into effector CD8 T cells that produced IFNγ and most were CD62Llo. However, these DC-33 primed effector CD8 T cells did not downregulate IL-7R or CD27 or upregulate KLRG1 and Granzyme B as commonly seen in acute LCMV and Listeria infections cells[13, 15] (Fig. 1A and B). Most of these cells appeared as KLRG1lo IL-7Rhi MPECs, and ~60% survived during the contraction phase with ~1×105 cells persisting as memory CD8 T cells 45 days later.
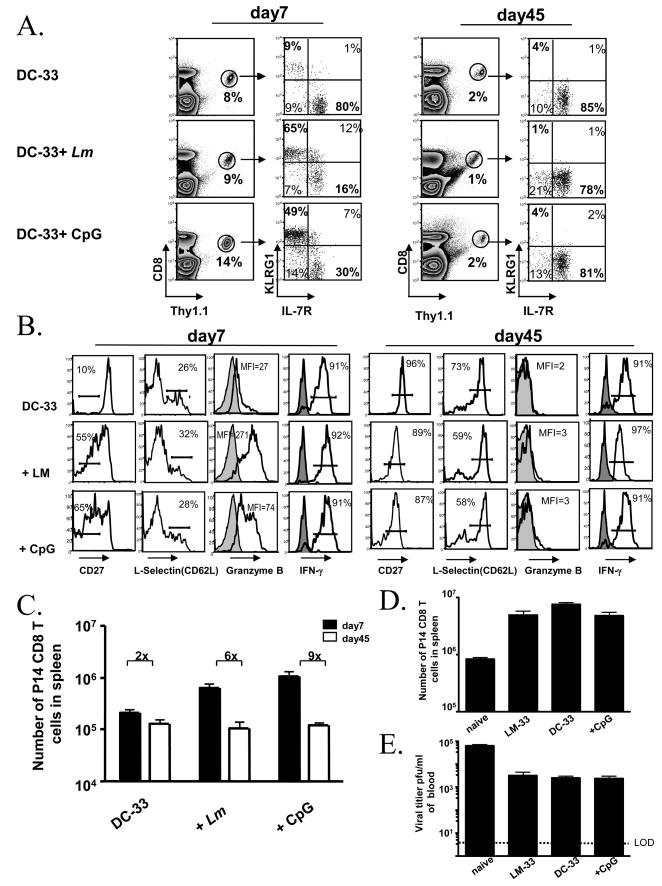
Figure 1. Inflammation enhanced effector CD8 T cell clone expansion and potency, but also reduced their memory potential during DC immunization.
(A and B) Naïve P14 chimeric mice were immunized with LPS-matured GP33–41-loaded bone marrow derived DCs (DC-33) alone or with heterologous Lm infection or CpG-B ODN. (A) FACS plots show expression of KLRG1 and IL-7R on splenic Thy1.1+ P14 CD8 T cells days 7 and 45 post-immunization. The percentage of P14 CD8 T cells within the total CD8 T cell population is indicated on contour plots. (B) Day 7 and 45 post immunization the P14 CD8 T cells were analyzed for expression of CD27, CD62L (L-selectin), Granzyme B and IFNγ. Histogram plots show expression of CD27, CD62L, Granzyme B directly ex vivo. For Granzyme B staining, CD44lo naïve CD8 T cells (shaded) are shown as controls. IFNγ production was assessed with (open) or without (shaded) 5 hr GP33–41 peptide stimulation in vitro. All plots are gated on donor Thy1.1+ P14 CD8 T cells. (C) Bar graphs show the number of splenic Thy1.1+ P14 CD8 T cells in (A) days 7 and 45 post-immunization. (D and E) Equal numbers of P14 naïve or memory CD8 T cells generated by DC-33 immunization alone, DC-33+CpG or by LM-33 infection were transferred into naïve mice and infected with LCMV clone13. Bar graphs show splenic numbers of memory CD8 T cells on day 8 p.i. (D) and viral titers on day 5 p.i. (E). LOD represents level of detection. Data are representative of at least two independent experiments.
When mice were immunized with DC-33+Lm or DC-33+CpG-B, effector CD8 T cell expansion was enhanced 3–4 fold compared to DC-33 alone (Fig. 1C). These effector T cells produced IFNγ and most were CD62Llo, but many cells also downregulated CD27 considerably and expressed more Granzyme B than those generated by DC-33 alone (Fig. 1B). When naïve CD8 T cells were primed with DC-33 plus Lm or CpG, at least two primary subsets of effector CD8 T cells could be distinguished based on KLRG1 and IL-7R expression. Similar to that found during acute infections [13], ~50–80% of the cells were KLRG1hi IL-7Rlo and ~20–30% were KLRG1lo IL-7Rhi (Fig. 1A). Nearly all of the KLRG1hi IL-7Rlo effector CD8 T cells generated were absent in the memory CD8 T cell population that formed 45 days later, suggesting these cells were short-lived like those found after LCMV and Listeria infection (Fig. 1A) The same was found in all tissues analyzed too (Supp. Fig. 1). Indeed, adoptive transfer of KLRG1hi IL-7Rlo effector cells, formed by DC-33+CpG priming, into naïve mice showed directly that these cells were short-lived because they disappeared within a few weeks after transfer (data not shown). For these reasons these cells will be referred to as SLECs hereafter. The phenotype of the P14 effector and memory CD8 T cells in the spleen was similar to that of the cells in multiple other organs (including blood, liver, lung and lymph nodes), indicating that these phenotypic changes were not specific to the spleen (data not shown). These data confirm the traditional effects of adjuvants in their ability to strongly augment effector CD8 T cell expansion and differentiation (i.e., increase effector cell potential), but illustrate a novel effect that in doing so, also promote terminal differentiation and reduce memory cell potential in this effector cell subset.
