Abstract
Prolonged, intense exercise causes immunosuppression, while moderate intensity exercise improves immune function and potentially reduces risk and severity of respiratory viral infection. Here, based upon available evidence, we present a model whereby moderate exercise-induced increases in stress hormones reduce excessive local inflammation and skew the immune response away from a Th1 and towards a Th2 phenotype, thus improving outcomes following respiratory viral infection.
Keywords: Physical activity, URTI, virus, influenza, inflammation
INTRODUCTION
Respiratory viral infections represent the most prevalent and pathogenic form of infectious disease, accounting over 7% of all deaths in both men and women in 2004 (17). Infection occurs when a host comes in contact with infected aerosolized droplets or contaminated surfaces, following which the virus invades and infects the host's upper and/or lower respiratory mucosal tissues. Illness duration typically lasts 7–14 days, and the usual symptoms include: cough, nasal congestion, fever, body aches, malaise, and in severe cases death. Deaths associated with respiratory viral infection occur most often in children, elderly, and other immune-compromised individuals, as their immune systems are incapable of handling the elevated viral load.
Respiratory viruses encompass a broad spectrum of virulence, ranging from rhinovirus (i.e., the “common cold”) to significantly more pathogenic viruses such as influenza (i.e., the “flu”). Every year, a seasonal flu epidemic occurs, beginning in October, and peaking in January. These yearly epidemics pose a significant health burden on the United States, ultimately being responsible for 200,000 hospitalizations and 36,000 deaths (9). In addition to the yearly epidemic, a pandemic influenza outbreak arises every 10–50 years culminating in the death of millions of people; a classic example is the “Spanish Flu” pandemic of 1918 which killed an estimated 40 million individuals. Influenza vaccination is the primary method of disease prevention, but often the vaccine is in short supply, and even when available, a large percent of the population fails to receive vaccination. To further compound vaccination problems, research suggests the vaccination efficacy in preventing hospitalization is roughly 75% and plummets to 45% and 30% for individuals over the age 65 and 75, respectively (32). Understanding how behaviors such as physical activity or exercise affect viral infection outcomes is of public health importance.
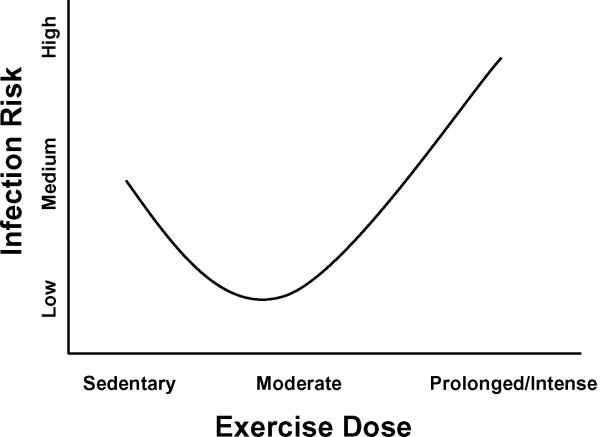
Cross-sectional and longitudinal data suggests persons who engage in regular moderate intensity exercise maintain a reduced risk of self-reported respiratory symptoms (14,18,23,34). Additionally, work from our laboratory and others demonstrates moderate intensity exercise performed prior to infection (6) or infectious symptoms (15) reduces respiratory virus-associated mortality in animals. In contrast, intense exercise before or during viral infection has been associated with greater morbidity and mortality (8,11,24). These findings have given rise to the `J-shaped' hypothesis (Fig. 1) relating exercise dose with infection risk (adapted from Nieman et al. (22)). The primary purpose of this brief article is to summarize current literature regarding exercise and viral respiratory infections and provide a platform for further investigation into the mechanisms mediating the protective effect of exercise against respiratory viral infections. It includes human epidemiological studies, human experimental trials, animal models, and highlights research from our laboratory providing insight into potential mechanisms through which regular exercise may be protective. While there is evidence that exercise can beneficially affect bacterial infection outcomes, viral infections through other portals, and responses to vaccinations, we are limiting the scope of this article to specifically focus on exercise and respiratory viral infections because there appears to be sufficient evidence to warrant conclusions.
Figure 1.
“J-shaped” model depicting dose-dependent effect of exercise on risk and severity of respiratory tract infections. Sedentary persons are considered to be at normal risk of URTI. Exercise of low-to-moderate intensity or frequency is associated with reduced risk of URTI (3,18,23,25,34) while high-intensity exercise is associated with an increased risk of infection (8,11,24). [Adapted from Nieman DC, Johanssen LM, Lee JW. Infectious episodes in runners before and after a roadrace. J Sports Med Phys Fitness. 1989;29(3):289–96. Copyright © 1989 BMJ Publishing Group Ltd. Used with permission.]
