Abstract
Background:
Limited data exist regarding the population-based epidemiology of idiopathic pulmonary fibrosis (IPF). The objective of the study was to describe the trends in the incidence, prevalence, and clinical course of IPF in the community.
Methods:
We conducted a population-based study of adult patients with IPF in Olmsted County, Minnesota, from 1997 to 2005. Two methods were used to identify IPF cases, as defined by the 2002 American Thoracic Society/European Respiratory Society consensus statement: (1) usual interstitial pneumonia (UIP) on a surgical lung biopsy specimen or a definite UIP pattern on a high-resolution CT image (narrow criteria) and (2) UIP on a surgical lung biopsy specimen or a definite or possible UIP pattern on CT image (broad criteria).
Results:
Of 596 patients screened for the possibility of pulmonary disease or pulmonary fibrosis over 9 years of follow-up, 47 cases had IPF. Of these, 24 met the narrow criteria. The age- and sex-adjusted incidence was 8.8/100,000 and 17.4/100,000 person-years, for narrow and broad criteria, respectively. The age-adjusted incidence was higher in men than in women, and among patients aged 70-79 years. During the study period, the incidence of IPF decreased (P < .001). On December 31, 2005, the age- and sex-adjusted prevalence was 27.9/100,000 and 63/100,000 persons by narrow and broad criteria, respectively. Thirty-seven patients experienced a total of 53 respiratory exacerbations (26 IPF related, 27 non-IPF related), and 34 (72%) patients died. The primary cause of death was IPF related in 16 (47%) patients. Median survival for narrow-criteria and broad-criteria incidence cases was 3.5 and 4.4 years, respectively.
Conclusions:
The incidence of IPF in Olmsted County decreased over the study period. Nonprimary IPF respiratory exacerbations are as frequent as primary IPF respiratory exacerbations and an important cause of death.
Idiopathic pulmonary fibrosis (IPF) is one of the most common forms of interstitial lung disease and is associated with substantial morbidity and mortality. Data on the current secular trends and anticipated burden of IPF are sparse. Data from population-based epidemiologic studies of IPF are essential to understand the disease trends and burden.
Current estimates of the incidence and prevalence of IPF are derived from patient registries that did not use or were published prior to the acceptance of the current diagnostic criteria for IPF.1-7 These studies do not distinguish incident and prevalent cases and are therefore subject to selection bias, which may influence their estimates of true incidence or prevalence of IPF. Further, some of these studies were conducted prior to the widespread use of high-resolution computed tomography (HRCT) scanning, which is more sensitive and specific than chest radiography to detect and diagnose IPF.8,9 Other studies are limited by their use of convenience sampling or by overreliance on diagnostic coding to identify and verify cases.10,11
The main objective of this study was to perform a population-based cohort study among residents of Olmsted County, Minnesota, between 1997 and 2005, with the intention of updating and describing the age- and sex-adjusted incidence of IPF, as defined by the American Thoracic Society and European Respiratory Society (ATS/ERS)12 consensus statement. Further, we analyze the subjects’ clinical course and survival and provide an estimate of recent prevalence with projected incidence of IPF for the United States through 2050.
Materials and Methods
Study Design and Settings
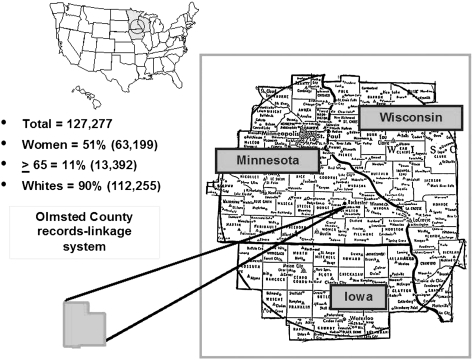
Following approval from the Mayo Foundation and Olmsted Medical Center Institutional Review Board, we conducted a community-based historical cohort study of IPF in Olmsted County (Fig 1). Population-based research is feasible in Olmsted County through the use of the unique resources of the Rochester Epidemiology Project (REP), a medical record linkage system for health-care providers to residents of Olmsted County.13,14 Medical care is essentially self-contained within this community of approximately 128,000 residents. The diagnoses, surgical procedures, radiographic reports, and pathology reports, including autopsies, recorded in these records are indexed, and all medical records are retrievable for review.13
Figure 1.