Greater numbers of effector CD8 T cells formed with CpG or Lm treatment, but similar numbers of memory CD8 T cells formed as compared to DC-33 alone because the number of MPECs that formed between the groups was comparable (Fig. 1C and data not shown). It is possible though, that the adjuvants had a qualitative effect on the memory CD8 T cells that formed and may have augmented their ability to fight a second infection. To test this idea, equal numbers (~5×104) of P14 memory CD8 T cells generated by DC-33 immunization alone or DC-33+CpG were transferred into naïve mice and infected with LCMV-clone 13. As controls, naïve and memory P14 CD8 T cells (generated by an acute infection with Lm expressing GP33–41 (Lm-33)) were also included. On days 5 and 8 p.i., the expansion of the memory CD8 T cells and their ability to control virus was analyzed. The results showed that the responses of the three memory CD8 T cell populations was, surprisingly, similar for both parameters (Fig. 1D and E). At face value, this suggested that in this experimental setting of DC vaccination the adjuvants primarily had an acute effect, to enhance the number and function of effector CD8 T cells. But, the adjuvants did not increase the quantity or quality of memory CD8 T cells and their precursors.
Antigenic stimulation and inflammation need to be coupled to induce SLEC formation
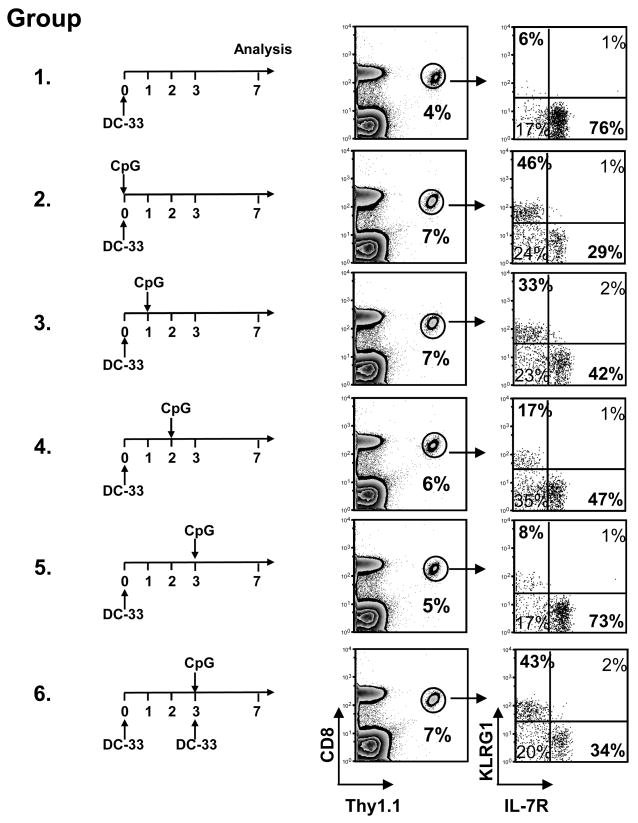
Because inflammation and antigenic signaling are intertwined during infection, we wanted to test if these signals could operate independently of one another or need to be coupled to influence SLEC formation. We immunized six groups of mice with DC-33 and then each group was treated once with CpG at different times thereafter (Fig. 2). The first group received DC-33 alone, the second group received DC-33+CpG simultaneously, and the other groups received CpG either 1, 2 or 3 days following DC-33 immunization. Our studies and some others[26] indicated that antigen and the donor DC-33 cells decay within 48–72 hrs after immunization (data not shown). This experiment showed very clearly that as the DC-33 decayed, so did the effects of CpG on effector CD8 T cell differentiation. Exposure of naïve CD8 T cells to CpG at the time of DC-33 immunization or even 24 hrs later was sufficient to induce a large population of SLECs. However, the ability of CpG treatment to induce this population of CD8 T cells decayed rapidly thereafter (Fig. 2). This was not because the activated CD8 T cells became refractory to the CpG-elicited signals because if the activated CD8 T cells, primed by DC-33 alone at day 0, were restimulated with DC-33+CpG on day 3, then SLECs were generated (Fig. 2, see group 6). These results suggested that the activated CD8 T cell needs to see both antigenic stimulation and inflammation simultaneously to receive the proper SLEC differentiation cues. Moreover, this model implies that TCR signaling adds a necessary component to the genetic pathways that guide SLEC development.
Figure 2. Antigen and inflammation need to be coupled to induce SLEC formation.
Six groups of P14 chimeric mice were immunized with DC-33, one group was left untreated as control. Each other group was treated once with CpG at different days after immunization (day 0, 1, 2, or 3), or treated with DC-33+CpG on day 3 post-immunization. FACS plots show expression of KLRG1 and IL-7R on splenic Thy1.1+ P14 CD8 T cells 7 days post immunization. Data are representative of two independent experiments.