IMMUNE DEFENSE AGAINST RESPIRATORY VIRAL INFECTIONS
Respiratory viruses such as influenza and rhinovirus are sub-microscopic, non-cellular infectious agents which invade respiratory mucosal tissue and replicate inside the host's living cells. Unlike bacterial infections, viruses are metabolically insufficient, relying completely on the host's cellular metabolism for replication and viral protein synthesis. Because viruses utilize host machinery, they often evade host immune surveillance, allowing rapid replication and increased viral load. The complexity of viral escape mechanisms selectively pressured the immune system to develop a broad spectrum of anti-viral responses which coordinate the recognition and clearance of viruses. Below, we will briefly highlight the major anti-viral defenses.
Respiratory viruses bind glycoproteins on the surface of mucosal epithelial cells, inducing receptor mediated endocytosis and ensuant infection of the host cell. In immunized individuals, salivary and mucosal immunoglobulins, primarily IgA, recognize and bind viral epitopes, blocking their entry into mucosal cells and reducing susceptibility to secondary infection. Virus invasion of the respiratory mucosa evokes an innate immune response through binding of pathogen-associated-molecular-patterns (PAMPs) to toll-like receptor (TLR) molecules on lung macrophages (Mϕ's), myeloid dendritic cells (mDC), and plasmacytoid dendritic cells (pDC). Specifically, TLRs 3,7, and 9 recognize single and double stranded mRNA characteristic of the viral genome and initiate signal transduction, leading to nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κβ) transcriptional activity, which promotes the synthesis of type I interferons α/β (IFN). Secretion of IFN-α and IFN-β by alveolar pDCs and Mϕ's induces host cell upregulation of two critical antiviral mechanisms: double stranded RNA-activated inhibitor of translation (DAI) and Mx proteins. DAI phosphorylates and inhibits eukaryotic initiation factor 2 (eiF-2), a protein required for initiation of viral mRNA translation, while Mx proteins prevent nucleocaspid assembly and inhibit viral polymerase activity. Together these antiviral mediators prevent viral replication and further infection of host cells, transiently halting viral activity until the adaptive cellular immune response eliminates the virus.
In addition to inducing antiviral activity in host cells, activated innate immune cells also secrete numerous pro-inflammatory cytokines including: interleukin (IL)-1, IL-6, IL-12 and tumor necrosis factor (TNF)-α which induce a local and systemic inflammatory response characterized by increased production of acute-phase opsonizing complement proteins, enhanced extravasation of leukocytes to infected tissues, and increased antigen presentation and cytotoxic capacity. These same cytokine communicate with the brain and are responsible for sickness behaviors associated with infection (4).
Of particular importance is IL-12 which bridges the gap between innate and adaptive immunity by driving the differentiation of naïve T helper cells (Th0) toward a Th1 phenotype characterized by the production of pro-inflammatory cytokines IL-2 and IFN-γ. Th1 secreted IL-2 promotes the maturation of antigen specific cytotoxic T-lymphocytes (CD8+ T cells) which recognize viral antigens on infected cells through the association of major histocompatibilty complex (MHC) I interactions with T cell receptors (TCR). Activation of CD8+ TCR induces cytotoxic killing of virally infected cells, CD8+ cell proliferation, production of antiviral cytokines such as IFN-γ and activation-induced cell death (AICD). IFN-γ has multiple, wide-ranging effects, including: synthesis of antiviral proteins, upregulation of MHC I receptors, and stimulation of natural killer (NK). NK cells induce apoptosis of infected cells, following which the dead cells are phagocytized by macrophages, myeloid dendritic cells, and pDCs, and the intracellular antigens cross-presented to CD4+ and CD8+ cells.
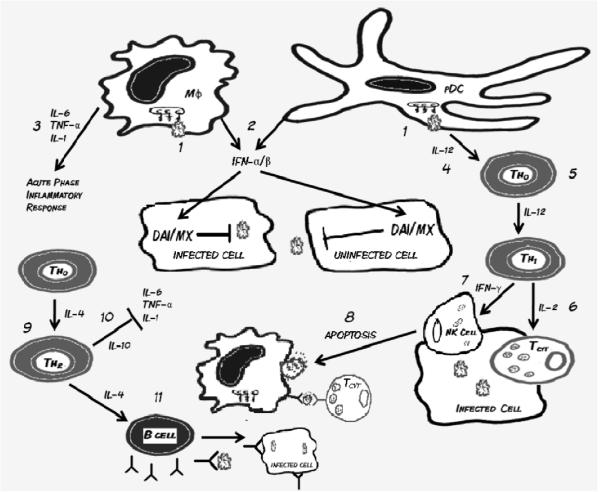
A strong Th1 response is necessary in the early stages of viral infection, as it promotes rapid clearance of the virus. Prolonged Th1 activity, however, may lead to respiratory tissue pathology, through increased cell damage and necrosis (31). Immune counter-regulatory mechanisms attempt to prevent Th1 induced pathology by shifting the Th cell phenotype towards Th2, characterized by the secretion of anti-inflammatory proteins IL-4 and IL-10. IL-10 acts to inhibit the effect of proinflammatory cytokines, while IL-4 stimulates naïve B cells to enlarge in size and upregulate synthesis of MHC II molecules important for antigen presentation. Circulating naïve B-cells recognize viral proteins and glycoproteins on the surface of infected cells. Antigen activation of the B-cell receptor, along with coactivation by Th2 cells causes the B cell to differentiate into plasma and memory B-cells. Plasma cells secrete large amounts of antiviral antibodies, particularly IgA, which binds viral epitopes to prevent viral entry into uninfected cells. Antibody binding of infected cells also induces natural killer (NK) cell antibody-dependent-cell-mediated cytotoxicity (ADCC) and complement protein activation, both of which lead to infected cell apoptosis. In summary, innate immune mechanisms (e.g., IFNs) hold viral replication and spread in check until adaptive immune responses (primarily cytotoxic CD8+ T cells) clear the infection; a process which takes place within 7–14 days. Primary infection leads to development of adaptive immune responses characterized by specific antibodies that prevent subsequent viral attachment and memory T cells which respond with greater vigor upon secondary exposure. A summary of immune defense mechanisms against respiratory viruses is presented in Figure 2.