Olmstead County records-linkage system.
Procedures
Cases were identified through the diagnostic index, using the International Classification of Diseases, 9th ed code 516.3 and the Hospital International Classification of Diseases-Adapted codes 517 and 519. For each patient identified, the complete (inpatient and outpatient) medical record in the community was concomitantly reviewed by a trained data abstractor and one of the investigators (E.R.F.P.), and quality control of the data was provided independently by C.E.D. and J.H.R. In addition, for each case identified, the HRCT images were reviewed by two thoracic radiologists (B.J.B. and T.E.H.) and decisions were reached by consensus. The reviewers ranked their certainty of the diagnosis of usual interstitial pneumonia (UIP) on a three-point scale (definite UIP, possible UIP, and unlikely UIP). For a definite diagnosis of UIP based on CT examination, the imaging findings had to include reticular infiltrates and honeycombing as predominant findings. Additionally, the findings had to have subpleural and basilar predominant distribution. The above description minus either honeycombing or a basilar predominant distribution was used to identify the possible UIP cases. Cases that were categorized as unlikely UIP lacked most or all of the imaging findings and the distribution attributed to the definite UIP category. All lung biopsy specimens were reviewed by a pulmonary pathologist (E.S.Y.) to confirm the diagnosis of UIP based on surgical lung biopsy specimens and according to standard criteria.12 During the medical record review, we identified all Olmsted County residents (ie, established residency in the county for more than 1 year before diagnosis) who met the ATS/ERS consensus statement criteria for probable (nonbiopsy cases) and definite IPF between January 1, 1997, and December 31, 2005. In addition, we queried all histopathologic information from the Mayo Clinic tissue registry database. Finally, we performed a text search of all clinical notes and pathology data containing textual information for the words “pulmonary fibrosis” and/or “lung fibrosis” from the electronic Mayo Clinic Life Sciences System. The system is a combination of data warehouse and search engine that stores patients’ clinical, laboratory, and radiographic data and facilitates searches to identify subsets of patients with specific features and medical histories.
Case Definition
IPF cases were identified by two methods: (1) evidence of UIP on surgical lung biopsy specimens or definite UIP pattern on HRCT images (narrow-case finding criteria) and (2) evidence of UIP on surgical lung biopsy specimens or a definite or possible UIP pattern on HRCT images (broad-case finding criteria, representing the entire patient study cohort). Patients under the first method met all major and minor ATS/ERS criteria for diagnosis of IPF used in the absence of surgical lung biopsy.12 The second method included all of those patients in the first method and a subgroup of patients that met the ATS/ERS criteria for diagnosis of IPF, but in whom the HRCT features were characterized as possible for UIP.
Statistical Analysis
Baseline characteristics were summarized for incidence cases using means and SDs or number and frequency percentages. These summaries were performed overall and also stratified by calendar year of IPF diagnosis. Incidence rates were calculated as of the date the patient met ATS/ERS diagnostic criteria for IPF. The denominator age- and sex-specific person-years for the population of Olmsted County residents aged 50 years or older were estimated from decennial census data, with interpolation between census years. Standard errors and 95% CIs were calculated for the rates, assuming that they follow a Poisson distribution. Incidence rates were directly adjusted for age or age and sex to the population structure of white persons in the United States in 2000. Poisson regression was used to compare male and female incidence rates adjusted for age. The projected number of newly diagnosed IPF cases in the United States per year through 2050 was estimated using the age-specific and sex-specific incidence rates for the final 3 years of the study period and the age-specific and sex-specific US population projections as indicated by the US Census Bureau.15
Incidence patients were followed using their records in the community until the date of their last known vital status. Survival after IPF diagnosis was estimated using the Kaplan-Meier method. Survival of incidence cases was compared with that expected of Minnesota’s white population, matched for age and sex, and tested using a one-sample log-rank test. The prevalence of IPF was calculated as of December 31, 2005, under the assumption that patients with incidence dates prior to 1997 would not have survived to this date. The rate of respiratory exacerbations following IPF diagnosis (overall, IPF related, non-IPF related) was calculated by dividing the observed number of events by the total person-years of follow-up. All analyses were performed using SAS software (SAS version 8; SAS Institute Inc; Cary, NC).