IL-12 is sufficient and necessary to induce SLEC formation during DC immunization
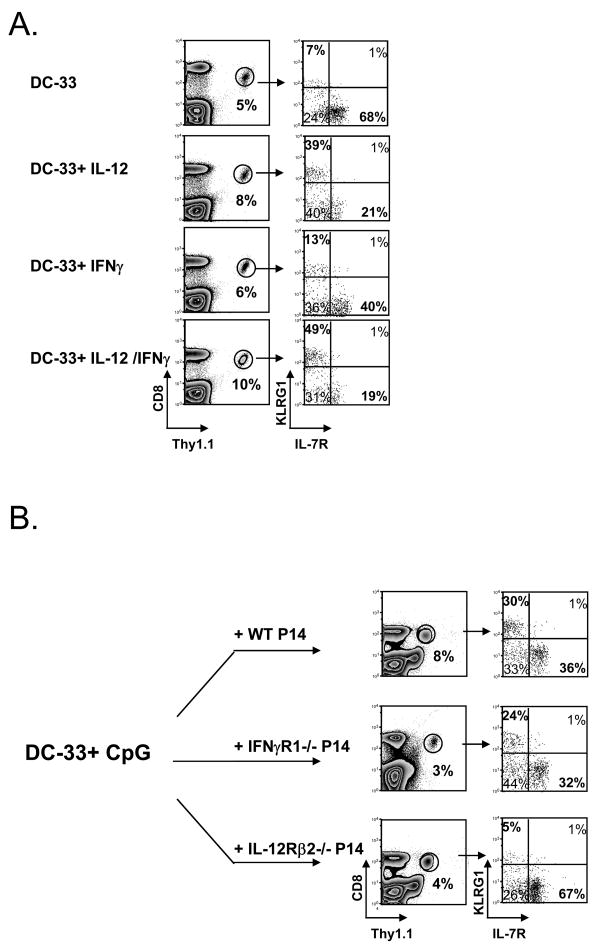
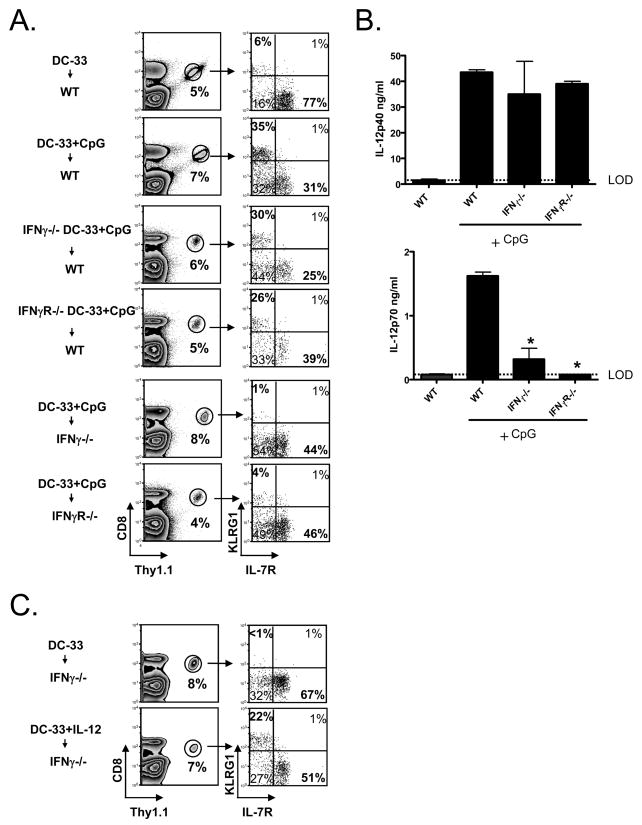
What are the necessary signals for inducing a terminally differentiated SLEC state in vivo during vaccination? Lm and CpG-B are potent inducers of IL-12 and IFNγ[27] (data not shown), and previous work showed that IFNγ promotes IL-7Rlo effector CD8 T cell development when primed with Lm or CpG-B[12]. Our past studies showed that in vitro stimulation with IL-12 induced formation of KLRG1hi IL-7Rlo effector CD8 T cells[13]. Therefore, to better understand the relationship between IFNγ and IL-12 in MPEC/SLEC fate decisions in vivo we immunized P14 chimeric mice with DC-33 and simultaneously treated with recombinant IL-12, IFNγ or IL-12+IFNγ. A sizable population of SLECs formed when the P14 CD8 T cells were primed with IL-12 or IL-12+IFNγ, however, IFNγ alone did not have this effect (Fig. 3A). Both IFNγ and IL-12 (alone or in combination) augmented effector CD8 T cell expansion and IL-12 also enhanced Granzyme B expression as previously reported[10, 28] (data not shown). Thus, IFNγ can influence effector CD8 T cell differentiation, but with regard to SLEC development, IL-12 appears to be a direct and more critical factor.
Figure 3. IL-12, but not IFNγ, is sufficient to induce SLEC formation during DC immunization.
(A) WT P14 chimeric mice were immunized with- DC-33 alone or with IL-12, IFNγor IL-12+IFNγ and analyzed 7 days later. (B) Small numbers of WT, IFNγR1−/− and IL-12Rβ2−/− P14 CD8 T cells were transferred into naïve mice that were then immunized with DC-33+ CpG. P14 CD8 T cells and were analyzed for expression of KLRG1 and IL-7R seven days later. Data are representative of three independent experiments.