Figure 2.
1) Respiratory viruses invade respiratory mucosal epithelial cells and residential innate immune cells, where they activate Toll-like receptors (TLR) and induce the innate immune response. 2) Activated macrophages (Mφ) and plasmacytoid dendritic cells (pDC) produce IFN-α/β, which prevents viral replication in host cells 3) Additionally, macrophages secrete pro-inflammatory cytokines (IL-6, TNF-α, and IL-1) which stimulate the acute phase inflammatory response. 4) Secretion of IL-12 by pDCs differentiates naïve Thelper (Th0) cells into 5) Th1 cells, which secrete IL-2 and IFN-γ. 6) IL-2 promotes maturation of Tcytotoxic cells, which recognize antigen presented through MHC 1 on infected cells, and promote apoptosis. 7) IFN-γ stimulates natural killer cells (NK) activity, also leading to increased apoptosis. 8) Apoptotic cells are phagocytized by Mφ, pDCs, and myeloid dendritic cells (mDCs) and the intracellular antigen is cross presented to Tcytotoxic cells. To counter-regulate the inflammatory Th1 response, naïve Th0 cells differentiate into 9) Th2 cells which secrete anti-inflammatory cytokines IL-10 and IL-4. 10) IL-10 down regulates the acute phase response, while IL-4 promotes differentiation of B cell into plasma and memory B lymphocytes. 11) Plasma B cells secrete antibodies, particularly IgA, which block viral entry and opsonize infected cells for antibody dependent cellular cytotoxicity.
EXERCISE AND VIRAL INFECTION IN HUMANS
Observational Evidence
A large body of epidemiological literature has examined the effects of differing amounts and intensities of physical activity/exercise on a wide variety of respiratory symptoms. An early study published by Heath et al. (11) found runners in the upper two quartiles of yearly mileage (>866 miles run during the 12 month follow-up period) had a significantly higher risk of self-reported upper respiratory tract infection (URTI) symptoms compared to runners in the lowest quartile (<486 miles). A recent study found low-to-moderate frequency of exercise reduced the risk of influenza-associated mortality in adults living in Hong Kong during 1998, while a high frequency of exercise (>4d/week) failed to reduce the risk of mortality when compared to the sedentary referent group (34). A retrospective study by Kostka et al. (14) found no relation between fitness level and self-reported weeks with URTI symptoms, although energy expenditure during leisure-time sports was significantly negatively correlated with total weeks of URTI symptoms as well as with number of episodes of URTI during a 1 year period. Matthews et al. (18) found a 29% decrease in incidence rate of URTI symptoms in adult men and women who engaged in moderate-to-vigorous physical activity of at least 11.96 MET·h·d−1 when compared with those individuals who were less physically-active.
These studies support the hypothesis that moderate exercise is protective against URTI symptoms and that there may be a differential dose-response effect such that intense, prolonged exercise or overtraining increase disease risk or symptom severity. Unfortunately, epidemiological and retrospective studies such as those above rely on subject recall of symptoms and self-reporting of a variety of important variables including training status and exercise intensity. In addition, due to the large subject numbers in such epidemiological studies and the often heterogeneous subject populations, it is difficult to control for potential confounders such as nutritional status or other environmental factors. Because of these restrictions, in-depth investigation of the effects of exercise on URTI has necessitated the undertaking of human and animal experiments which restrict subject populations and conditions to allow for better control of these potential confounding factors.