Results
During the 9-year study period, 596 Olmsted County residents aged 18 years or older were initially screened and identified to have possible pulmonary fibrosis (89% > 50 years old). After review of the medical records, 499 patients were excluded because they did not have idiopathic interstitial pneumonia (eg, chronic pulmonary infections), 42 had a known cause for pulmonary fibrosis (eg, connective tissue disease), three had an unlikely UIP pattern on CT image (with no lung biopsy), and five had IPF first identified before January 1, 1997 (and were therefore not incident cases). Thus, the study population consisted of a total of 47 new cases of IPF (19 women and 28 men, mean age at diagnosis 73.5 ± 7.9 years), fulfilling the broad-case criteria for IPF (194, person-years of follow-up; median length of follow-up, 3.5 years). Fourteen (29%) had histologically proven UIP. Of those without histologically proven UIP, 10 (30%, 10/33) had a definite UIP pattern on HRCT examination (Table 1). The baseline characteristics are displayed in Table 1. None of the study patients during the study period and follow-up period underwent lung transplantation.
Table 1.
—Baseline Characteristics of Incident Cases Stratified by Calendar-Year of IPF Diagnosis
| Variable | Calendar-Year of IPF Diagnosis | |||
| Overall, 1997-2005, N = 47 | 1997-1999, n = 20 | 2000-2002, n = 16 | 2003-2005, n = 11 | |
| Age, y | ||||
| Mean and SD | 73.5 ± 7.8 | 74.6 ± 8.9 | 72 ± 7.2 | 74.2 ± 7.0 |
| 50-59 | 2 (4) | 1 (5) | 1 (6) | 0 |
| 60-69 | 15 (32) | 6 (30) | 5 (31) | 4 (36) |
| 70-79 | 20 (42) | 8 (40) | 8 (50) | 4 (36) |
| > 80 | 10 (22) | 5 (25) | 2 (12) | 3 (27) |
| Men | 28 (59) | 11 (55) | 11 (69) | 6 (54) |
| BMI, kg/m2 | 27.1 ± 4.9 | 26.1 ± 3.8 | 29.2 ± 6 | 26.2 ± 2 |
| Surgical lung biopsy examination-proven UIP | 14 (29) | 5 (25) | 5 (31) | 4 (36) |
| CT fibrosing score, % | 29.8 ± 14 | 29.2 ± 14 | 30 ± 10 | 31 ± 18 |
| Definite UIP on CT pattern | 11 (23) | 6 (30) | 3 (19) | 2 (18) |
| Nonbiopsy cases with definite UIP CT pattern | 10 (21) | 6 (30) | 2 (12) | 2 (18) |
| Smoking, pack-years | ||||
| < 20 | 5 (11) | 1 (5) | 3 (19) | 1 (9) |
| 20-40 | 14 (29) | 5 (25) | 6 (37) | 3 (27) |
| > 40 | 9 (19) | 3 (15) | 3 (19) | 3 (27) |
| Never | 19 (40) | 11 (55) | 4 (27) | 4 (36) |
| Pulmonary function tests, % predicted | ||||
| TLC | 72.1 ± 15 | 68 ± 13.1 | 75.2 ± 12.8 | 74.3 ± 20 |
| FVC | 68.8 ± 18.8 | 65.2 ± 17.3 | 68.1 ± 20.2 | 74.2 ± 19.4 |
| FEV1 | 72.5 ± 17.7 | 69 ± 17.7 | 73.1 ± 20.5 | 77.3 ± 13.2 |
| FEV1/FVC | 82.5 ± 8.5 | 83.2 ± 9.4 | 82.3 ± 8.8 | 82.1 ± 7.3 |
| DLCO | 49.1 ± 16.4 | 49.8 ± 14.9 | 49.8 ± 19.3 | 47.1 ± 15.5 |