To further test this point, we analyzed the formation of SLECs in CD8 T cells deficient in the receptors for IL-12 or IFNγ during DC-33+CpG vaccination. WT mice containing small numbers of IL-12Rβ2−/− or IFNγR1−/− P14 CD8 T cells were immunized with DC-33+CpG and analyzed seven days later. This experiment showed that both mutant CD8 T cell populations had reduced clonal expansion compared to WT cells, however, SLECs developed in CD8 T cells lacking IFNγR1, but not the IL-12Rβ2 (Fig. 3B). When combined, these results indicate that IL-12, but not IFNγ, is a primary SLEC inducing signal that acts directly on activated CD8 T cells during DC immunization.
Bystander production of IL-12 is sufficient to induce SLECs during both DC immunization and Listeria infection
IL-12 plays an instructive role in CD4 Th1 development and it is generally thought that, when conjugated via the immunological synapse, the antigen-presenting cell (APC) delivers this lineage-determining cytokine directly to the T cell. To determine if the donor DC directly delivered IL-12 to the CD8 T cells during DC vaccination to drive SLEC formation, we tested the requirement of IL-12 in antigen-presenting DCs using IL-12p35−/− bone marrow derived DCs. P14 chimeric mice were immunized with WT or IL-12p35−/− DC-33+CpG and seven days later we observed that both populations of DCs induced a similar percentage (~30%) of KLRG1hi IL-7Rlo effector CD8 T cells (Fig. 4A). This result suggested that IL-12 production from the antigen-presenting DC was not required during priming with CpG. Although LPS-matured bone marrow derived DCs can produce IL-12 upon CpG stimulation in vitro (data not shown), SLEC generation seems largely independent of this cell source during DC immunization in vivo.
Figure 4. Production of IL-12 from non-APCs is sufficient to induce KLRG1hi IL-7Rlo SLECs during DC-33+CpG immunization and Lm infection.
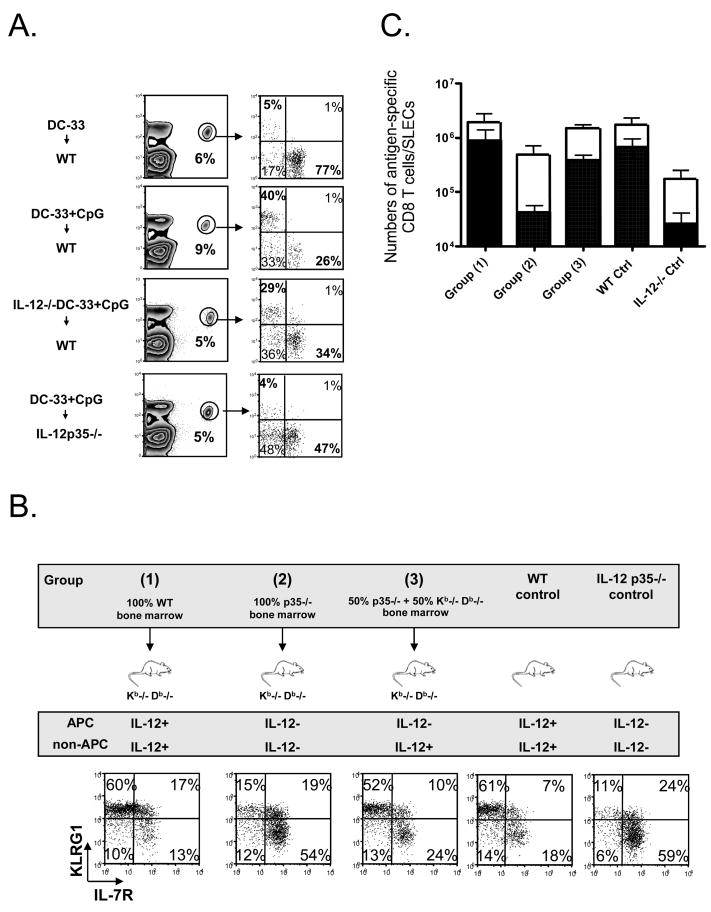
(A) WT P14 chimeric mice were immunized with WT DC-33 alone or with WT or IL-12p35−/− DC-33 + CpG, and IL-12p35−/− P14 chimeric mice was immunized with WT DC-33+CpG. P14 CD8 T cells were analyzed for expression of KLRG1 and IL-7R seven days later. (B and C) Kb−/− Db−/− mice were lethally irradiated and reconstituted with the following types of bone marrow cells: 100% WT (group 1), 100% IL-12p35−/− (group 2) or a 50%:50% mixture of IL-12p35−/− and Kb−/− Db−/−. The top panel outlines these different groups of mice and summarizes the cell types capable of producing IL-12 in each case. Unmanipulated WT or IL-12p35−/− mice were also used as controls. Approximately two months after reconstitution, ~1×104 Thy1.1+ P14 CD8 T cells were transferred into the different groups of mice and they were infected with ΔActA-Lm-33. Seven days later, splenic Thy1.1+ P14 effector CD8 T cells were analyzed for KLRG1 and IL-7R expression (B), and numbers of P14 CD8T cells (open bar) and IL-7RloKLRG1hi SLECs (solid bar) were calculated and plotted in the graph (C). Numbers are pooled from three independent experiments.