A number of longitudinal studies have sought to determine the effects of specific intensities and durations of exercise training on the incidence of various URTI symptoms including rhinovirus and influenza. Several early studies examined the effects of intense bouts of exercise in competitive athletes on the risk for later development of URTI symptoms. A pair of studies from the laboratory of David Nieman examined the incidence of URTI in athletes who participated in a variety of running races. A comparison of runners competing in a 5 km, 10 km, or 21.1 km race (23) found athletes who ran over 15 miles per week had reduced incidence of respiratory symptoms when compared with athletes training at a lesser volume, and that runners training for the 21.1 km race had fewer infections than their shorter-distance counterparts in the two months prior to the race. In addition, there was no increased reporting rate of URTI symptoms in the 7 days following the races when compared to the 7 days prior to the races. A second study examined more than 2000 athletes competing in the Los Angeles Marathon (24). Runners who trained >97 km per week had twice the risk of the development of URTI symptoms when compared to the referent group which trained <32 km per week. Furthermore, runners who completed the marathon increased their risk for URTI nearly 6-fold during the week following the race when compared with similarly-trained controls that registered, but did not participate in the marathon. In a more recent study, Ekblom et al. (8) found that 19% of marathon finishers experienced URTI symptoms during a 3 week period following race completion. However, when compared to the 16% of marathoners who experienced URTI symptoms in the 3 week period before the race, the investigators concluded a single strenuous bout of activity does not increase risk of respiratory infection. However, 33% of runners who experienced an infectious episode before the race had a recurrence of URTI symptoms after the race, suggesting a strenuous bout of exercise potentially increases the risk of subsequent infection if the exercise is performed soon after an initial infection.
Nieman et al. (25) also examined the effect of 15 weeks of moderate-intensity exercise training on symptoms of URTI. Exercising subjects demonstrated shorter infectious episodes compared to their sedentary controls as measured by number of symptom-days per infectious episode. In addition, URTI symptom-days were negatively-correlated with increases in fitness. A recent 12-month study examined the effects of moderate-intensity exercise on the development of cold symptoms in obese, sedentary postmenopausal women (3), a subject population known to have reduced immune function. Exercising subjects were found to have a significantly reduced number of incidences of self-reported cold symptoms compared to their stretching controls, with the largest differences between groups coming during months 6–12 of the study. These results of these studies support the hypothesis of the J-shaped curve (Fig. 1) in which moderate-intensity exercise is protective against respiratory viral infection while high-intensity exercise increases the risk of infection.
Concentrations of salivary IgA (s-IgA) have been examined as a means of explaining the incidence rates of URTI in athletes because s-IgA binds to and opsonizes foreign organisms including respiratory viruses. Salivary IgA is considered a first line of defense in subjects that have been previously exposed to specific pathogens. It appears that long duration and high intensity exercise, in both an acute and chronic fashion, decreases salivary IgA and is associated with increased respiratory symptoms (9). In contrast, limited research suggests both acute and chronic moderate intensity exercise increases s-IgA levels providing protection against respiratory symptoms. Klentrou et al. found 12 weeks of moderate exercise training reduced self-reported infections and decreased URTI symptoms, which were correlated with increased s-IgA (12). Additional research by Shimizu et al. demonstrated a similar S-IgA response in elderly subjects, further supporting exercise training as an adjunct method to protect against respiratory virus infections. (27). A definitive role for s-IgA in explaining exercise-induced changes in respiratory tract infection symptoms has not yet been provided, and necessitates further research.
Experimental Evidence
Some researchers have questioned the validity of the `J-shaped model' describing the effects of exercise on viral infection risk (28) because most of the studies on which the model is based examined only the symptoms associated with URTI and not clinically diagnosed infection with a particular pathogen. Because of these deficiencies, several studies have attempted to more closely examine the effects of exercise on the pathogenesis and clinical outcomes attributed to specific respiratory viruses or bacteria using both human and animal models.
An important study by Spence et al. (28) tested elite athletes, recreational athletes, and sedentary individuals over a 5 month surveillance period. Individuals who reported symptoms of URTI were tested for several common viral and bacterial pathogens by nasopharyngeal and throat swabs. Nine sedentary, 7 recreational athletes, and 21 elite athletes developed URTI symptoms during the course of the study, a result which supports the “J” shaped curve hypothesis. The subjects showing URTI symptoms were tested for the majority of the major pathogens responsible for URTI in humans, including adenovirus, influenza virus A and B, several types of parainfluenza virus, respiratory syncytial virus, and others. Actual pathogens, however, were detected in only 30% of all of the cases, with the percentages of positive findings distributed approximately equally between the three groups. This finding suggested that, although the symptoms were consistent with URTI, the underlying cause of the symptoms seen with different intensities of exercise training may not be due to common respiratory pathogens, but instead to other phenomena such as allergic reactions or idiopathic causes such as airway hypersensitivity and asthma associated symptoms.