| Spo2, rest | 94.5 ± 1.8 | 94 ± 2 | 95.1 ± 1.7 | 94.6 ± 1.5 |
| Spo2, exercise | 88.7 ± 4.5 | 87 ± 4.7 | 90.4 ± 3.8 | 89 ± 4.6 |
| Comorbidities | ||||
| Pulmonary hypertensiona | 25 (53) | 11 (55) | 9 (56) | 5 (45) |
| COPD | 13 (28) | 5 (25) | 5 (31) | 3 (27) |
| Obstructive sleep apnea | 8 (17) | 2 (10) | 4 (25) | 2 (18) |
| Gastroesophageal reflux | 26 (55) | 9 (45) | 12 (75) | 5 (45) |
| Coronary artery disease | 21 (45) | 7 (35) | 8 (50) | 6 (54) |
| Diabetes mellitus | 8 (17) | 3 (15) | 4 (25) | 1 (9) |
| Hypertension | 31 (66) | 9 (45) | 15 (94) | 7 (63) |
| Lung cancer | 4 (8) | 3 (15) | 1 (7) | 0 |
| Hypothyroidism | 14 (29) | 7 (35) | 4 (25) | 3 (27) |
| Congestive heart failure | 5 (11) | 3 (15) | 0 | 2 (18) |
| Atrial fibrillation | 9 (19) | 2 (10) | 4 (25) | 3 (27) |
| Depression | 5 (11) | 2 (10) | 2 (12) | 1 (9) |
| Dementia | 2 (4) | 1 (5) | 1 (6) | 0 |
| Transthoracic echocardiogram | ||||
| Right ventricular systolic pressure, mm Hg | 47.8 ± 15.9 | 53.8 ± 18 | 49.7 ± 15 | 37.7 ± 7.3 |
| Peak tricuspid regurgitation, m/s | 3 ± 0.6 | 3.4 ± 0.7 | 2.9 ± 0.6 | 2.8 ± 0.3 |
| Ejection fraction, % | 58.7 ± 11.5 | 57.5 ± 14 | 58.2 ± 12.4 | 61.1 ± 4.8 |
| New York Heart Association Class | ||||
| 1-2 | 40 (85) | 16 (80) | 14 (87) | 10 (90) |
| 3-4 | 7 (15) | 4 (20) | 2 (12) | 1 (9) |
Values are given as mean ± SD or n (%). DLCO = diffuse capacity of lungs for carbon monoxide; IPF = idiopathic pulmonary fibrosis; Spo25pulse oximetry; TLC = total lung capacity; UIP = usual interstitial pneumonia.
Right ventricular systolic pressure ≥ 40 mm Hg and peak tricuspid regurgitation ≥ 2.9 m/s on trans thoracic echocardiographic examination.
Incidence, Secular Trends, and Future Projections of IPF
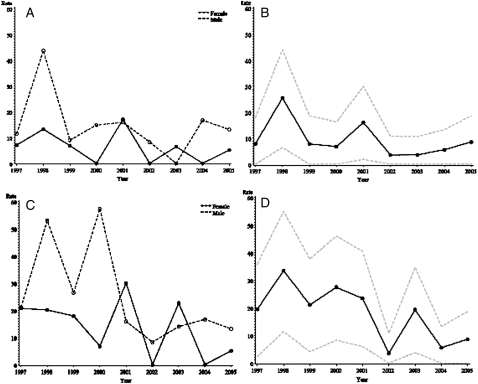
The age- and sex-adjusted incidence rate of IPF among residents aged 50 years or older diagnosed using the narrow-case criteria in Olmsted County between 1997 and 2005 was 8.8 cases per 100,000 person-years (95% CI, 5.3-12.4) (Table 2). Based on the broad-case criteria, the adjusted incidence rate was 17.4 cases per 100,000 person-years (95% CI, 12.4-22.4) (Table 2). The age-adjusted incidence rate, by both narrow-case and broad-case finding criteria, was higher in men than in women, with the highest incidence among those aged 70-79 years. Plots of the annual age-adjusted and sex-adjusted incidence rates (Fig 2) demonstrate that the observed incidence of IPF decreased over the study period (P < .001). Over the last 3 years of the study period (2003-2005), the age-adjusted and sex-adjusted incidence rate was 6.0 (95% CI, 1.2-10.8) and 11.0 (95% CI, 4.5-17.6) for narrow-case and broad-case criteria, respectively.
Table 2.