Next, we tested if the donor DCs were sufficient to induce SLECs by immunizing IL-12p35−/− mice, containing P14 CD8 T cells, with WT DC-33+CpG. These experiments clearly showed that the donor DC-33 cells were not sufficient to induce SLECs during DC-33+CpG priming, but rather, host-derived IL-12 was required (Fig. 4A). This result suggested that IL-12 production by “bystander cells” (non-APCs) played a dominant role in the CD8 T cell priming during DC vaccination with adjuvants.
To determine if this bystander effect extends to settings of infection too, we restricted the production of IL-12 to non-APCs during infection with an attenuated form of Lm that produces an abortive infection and expresses the Db-restricted epitope GP33–41 (ΔActA Lm-33). In this infection, similar to the adjuvant CpG, the formation of KLRG1hi IL-7Rlo SLECs was dependent on IL-12 (Fig. 4B). Three groups of mice were generated in which irradiated Kb−/− Db−/− mice were reconstituted with either (1) 100% WT, (2) 100% IL-12p35−/− or (3) a 50%:50% mixture of IL-12p35−/− and Kb−/− Db−/− donor bone marrow (Fig. 4B). In group (1) the APCs could present GP33–41 peptide and IL-12; in group (2) the APCs could present antigen, but not IL-12; in group (3) only IL-12-deficient APCs could present antigen and therefore, the source of IL-12 was restricted to non-APCs. Unmanipulated WT and IL-12p35−/− mice infected with ΔActA Lm-33 served as additional controls. After a two-month recovery, we transferred small numbers of purified P14 CD8 T cells into the different groups of mice, infected them with ΔActA Lm-33 and seven days later analyzed the effector CD8 T cells for formation of KLRG1hi IL-7Rlo SLECs and KLRG1lo IL-7Rhi MPECs. SLEC development was normal in group (1) and defective in group (2) animals as expected. Similar with IL-12p35−/− control mice, group (2) mice also had reduced effector CD8 T cell expansion (Fig. 4B and 4C). Interestingly, SLEC development was relatively normal in the mice in group (3) (Fig. 4B and 4C). These data show that bystander cells, which do not form a TCR:Ag immunological synapse with the antigen-specific CD8 T cells, can represent a prominent and physiologically relevant source of IL-12 for CD8 T cells priming during infection.
IFNγ is required for optimal IL-12 production in vivo
Although exogenous IFNγ did not directly induce SLEC formation (Fig. 3) it was possible that IFNγ was involved indirectly in this process and this would explain the increase of IL-7Rhi effector CD8 T cells found in IFNγ −/− mice infected with Lm[23]. To explore this we first tested if the antigen-presenting DCs needed to produce or see IFNγ to induce SLECs by immunizing mice with IFNγ −/− or IFNγR1−/− DC-33+CpG, and as predicted from earlier data, this was also not the case (Fig. 5A). Next, we tested if host cells needed to produce or “see” IFNγ to generate SLECs by immunizing IFNγ −/− and IFNγR1−/− mice, containing small numbers of P14 CD8 T cells, with WT DC-33+CpG, and this showed that SLEC development was dependent on host expression of IFNγ and IFNγR (Fig. 5A). Together these data suggested that IFNγ was necessary to influence SLEC/MPEC differentiation in vivo, but it acted indirectly on the CD8 T cells in this process.
Figure 5. IFNγ is required for optimal IL-12p70 production, thus indirectly induced SLEC formation in vivo during CpG-B treatment.
(A) WT P14 chimeric mice were immunized with WT, IFNγ−/− or IFNγR1−/− DC-33+CpG, and IFN−/− or IFNγR1−/− P14 chimeric mice were immunized with WT DC-33+CpG. P14 CD8 T cells were analyzed seven days later. (B) Bar graphs show the concentration of IL-12p40 (top panel) and IL-12p70 (bottom) in the serum of WT, IFNγ −/− and IFNγR1−/− 6h after CpG injection as measured by ELISA. (C) IFNγ −/− mice containing P14 CD8 T cells were immunized with DC-33 alone or plus recombinant IL-12p70 (1ug/mouse). After 7 days, splenic Thy1.1+ P14 CD8 T cells were analyzed for KLRG1 and IL-7R. LOD=level of detection. Asterisk denotes P<0.05. Data are representative of at least two independent experiments.
Next, we investigated if IFNγ and IL-12 act in a parallel or linear fashion in this process. IL-12 is a heterodimeric cytokine, consisting of IL-12p35 and IL-12p40, and the expression of each subunit can be coordinately regulated by different stimuli, one of which is IFNγ. For example, TLR activation triggers IL-12p40 expression, but expression of IL-12p35 often requires a second signal (in the form of IFNγ, IFNα/β or CD40:CD40L)[29–32]. We considered that in the absence of IFNγ, perhaps, the production of IL-12p70 was suboptimal during DC+CpG priming. To explore this possibility we measured serum levels of IL-12p40 and IL-12p70 in WT, IFNγ −/− and IFNγR1−/− mice 6 hrs after CpG treatment by ELISA (Fig. 5B). This showed that IL-12p40 was induced in all three groups of animals, but only WT animals produced substantial amounts of IL-12p70 (Fig. 5B). This finding indicated that during DC+CpG vaccination the production of bioactive IL-12p70 required IFNγ signaling, and explains the impaired SLEC formation in IFNγ −/− and IFNγR−/− hosts (Fig. 5A).