Due to ethical considerations, there are very few experimental studies examining the effects of exercise on viral infection in people with one notable exception. Weidner et al. (33) inoculated college-aged subjects with rhinovirus, after which half of the subjects performed six exercise bouts at 70% heart rate reserve over a 10-day period while the other half remained sedentary. There were no significant differences in symptom severity or mucous weights between the exercising and non-exercising groups at any time point during the course of the study. Thus, this experimental study refutes epidemiological and longitudinal evidence reporting beneficial effects of moderate exercise on URTI. The reason for the discrepancy between this study and the others is unknown. However, most of the subjects in the Weidner study engaged in regular physical activity before the study began, baseline fitness is likely to have blunted any potential beneficial effects of moderate exercise on immune function that might have been expected based on the epidemiological and observational data which has already been discussed. Additionally, the restriction in human experiments such as the Weidner study to viruses that cause only mild symptoms precludes investigation of other common but more serious respiratory infections such as the influenza virus as well as less common respiratory symptom-associated adenoviruses and enteroviruses. Clearly, within the confines of ethical considerations, more human experimentation is warranted to definitively test whether exercise dose affects respiratory viral infection course.
EXERCISE AND VIRAL INFECTION IN ANIMAL MODELS
Due to the difficulties in experimentally establishing the effects of exercise on specific viral pathogens in humans, studies in mice, including work performed in our laboratory, have been undertaken. One limitation of all these animal studies is that they have all examined primary, and not secondary (after immunological memory has been obtained), responses to infection. Clearly, the effects of exercise on responses to secondary respiratory pathogen exposure need to be performed.
Davis et al. (5) inoculated mice with herpes simplex virus-1 (HSV-1) 15 minutes after either a single bout of 30 minutes of moderate-intensity (18 m·min−1) or high-intensity (gradually increasing from 18 m·min−1 to 36 m·min−1) treadmill running to volitional fatigue. Sedentary mice served as controls and all mice remained in their home cages for 21 days post-infection. At the 21 day time point, 41% of the mice which exercised to fatigue had died; significantly higher than the 16% mortality rate experienced by the control group. There was no statistical difference in mortality rate between the control and moderately exercised group (9% mortality at 21 days). These results demonstrated that a single bout of intense exercise can cause serious deficiencies in immune defense when a potentially lethal viral infection is contracted. Follow-up studies have confirmed this finding (2), although one study has also indicated that short-term moderate exercise prior to infection in mice can be protective against viral infection-associated mortality (6). Unfortunately, HSV-1 is not a true respiratory pathogen, thus this model is limited in the amount of information it can provide regarding the effects of exercise on common respiratory tract infection pathogenesis in people.
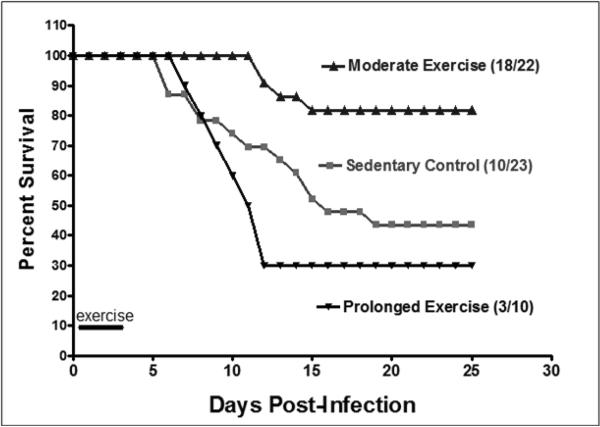
As a result of this limitation, our lab conducted a study (15) which tested the effects of exercise on influenza virus infection. We utilized the common laboratory influenza strain A/Puerto Rico/8/34 at a dose designed to induce approximately 50% mortality in sedentary control Balb/cByJ mice. Four hours after infection, mice began a 4 day exercise program of either 30 (moderate) or 150 (prolonged) minutes of exercise daily. We reasoned that, by applying exercise after infection but before symptom onset, we would impart exercise effects on the developing immune response better than if exercise was applied at some point prior to infection. Moreover, because no one knows exactly when they become infected, we thought this paradigm was more realistic than a single bout of exercise followed by infection. The exercise program was terminated after Day 4 when mice began exhibiting symptoms (e.g., lethargy, reduced nest building) of infection. We found that mice assigned to moderate exercise had significantly lower mortality rates than did the control, non-exercising group (18% vs. 56% mortality, respectively) after 25 days (Fig. 3). Mice in the prolonged exercise group did not have a statistically different mortality rate than controls; although prolonged exercise did result in a greater percentage of mortality than controls (70% vs. 56% mortality, respectively). Indeed, the detrimental effect of intense, prolonged exercise on mortality due to influenza virus has recently been established (21). Mice in the prolonged exercise group also had greater influenza-associated composite morbidity score (as measured by observed physical activity, response to handling, and physical appearance) prior to death than did their control and moderate exercise counterparts; although morbidity scores were not different at any time point between the latter two groups. This study was the first to indicate that moderate exercise initiated after infection but before symptom onset can have a beneficial effect on susceptibility to a true human respiratory pathogen. It is important to note that the dose at which the influenza virus was given induced a lower-respiratory tract infection, thus the results of this study may have implications on viral infections that are considerably more serious than normal infections to which athletes and exercisers are routinely exposed.
Figure 3.