—Incidence per 100,000 Person-Years of IPF Stratified by Calendar-Year of IPF Diagnosis by Narrow-Case Finding Criteria and Broad-Case Criteria
| Narrow-Base Criteria | ||||
| Sex and Age | Calendar-Years of IPF Diagnosis | |||
| Overall, 1997-2005, N = 24 | 1997-1999, n = 11 | 2000-2002, n = 7 | 2003-2005, n = 6 | |
| Overall, age and sex adjusted incidence | 8.8 (24) | 13.68 (11) | 7.52 (7) | 5.96 (6) |
| 95% CI | 5.28-12.38 | 5.57-21.80 | 1.92-13.11 | 1.15-10.76 |
| Men, age adjusted incidence | 13.38 (15) | 21.31 (7) | 10.04 (4) | 9.90 (4) |
| 95% CI | 6.51-20.24 | 5.31-37.30 | 0.00-20.08 | 0.11-19.70 |
| Age, y | ||||
| 50-59 | 1.64 (1) | 0.00 (0) | 4.94 (1) | 0.00 (0) |
| 60-69 | 10.69 (4) | 17.87 (2) | 8.07 (1) | 7.24 (1) |
| 70-79 | 21.44 (5) | 42.28 (3) | 12.80 (1) | 11.88 (1) |
| > 80 | 41.26 (5) | 57.99 (2) | 25.13 (1) | 42.64 (2) |
| Women, age adjusted incidence | 6.08 (9) | 9.03 (4) | 5.68 (3) | 3.88 (2) |
| 95% CI | 2.08-10.08 | 0.17-17.89 | 0.00-12.18 | 0.00-9.27 |
| Age, y | ||||
| 50-59 | 1.55 (1) | 5.31 (1) | 0.00 (0) | 0.00 (0) |
| 60-69 | 4.98 (2) | 0.00 (0) | 7.62 (1) | 6.73 (1) |
| 70-79 | 16.72 (5) | 31.72 (3) | 9.95 (1) | 9.63 (10) |
| > 80 | 3.93 (1) | 0.00 (0) | 11.81 (1) | 0.00 (0) |
| Broad-Case Criteria | ||||
| Sex and Age | Calendar-Years of IPF Diagnosis | |||
| Overall, 1997-2005, N = 47 | 1997-1999, n = 20 | 2000-2002, n = 16 | 2003-2005, n = 11 | |
| Overall, age and sex adjusted incidence | 17.43 (47) | 24.65 (20) | 17.79 (16) | 11.04 (11) |
| 95% CI | 12.42-22.44 | 13.81-35.49 | 9.03-26.55 | 4.47-17.61 |
| Men, age adjusted incidence | 24.02 (27) | 33.50 (11) | 26.26 (10) | 14.41 (6) |
| 95% CI | 14.84-33.20 | 13.45-53.56 | 9.72-42.80 | 2.75-26.07 |
| Age, y | ||||
| 50-59 | 1.64 (1) | 0.00 (0) | 4.94 (1) | 0.00 (0) |
| 60-69 | 21.39 (8) | 26.81 (3) | 24.20 (3) | 14.47 (2) |
| 70-79 | 42.88 (10) | 70.47 (5) | 38.41 (3) | 23.76 (2) |
| > 80 | 66.02 (8) | 86.98 (3) | 75.40 (3) | 42.64 (2) |
| Women, age adjusted incidence | 13.43 (20) | 19.57 (9) | 12.22 (6) | 9.15 (5) |
| 95% CI | 7.50-19.37 | 6.72-32.43 | 2.37-22.07 | 1.05-17.26 |
| Age, y | ||||
| 50-59 | 1.55 (1) | 5.31 (1) | 0.00 (0) | 0.00 (0) |
| 60-69 | 12.44 (5) | 16.39 (2) | 7.62 (1) | 13.46 (2) |
| 70-79 | 36.79 (11) | 52.87 (5) | 39.79 (4) | 19.25 (2) |
| > 80 | 11.78 (3) | 12.62 (1) | 11.81 (1) | 11.03 (1) |
Data are presented as incidence rate per 100,000 person-years among people aged 50 or older, followed in parentheses by actual number of cases observed. See Table 1 for expansion of the abbreviation.
Figure 2.