If IL-12p70 is the primary cytokine lacking in IFNγ −/− mice during DC+CpG vaccination, then one should be able to bypass the need of IFNγ to induce the formation of SLECs in these mice by providing supplemental IL-12p70 during DC-33 immunization. To test this idea, we immunized IFNγ −/− mice with DC-33+IL-12p70 and found this could restore SLEC development (Fig. 5C). When combined, these data suggest IFNγ and IL-12 act linearly, whereby IFNγ→IL-12p70 →SLECs. However, this model does not exclude that IFNγ can act directly on effector CD8 T cells for other processes such as clonal expansion[11].
Identification of bystander cells that produce IL-12 and IFNγ
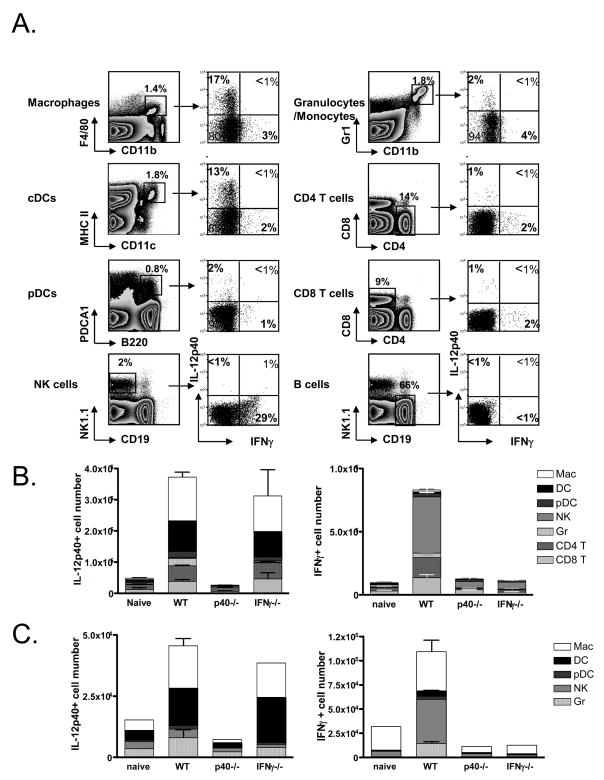
Given the above data, we sought to better characterize the IL-12- and IFNγ-producing “bystander” cell populations during immunizations with Lm and CpG. First, we infected WT, IL-12p40−/− and IFNγ −/− mice with Lm for 24h and stained the splenocytes isolated directly ex vivo for intracellular IL-12p40 and IFNγ. As expected, this showed that the principle producers of IL-12 were macrophages (CD11b+ F4/80+) and conventional DCs (MHCII+ CD11c+) and these cells comprised ~70% of the total IL-12 producing cells in the spleen at this time (Fig. 6). Very little to no IL-12 was produced by plasmacytoid DCs, granulocytes/monocytes, NK cells, T and B cells. When the mice were immunized with DC+CpG the profile of IL-12 producing cells was virtually the same, although the actual numbers of IL-12 producing cells was substantially lower (Fig. 6B).
Figure 6. Identification of the IL-12 and IFNγ producing cells during DC vaccination and Lm infection.
WT, IL-12p40−/− and IFNγ −/− mice were immunized with Lm for 24hrs or CpG for 6 hrs and then the splenocytes were cultured in BFA for an additional 6 hrs and stained for surface markers and intracellular IL-12p40 and IFNγ. (A) Left contour plots show the gating criteria used to identify the different cell populations and right dot plots show the expression of IL-12p40 and IFNγ in these cells 24hrs after Lm infection. Note: similar types of cells, albeit at lower frequencies, produced these cytokines after 6hrs of CpG treatment (data not shown). (B and C) Bar graphs indicate numbers of IL-12- and IFNγ-producing cells in the spleens of WT, IL-12p40−/− and IFNγ −/−mice after Lm infection (B) or CpG immunization (C).
The predominant IFNγ producing cells during both Lm infection and CpG immunization were NK cells as they accounted for ~60% of the total IFNγ producing cells (Fig. 6). Again, the overall number of IFNγ-producing cells was lower with CpG treatment compared to Lm infection. Macrophages were also a significant source of IFNγ after CpG immunization and T cells, cDCs and macrophages represented a small, but detectable, source during Lm infection (Fig. 6B). Interestingly, the ability of NK cells to produce IFNγ was dependent on IL-12, highlighting a synergistic relationship between the IFNγ and IL-12-producing cells (Fig. 6B). These results agree with previous descriptions of the innate immune cells that respond early to Lm infection and CpG[33–36], and signify the physiologically-relevant “bystander” cell populations that are likely recruited to the sites of CD8 T cell priming to regulate effector CD8 T cell fate decisions in vivo during these types of infections and vaccinations.