Influence of exercise on mortality due to influenza (H1N1 Puerto Rico A/8/34) in male 20–24 wk old Balb/c mice. Mean survival was 14 ± 1, 17 ± 2, and 16 ± 3 days for control, moderate and prolonged exercise, respectively. There was a statistically significant difference between control and moderate exercise (log rank = 7.3; p = 0.007), but not between control and prolonged exercise (log rank = 1.1; p = 0.29). (Reprinted from Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19(5):377–80. Copyright © 2005 Elsevier. Used with permission.)
We conducted a further experiment to define potential mechanisms through which exercise improves survival in this influenza virus model (16). Using the identical exercise and influenza infection model described above, we analyzed cellular infiltration in lungs, spleen, and draining mediastinal lymph nodes and cytokine gene and protein expression at several time points post-infection. We hypothesized exercise training would promote an anti-inflammatory shift from a Th1 dominated phenotype towards a Th2-type immune response; while not totally abrogating Th1-mediated immunity. Th1 inflammatory immune responses induce upregulation of pro-inflammatory cytokines, particularly IFN-γ, which initiates improved Mϕ antigen presentation and enhanced phagocytotic/cytotoxic activity. A sufficient Th1 response is essential for early anti-viral activity and promotes elevated immune surveillance, enhanced viral clearance, and memory responses. Evidence suggests, however, that a prolonged or exaggerated Th1 inflammatory response triggers tissue damage causing lung pathology ultimately reducing survival rates (31).
We found that the same moderate exercise that improved survival also resulted in significantly lower cell infiltration into the lungs and draining lymph nodes and reduced (but not absent) IFN-γ mRNA and protein expression 3 and 5 days post influenza infection. Qualitative protein expression analysis (e.g., antibody array) revealed a two-fold reduction in Th1 type cytokines and chemokines including IFN-γ, IL-17, IL-13, interferon-inducible T-cell alpha chemoattractant (ITAC), leptin, stromal cell-derived factor-1 (SDF-1), and lipopolysaccharide-inducible CXC chemokine (LIX). Contradicting our hypothesis, however, was an observed increase in IL-12, and no change in IL-2, both hallmark cytokines of a Th1 response. In regard to IL-12, our data revealed that the protein was expressed at extremely low levels in the lung tissue. IL-2 plays a critical role in the differentiation and maturation of T regulatory cells (CD4+CD25+), which are anti-inflammatory immune cells which play a crucial role in controlling Th1-type inflammatory responses. A possible explanation is that exercise may not reduce IL-2, but instead improves Treg maturation, promoting an anti-inflammatory environment in the lungs. This hypothesis has yet to be tested.
In parallel with the reduction in many (but not all) Th1 cytokines, we observed a shift toward Th2-type anti-inflammatory cytokine profile. IL-4 lung protein levels were 2-fold higher for the exercising mice compared to sedentary mice 3 days post-infection. IL-4 promotes the differentiation of naïve Th cells to a Th2 phenotype, which then co-stimulate B cells, initiating the production of virus neutralizing antibodies. Viral antibodies reduce viral load by inhibiting infection of uninfected cells and opsonizing virus-infected cells for ADCC. Further supporting the anti-inflammatory hypothesis, exercise increased soluble TNF-α receptor (sTNFrII), which binds circulating TNF-α, preventing membrane binding and the subsequent activation of NF-κβ signaling pathways. In addition to elevated expression of Th2-type cytokines, exercise increased expression of eosinophil chemoattractants, which induce extravasation of eosinophils into viral infected tissue, where their ribonucleases can degrade viral single-stranded RNA, inhibiting virus replication. In summary, we found that moderate intensity exercise following influenza infection reduced lung inflammation by inducing a shift from Th1-type inflammatory response to an anti-inflammatory Th2-type response and this was associated with a reduction in morbidity and mortality. Our findings are consistent with a study by Kohut et al. who demonstrated that intense, prolonged exercise resulted in reduced IFN-γ and pro-inflammatory cytokines in splenocytes stimulated ex vivo with HSV-1 (13). In contrast, they also reported reduced IL-2 production. Comprehensive quantitative analysis of the Th1 and Th2 response in the lungs and respiratory tract of infected mice is needed to definitively determine whether exercise skews cytokine balance.