Incidence rate of IPF adjusted to 2000 US white population per 100,000 person-years among residents of Olmstead County, Minnesota, from 1997 to 2005. Age-adjusted (A) and age-adjusted and sex-adjusted (B) incidence of narrow criteria cases of IPF with confidence intervals (N = 24). Age-adjusted (C) and age-adjusted and sex-adjusted (D) incidence of broad criteria cases of IPF with confidence intervals (N = 47). IPF = idiopathic pulmonary fibrosis.
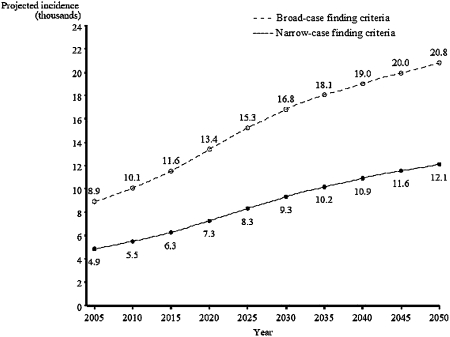
The number of new IPF cases is projected to increase over time, doubling by 2030; this corresponds to the large increase in the US population of people in older age groups (aged 65-84 years).14 On the basis of the age-specific incidence rate that we observed between 2003 and 2005 and projections of the future demographic characteristics of the US population, we estimate that by 2050 the annual number of new cases of IPF may be between 12,000 and 21,000 cases (Fig 3).
Figure 3.
Projected number of individuals with IPF in the United States between 2005 and 2050, assuming no further increase in age-adjusted IPF incidence rate, as evident in 2003 to 2005. See Figure 2 legend for expansion of the abbreviation.
Prevalence of IPF
On December 31, 2005, there were 32 Olmsted County residents alive with a diagnosis of IPF (10 in the narrow-case finding criteria and 22 in the broad-case finding criteria). The age-adjusted and sex-adjusted prevalence among people aged 50 years or older by narrow-case finding criteria was 27.9 cases per 100,000 persons (95% CI, 10.4-45.4) and 63 cases per 100,000 persons (95% CI, 36.4-89.6) by using broad-case finding criteria.
Clinical Course and Patient Survival
Ten patients had a slowly progressive respiratory decline without evidence for acute respiratory worsening that required hospitalization. Thirty-seven (79%, 37/53) patients experienced 53 hospital admissions for acute respiratory worsening. Twenty-six (49%, 26/53) of these episodes of acute respiratory worsening, occurring in 17 patients, were without clinically apparent infection, heart failure, pulmonary embolism, or other identifiable cause (ie, acute exacerbation of IPF). The rate of IPF-related exacerbation was 0.13 per person-year. The frequencies of IPF-related and non-IPF-related respiratory exacerbations were similar, and these rates remained stable over time following diagnosis (ie, the rates did not increase or decrease as a function of time after diagnosis) (Table 3).
Table 3.
—Respiratory Exacerbations After IPF Diagnosis
| Time After Diagnosis | ||||
| Overall | 0-2 y | 2-4 y | > 4 y | |
| Person-years of follow-up | 194.8 | 78.3 | 56.0 | 60.5 |
| Exacerbations | ||||
| IPF | 26 (0.13) | 9 (0.11) | 6 (0.11) | 11 (0.18) |
| Non-IPF | 27 (0.14) | 11 (0.14) | 10 (0.18) | 6 (0.10) |
| Total | 53 (0.27) | 20 (0.26) | 16 (0.29) | 17 (0.28) |
Forty-seven patients were followed for a total of 194.8 person-years (median 3.5; range 0.2-10.8 person-years) after IPF diagnosis. During follow-up, there were 37 patients who experienced a total of 53 respiratory exacerbations requiring hospitalization (26 IPF related, 27 non-IPF related). Seventeen patients had 26 IPF-related exacerbations, and 20 patients had 27 non-IPF-related exacerbations. The data presented represent the number (rate/person-year) of exacerbations. Data are presented overall and separately for three distinct time intervals after IPF diagnosis. See Figure 1 for expansion of the abbreviation.