Discussion
Inflammatory cytokines elicited by the innate recognition of PAMPs during infection or vaccination are critical for clonal expansion and differentiation of effector T cells, and data is emerging on how they also regulate memory CD8 T cell potential[13, 14, 17, 37]. The aim of this study was to focus on a model PAMP, CpG-B, during DC vaccination to understand how the primary cytokines elicited by this adjuvant, IL-12 and IFNγ, integrate with antigenic stimulation to regulate effector CD8 T cell differentiation in vivo. We found that the presence of a bystander Lm infection, CpG or IL-12 during DC vaccination provided several notable benefits to the host such as formation of significantly larger numbers of effector CD8 T cells with elevated expression of granzyme B, but this was accompanied by an increase in the numbers of terminally differentiated SLECs. However, the number of putative memory cell precursors that developed was comparable between animals treated with or without adjuvants, and likewise, so was the size, form and function of the memory CD8 T cell population (Fig. 1).
Recent studies have shown that adjuvants such as CpG or bystander Lm infection can slow down the rate at which activated CD8 T cells acquire memory T cell properties[12]. In the absence of inflammation, the activated cells found at peak of expansion already display “mature” memory CD8 T cell properties such as a high proliferative potential and increased expression of IL-7R, CD27 and IL-2[12, 15]. Inflammation also promoted contraction of the effector CD8 T cells and this was largely dependent on IFNγ[12, 23]. Our studies support and extend from these observations by suggesting that CpG, and in particular IL-12, induce terminally differentiated SLECs to form, which have a reduced proliferative capacity and decreased expression of IL-7R, CD27 and IL-2[12, 13]. In the absence of IL-12 (and possibly other signals) fewer terminally differentiated effector CD8 T cells form and the effector cells are more similar to a mature memory T cells[16, 17]. Because these inflammatory cytokines seem to act as a double-edged sword with both positive and negative effects on effector and memory CD8 T cell differentiation, it will be interesting to dissect the genetic pathways controlling these key processes. In the future, one may be able to maximize expansion, but limit terminal differentiation to create stronger and longer-lasting immunity.
This study better elucidates the complex interaction between IFNγ and IL-12 on effector CD8 T cell differentiation during DC vaccination with CpG. We show here that IL-12, but not IFNγ, can directly instruct effector CD8 T cells to differentiate into SLECs. Furthermore, release and capture of IFNγ by cell types other than the APC was required in vivo for optimal production of IL-12 in CpG treated animals. These results suggested a linear pathway in which IFNγ→IL-12→SLECs during CD8 T cell priming and perhaps, this was best illustrated by rescue of SLEC formation in IFNγ −/− animals treated with recombinant IL-12p70. However, it is well known that IFNγ production is augmented by IL-12 through a positive feedback loop[29, 30] and as seen in our data (Fig. 6), so perhaps a more accurate representation of this pathway is IFNγ IL-12→SLECs. It is also important to note that IFNγ can act directly on CD8 T cells during LCMV infection to promote their clonal expansion and formation of memory CD8 T cells[11, 28]. Activated CD8 T cells also reduce their responsiveness to IFNγ by down regulation of IFNγR2 expression shortly after activation[38]. Therefore, IFNγ has multiple effects on effector CD8 T cell differentiation and how this varies according to the nature of the immune response needs to be better examined.
IL-12→SLECs. It is also important to note that IFNγ can act directly on CD8 T cells during LCMV infection to promote their clonal expansion and formation of memory CD8 T cells[11, 28]. Activated CD8 T cells also reduce their responsiveness to IFNγ by down regulation of IFNγR2 expression shortly after activation[38]. Therefore, IFNγ has multiple effects on effector CD8 T cell differentiation and how this varies according to the nature of the immune response needs to be better examined.
A key element that emerged from these studies was the profound influence of “bystander” cells (non-APCs) on CD8 T cell priming during both DC vaccination and bacterial infection. Our data suggest that these bystander cells are sufficient, and necessary in the case of DC vaccination, for producing IFNγ and IL-12 to alter effector CD8 T cell differentiation. Our characterization of these cells suggested that conventional DCs and macrophages are the primary producers of IL-12 whereas NK cells were the major source of IFNγ. This information presents a novel viewpoint that differs from the traditional view of single APC:T cell conjugates, where the APC supplies both activation and polarization signals to the T cells[39]. Rather, these findings suggest a multicellular model in which NK cells produce IFNγ to augment production of IL-12 by neighboring DCs and macrophages (and vice versa), and then these cells communicate with newly activated T cells to deliver IL-12 and regulate their expansion and effector differentiation. This model does not necessitate that all cell types directly contact each other simultaneously. The critical involvement of bystander NK cells in Th1 and basophils in Th2 CD4 T cell priming have also recently been described40− [40] In these studies, NK cells and basophils are recruited to the site of T cell priming and supply IFNγ and IL-4/TSLP to instruct Th1 or Th2 development, respectively. These data do not rule out that during infection the APC alone can suffice to provide all three signals, nor do they detract from the exquisite role of the immunological synapse in naïve T cell activation. But, they do offer a new and more complex view on T cell priming that differs from the single APC:T cell conjugate paradigm. These findings become even more relevant when one considers the first encounter(s) of T cells with APCs early during infection because a recent study suggested that an asymmetric separation of daughter cells, during the first T cell division, rendered the daughter cell synapsed with the APC with less memory cell developmental potential[41]. If true, our work would suggest that somehow this proximal cell has a greater advantage to communicate with bystander IL-12 producing cells during T cell priming, perhaps through their rapid up-regulation of IL-12 receptor.