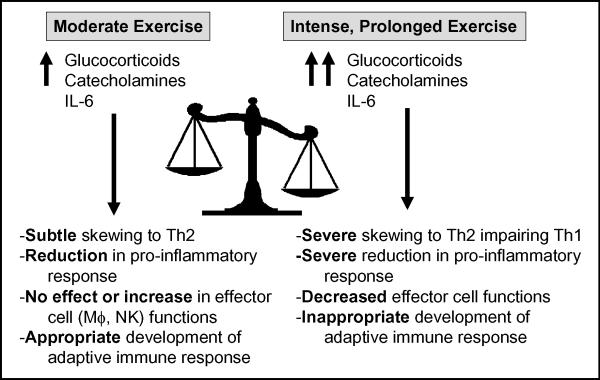
Explanation of the hormetic effect (e.g., favorable biological responses at low doses, unfavorable responses at higher doses) of exercise on survival in response to a primary viral infection is a difficult task. Although speculative, it may be that intense, prolonged exercise affects the cytokine balance and immune cell function more dramatically than moderate exercise. Based upon our data, our current hypothesis (Fig. 4) maintains that moderate exercise causes a subtle shift away from Th1 and toward a Th2 response, enhancing recovery and improving survival rates in cases where viral load and morbidity/mortality risk is high. Under these conditions, an exaggerated inflammatory response to the high viral load contributes to the pathology seen in the lung and, ultimately, increased morbidity and mortality. There appears to be a point of diminishing return however; as intense, prolonged exercise leads to a suppression of inflammation and reduction in critical anti-viral effector functions, including those of alveolar Mϕ's (5) and perhaps NK cells (22) resulting in increased morbidity and mortality. Indeed, a role for exercise-induced modulation of alveolar Mϕ function in response to HSV-1 infection has been elegantly described by Murphy et al. (20). In that study, intranasal treatment with clodronate liposomes (which depleted alveolar Mϕ's) completely inhibited the protective effect of exercise on HSV-1 mortality and morbidity, suggesting a critical role of lung Mϕ's in the initial recognition and clearance of that virus. This contrast between moderate intensity exercise and prolonged or high-intensity exercise is supported by numerous studies (1,10,19) which demonstrate a highly polarized Th2 response, as observed during prolonged intense exercise (29,30), may be detrimental to influenza recovery. Together, these findings suggest influenza outcomes are primarily mediated by the Th1/Th2 balance, and a moderate exercise-induced counter-regulatory shift toward Th2 response, without profound suppression of Th1 response, may reduce mortality and morbidity in high risk individuals or in response to inoculums that are quite large (as were the one's used in the above animal studies). It is important to note, however, that our hypothesized model of exercise and respiratory viral infection may not hold true for lower inoculums of virus or secondary exposures.
Figure 4.
Hypothetical model describing the exercise dose-response effect on Th1 and Th2 immune responses to respiratory viral infection. Moderate exercise transiently increases glucocorticoids, catecholamines and IL-6 to moderate levels resulting in a subtle skewing away from Th1 and towards Th2 while either not affecting or increasing key effector cell functions and allowing for development of an appropriate adaptive immune response. Conversely, intense, prolonged exercise results in a greater, longer lasting increase in glucocorticoids, catecholamines and IL-6 resulting in severe skewing away from Th1 towards Th2, decreased effector cell function and failure to develop appropriate adaptive immune responses.
As for the direct modulators responsible for a skewing of the immune response, exercise and other physical/physiological stressors promote upregulation of stress hormones, particularly catecholamines and glucocorticoids, which are capable of binding immune cells and influencing anti-viral immune functions. Dhabhar et al. suggests stress hormones exert a bi-directional effect on immune function, with the slightly elevated concentrations of glucocorticoids and catecholamines observed during acute stress providing crucial immunoenhancing and anti-inflammatory effects during pro-inflammatory reactions (7). In contrast, chronic stress, which affects circadian rhythms and significantly elevates stress hormone concentration for prolonged periods, exerts immunosuppressive effects and increases susceptibility to infection. Indeed, adrenalectomy and glucocorticoid/catecholamine blockade exacerbates inflammatory diseases and eliminates stress-induced enhancement of skin delayed-type hypersensitivity (DTH) reactions (7). In addition to stress hormones, exercise increases IL-6 locally in muscle and systemically in blood (26) which subsequently induces IL1-ra, sTNF receptor and IL-10 that may limit excessive inflammation induced by respiratory virus infection (Fig. 4). It appears that the balance between inadequate and excessive stress responses is the result of evolutionary selective pressure. Acute stressors of limited duration, such as moderate intensity exercise or being chased by a predator, stimulate “fight or flight” responses priming the immune system for potential challenges imposed by the stressor. Chronic stressors, on the other hand, may be evolutionarily adaptive in that immunosuppression conserves energy potentially utilized for coping with the stressor; albeit at the cost of increased risk for infection (7). Moderate intensity exercise may provide an appropriate stress response that leads to immunopotentiation and anti-inflammatory actions resulting in improved recovery and survival following respiratory viral infection.
CONCLUSIONS
This article has provided evidence to support the hypothesis that moderate intensity exercise reduces inflammation and improves the immune response to respiratory viral infections. We hypothesize that acute and chronic moderate exercise induces a level of stress hormones that down-regulates excessive inflammation within the respiratory tract and aids in activating innate anti-viral immunity shifting the immune response towards a Th2 profile (Fig. 4), thereby balancing the Th1/Th2 responses to prevent an excessive Th1 immune reaction to these pathogens. Prolonged, intense exercise may do this as well but may shift the balance too much towards Th2 and away from Th1 actually allowing the virus to gain a better foothold and cause greater pathology. Further research is necessary to examine cellular and molecular mechanisms through which exercise modulates immune function. Additionally, human studies should attempt to elucidate the most common respiratory pathogens responsible for infections associated with high intensity exercise training and athletic competitions, and the methods they employ to evade immune response, as well as attempt to translate mechanistic studies to a human experimental model. Based upon the available evidence, moderate intensity exercise training should be used as an adjunct to other preventative measures against respiratory tract viral infection.