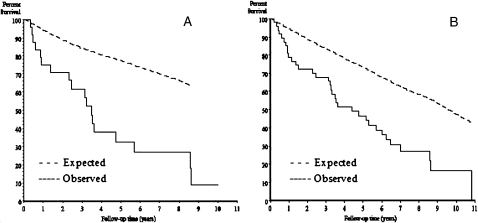
Thirty-four (72%) patients died during the 9-year observation period. The primary cause of death was IPF related in 16 (47%) patients (Table 4). The observed survival for narrow-case and broad-case criteria was significantly different from the expected survival for residents of Olmsted County (P < .001). Median survival for narrow- and broad-case criteria was 3.47 years and 4.37 years, respectively (Fig 4).
Table 4.
—Primary Causes of Death
| Causes of Death, n = 34 | Acute Onset of Dyspnea, < 4/wk | Subacute Onset of Dyspnea, 4-52/wk |
| Progression of IPF | 5 (29) | 10 (59) |
| Progression after video-assisted thoracoscopic surgery | 1 (6) | … |
| Cardiogenic pulmonary edema | 1 (6) | |
| Ischemic heart disease | 4 (23) | |
| Pulmonary embolism | 1 (6) | … |
| Arrhythmia | 1 (6) | … |
| Cor pulmonale | 1 (6) | … |
| Pneumonia | 1 (6) | 2 (12) |
| Sepsis | 1 (6) | … |
| Cerebrovascular accident | 1 (6) | … |
| Upper gastrointestinal bleed | 1 (6) | … |
| Complications after pleurodesis | … | 1 (6) |
| Unknown | … | 3 (17) |
| Total | 17 (100) | 17 (100) |
Values are given as n (%). See Table 1 for expansion of the abbreviation.
Figure 4.
Observed and expected cumulative survival of patients from time of IPF diagnosis, between 1997 and 2005, among residents of Olmstead Country, Minnesota. Narrow criteria cases of IPF (log rank, P < .0001) (A). Broad criteria cases of IPF (log rank, P < .0001). See Figure 2 legend for expansion of the abbreviation.
Discussion
To our knowledge, this is the first population-based study that provides an epidemiologic update of trends from the recent years in a general population of patients with IPF using ATS/ERS diagnostic criteria. We found that the adjusted incidence rate of IPF decreased during the 9-year study period, with peaks in 1998 and 2001.
The cause of the two distinct peaks observed in IPF incidence rate (1998 and 2001) is uncertain. Several explanations exist and include a reduction in disease latency following the introduction of ATS/ERS diagnostic criteria, changes in physicians’ threshold for referral for definitive diagnostic studies, a longer presymptomatic stage from 1997 to 2001, or a function of the small number of cases.
Heightened physician awareness after the introduction of the ATS/ERS consensus statement may explain the slight increase in IPF cases observed after 2002. If we assume that some of the incident ATS/ERS IPF cases in the early years (1997-2001) are prevalent undiagnosed cases before the 9-year study period, then the incidence and prevalence pattern in recent years (2003-2005) may represent more precise estimates.
The observed secular trends in incidence are not likely due to differences in disease ascertainment, implying alternating periods of high and low ascertainment. The initial disease diagnosis in most cases was clinical, complete medical records for all cases were available for review, and stringent but consistent ATS/ERS diagnostic criteria were applied, making overascertainment or screening bias after screening for IPF in individuals who did not soon come to clinical attention unlikely. Similarly, underestimation of the total burden of IPF (ie, undiagnosed or misdiagnosed cases) is unlikely given the valid screening method used (ie, REP) and the clinical, radiographic, and pathologic review of each excluded case.
Our observed changes in incidence rates are not due to a change in the demographics of the Olmsted County population at risk because all of the incidence rates were age-adjusted and sex-adjusted to a standard population. Trends in migration also are unlikely to explain our results because we required confirmation of residency in Olmsted County at the time of onset of IPF. Moreover, our entire cohort consisted of Olmsted County residents for at least 1 year prior to the diagnosis of IPF and at the end of study follow-up. Accordingly, migration into Olmsted County for reasons related to health care is an unlikely explanation for the incidence trends observed in our study. No significant change in smoking prevalence was observed during the study period, and detailed information on occupations in this cohort was beyond the scope of this study; however, we observed no particular trends to explain changes in incidence rates.