Many approaches have been tested to enhance IL-12 expression in DCs to improve vaccination including treating DCs in culture with cytokine cocktails, CD40 agonist antibody and TLR ligands[42, 43]. However, our work points out that use of in vitro matured BMDCs activated T cells, but did not provide sufficient amounts of IL-12 to affect effector CD8 T cell expansion, differentiation or cell-fate decisions. Rather, neighboring non-APCs provided these instructive signals in response to exogenously provided PAMPs. Perhaps, other types of treatments and/or DC populations would play a more active role in this process, but using these standard approaches we find the bystander cells are more prominent. Therefore, this point should be taken into consideration when designing DC therapies similar to that used here.
CpG oligodeoxynucleotides are also currently being tested in a variety of human vaccine trials, which have shown to enhance antigen presentation, accelerate antibody production and bias Th1 responses[44]. A recent study comparing TLR9 vs. TLR7/8 agonists during HIV Gag protein vaccination in non-human primates found that the TLR7/8 adjuvant generated a larger proportion of IL-2+ IFNγ+ memory T cells that showed greater recall responses than CpG after the rAD-Gag boost [45]. These findings, coupled with those here, illustrate the need to further investigate how different adjuvants, and the cytokines they produce, affect the development of effector and memory T cells both qualitatively and quantitatively to provide a better framework for designing new vaccines.
Supplementary Material
Acknowledgments
We thank Drs. Jason K. Whitmire and J. Lindsay Whitton for generously providing P14 IFNγR1−/− Thy1.1+ CD8 T cells and Dr. Hongmei Li for technical support for generation of bone marrow chimeras. We thank Kaech lab members for their thoughtful discussions and suggestions on this manuscript.
This work was supported by the Burroughs-Wellcome Fund 1004313 (S.M.K.), NIH RO1 AI066232-01 (S.M.K.).
Abbreviations in this paper
- DC
dendritic cells
- Lm
Listeria monocytogenes
- WT
wild type
- KLRG1
Killer cell lectin-like receptor G1
- ODNs
oligodeoxynucleotides
Footnotes
Authorship
W.C. designed and performed experiments, analyzed the data, and contributed to writing and editing of the manuscript; N.S.J. contributed to the design of studies and editing of the manuscript; A.J. contributed new reagents; and S.M.K. designed the studies, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006 Feb 24;124(4):849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature reviews. 2002 Apr;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Letvin NL. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity. 2007 Sep;27(3):366–9. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz K, Meijerink E, Speiser DE, Tissot AC, Cielens I, Renhof R, et al. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. European journal of immunology. 2005 Mar;35(3):816–21. doi: 10.1002/eji.200425755. [DOI] [PubMed] [Google Scholar]
- 5.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nature medicine. 2005 Apr;11(4 Suppl):S63–8. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 6.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005 Sep 5;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunological reviews. 2006 Jun;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 8.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006 Jul;25(1):19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006 Aug 1;177(3):1746–54. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. The Journal of experimental medicine. 2003 May 5;197(9):1141–51. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. The Journal of experimental medicine. 2005 Apr 4;201(7):1053–9. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005 Jul;11(7):748–56. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 13.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007 Aug;27(2):281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007 Sep;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007 Jul 1;179(1):53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006 Dec 1;177(11):7515–9. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 17.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007 Aug 15;179(4):2074–81. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003 Dec;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 19.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 13;101(15):5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001 Nov 1;167(9):4838–43. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 21.Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006 Jan 1;176(1):507–15. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 22.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 10;104(28):11730–5. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nature immunology. 2002 Jul;3(7):619–26. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 24.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002 Aug 29;418(6901):988–94. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 25.Thomas S, Kolumam GA, Murali-Krishna K. Antigen presentation by nonhemopoietic cells amplifies clonal expansion of effector CD8 T cells in a pathogen-specific manner. J Immunol. 2007 May 1;178(9):5802–11. doi: 10.4049/jimmunol.178.9.5802. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jan 3;103(1):147–52. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annual review of immunology. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 28.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007 Jul 15;179(2):1190–7. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nature reviews. 2007 Mar;7(3):179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. The Journal of experimental medicine. 1996 Jan 1;183(1):147–57. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. The Journal of experimental medicine. 2005 May 2;201(9):1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000 Oct;13(4):453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006 Aug 1;177(3):1618–27. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- 34.Way SS, Wilson CB. Cutting edge: immunity and IFN-gamma production during Listeria monocytogenes infection in the absence of T-bet. J Immunol. 2004 Nov 15;173(10):5918–22. doi: 10.4049/jimmunol.173.10.5918. [DOI] [PubMed] [Google Scholar]
- 35.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998 Nov 15;161(10):5600–6. [PubMed] [Google Scholar]
- 36.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001 Jan 15;166(2):1097–105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 37.Joshi NS, Kaech SM. Effector CD8 T Cell Development: A Balancing Act between Memory Cell Potential and Terminal Differentiation. J Immunol. 2008 Feb 1;180(3):1309–15. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 38.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005 Jun 1;174(11):6791–802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 39.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 40.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, et al. Basophils enhance immunological memory responses. Nature immunology. 2008 Jul;9(7):733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 41.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science (New York, NY. 2007 Mar 23;315(5819):1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 42.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature reviews. 2005 Apr;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 43.Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001 May;14(5):495–8. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 44.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006 Jun;5(6):471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 45.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005 Jun 15;174(12):7676–83. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.