Acknowledgments
This work supported by NIH grant AG-13928 (to J.A. Woods), American College of Sports Medicine Student Research Grant (to T.W. Lowder) and a grant from the University of Illinois Research Board
REFERENCES
- 1.Bot A, Holz A, Christen U, et al. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000;269(1):66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, Davis JM, Murphy EA, Carmichael MD, Ghaffar A, Mayer EP. Gender differences in viral infection after repeated exercise stress. Med Sci Sports Exerc. 2004;36(8):1290–5. doi: 10.1249/01.mss.0000135798.72735.b3. [DOI] [PubMed] [Google Scholar]
- 3.Chubak J, McTiernan A, Sorensen B, et al. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119(11):937–42. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis JM, Kohut ML, Colbert LH, Jackson DA, Ghaffar A, Mayer EP. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol. 1997;83(5):1461–6. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, Murphy EA, Brown AS, Carmichael MD, Ghaffar A, Mayer EP. Effects of oat beta-glucan on innate immunity and infection after exercise stress. Med Sci Sports Exerc. 2004;36(8):1321–7. doi: 10.1249/01.mss.0000135790.68893.6d. [DOI] [PubMed] [Google Scholar]
- 7.Dhabhar FS. Stress-induced augmentation of immune function--the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16(6):785–98. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 8.Ekblom B, Ekblom O, Malm C. Infectious episodes before and after a marathon race. Scand J Med Sci Sports. 2006;16(4):287–93. doi: 10.1111/j.1600-0838.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR7):1–60. [PubMed] [Google Scholar]
- 10.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180(4):1273–82. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath GW, Ford ES, Craven TE, Macera CA, Jackson KL, Pate RR. Exercise and the incidence of upper respiratory tract infections. Med Sci Sports Exerc. 1991;23(2):152–7. [PubMed] [Google Scholar]
- 12.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87(2):153–8. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 13.Kohut ML, Boehm GW, Moynihan JA. Prolonged exercise suppresses antigen-specific cytokine response to upper respiratory infection. J Appl Physiol. 2001;90(2):678–84. doi: 10.1152/jappl.2001.90.2.678. [DOI] [PubMed] [Google Scholar]
- 14.Kostka T, Berthouze SE, Lacour J, Bonnefoy M. The symptomatology of upper respiratory tract infections and exercise in elderly people. Med Sci Sports Exerc. 2000;32(1):46–51. doi: 10.1097/00005768-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19(5):377–80. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Lowder T, Padgett DA, Woods JA. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12(97–111) [PubMed] [Google Scholar]
- 17.Mathers C, Fat DM, Boerma JT, World Health Organization . The global burden of disease: 2004 update. World Health Organization; Geneva, Switzerland: 2008. p. vii.p. 146. [Google Scholar]
- 18.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34(8):1242–8. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70(8):5230–5. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy EA, Davis JM, Brown AS, et al. Role of lung macrophages on susceptibility to respiratory infection following short-term moderate exercise training. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1354–8. doi: 10.1152/ajpregu.00274.2004. [DOI] [PubMed] [Google Scholar]
- 21.Murphy EA, Davis JM, Carmichael MD, Gangemi JD, Ghaffar A, Mayer EP. Exercise stress increases susceptibility to influenza infection. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26(2):128–39. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Nieman DC, Johanssen LM, Lee JW. Infectious episodes in runners before and after a roadrace. J Sports Med Phys Fitness. 1989;29(3):289–96. [PubMed] [Google Scholar]
- 24.Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30(3):316–28. [PubMed] [Google Scholar]
- 25.Nieman DC, Nehlsen-Cannarella SL, Markoff PA, et al. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11(6):467–73. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu K, Kimura F, Akimoto T, et al. Effects of exercise, age and gender on salivary secretory immunoglobulin A in elderly individuals. Exerc Immunol Rev. 2007;13(55–66) [PubMed] [Google Scholar]
- 28.Spence L, Brown WJ, Pyne DB, et al. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc. 2007;39(4):577–86. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Nakaji S, Kurakake S, et al. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc Immunol Rev. 2003;9(48–57) [PubMed] [Google Scholar]
- 30.Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8(6–48) [PubMed] [Google Scholar]
- 31.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;74(1–2):109–16. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 32.Vu TFS, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 33.Weidner TG, Cranston T, Schurr T, Kaminsky LA. The effect of exercise training on the severity and duration of a viral upper respiratory illness. Med Sci Sports Exerc. 1998;30(11):1578–83. doi: 10.1097/00005768-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wong CM, Lai HK, Ou CQ, et al. Is exercise protective against influenza-associated mortality? PLoS ONE. 2008;3(5):e2108. doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]