The age-adjusted incidence rate in persons aged 50 years or older using narrow-case finding criteria of 9.9 (95% CI, 0.1-19.7) in men and 3.9 (95% CI, 0.0-9.3) in women per 100,000 person-years from 2003 to 2005 is similar to that reported from the New Mexico Interstitial Lung Disease registry in Bernalillo County between 1988 and 1990, in which the overall age-unadjusted incidence rate of IPF was estimated to be 10.7 for men and 7.4 for women per 100,000 person-years.9 Although, we are tentative to suggest that the incidence rate of IPF has remained relatively stable over the past two decades, the comparison of the results obtained in the present study with those of Coultas et al9 is problematic because of significant methodologic differences between studies. Our findings are consistent with prior study estimates of survival among persons with IPF (median, 3-5 years) as well as the preponderance in men and in persons aged 60 years or older.16-18 Referral bias is unlikely with the latter, as medical care in Olmsted County is readily available and the quality is comparable for elderly men and women.13,14
The current study provides a population-based estimate on the rate of acute exacerbation in patients with IPF. Interestingly, nonprimary IPF respiratory exacerbations were as frequent as primary IPF respiratory exacerbations, and were an important cause of death. Clinical progression of IPF preceded death in 47% (16/34) of patients (see Table 4). While the optimal therapy for IPF remains yet to be identified, the effectiveness of treatment strategies targeting the longitudinal interactions among prevalent comorbid conditions such as pulmonary hypertension, gastroesophageal reflux, and cardiovascular risk factors19-22 on the prevention of primary or non-IPF respiratory exacerbations needs further research.
The main strengths of this study are that it was population-based, it used predefined and accepted criteria to identify patients with IPF, and the ascertainment of cases within a defined geographic region approached 100%. The REP provides a wealth of clinical detail, enabling us to more specifically identify true IPF cases. This study illustrates the difficulty of previous studies that rely on diagnostic codes and surveys in identifying IPF cases. Less then 10% of the original cases identified proved to be IPF by ATS/ERS search criteria.
A potential limitation of the study is the use of CT examinations to make a definitive diagnosis of UIP in those cases in which biopsy specimens were not available. However, in a prospective study by Hunninghake et al,23 it was shown that by using the criteria outlined in the procedures section to diagnose UIP based on CT findings, when a definite diagnosis of UIP is made, the radiologist is correct 96% of the time. This was shown to be comparable to the rate of correct diagnosis of the disease in the same study. Therefore, it was the conclusion of Hunninghake et al that when the CT findings were compatible with a definite diagnosis of UIP, then in the appropriate clinical setting a biopsy was not necessary to confirm the diagnosis. For the possible UIP cases diagnosed using CT results, the correlation is not as strong, and it is possible that in the broad-case finding criteria group there were a small number of cases that were not UIP that were included in the numbers.
Our study is limited by its focus on a small Midwestern community that is predominantly white, and these results might not be generalizable to locations with larger minority populations. However, the socio-demographic characteristics of Olmsted County residents are generally similar to other white populations in the United States. The study was based on 47 case-patients diagnosed by ATS/ERS criteria, which could be viewed as too small for reliability. Although this may be a relatively low number of case-patients in comparison with other studies in the literature that address the epidemiology of IPF, the precision and validity of our results are based on the availability of the original medical records through the REP,13,14 which enables the identification and reclassification of potential study subjects based on contemporary diagnostic criteria, irrespective of the patients’ assigned diagnoses at the time of their medical care.14 Lastly, uncertainty remains with respect to the validity of projecting the incidence estimated in our study to the US population because of the small number of cases and especially because we cannot quantify potential errors in the US Census age-specific and sex-specific population projections and errors inherent in our underlying assumptions.
On the basis of our results, the community-based, age-adjusted incidence rate of IPF decreased over the study period. Nonprimary IPF respiratory exacerbations are as frequent as primary IPF respiratory exacerbations and are an important cause of death.
Acknowledgments
Author contributions: Dr Fernández Pérez: performed the study conceptualization and design; assisted in data collection; worked on clinical, radiologic, and pathologic case characterization; performed statistical analyses; and assisted in manuscript preparation, revision, and final approval.
Dr. Daniels: worked on clinical, radiologic, and pathologic case characterization and assisted in manuscript preparation, revision, and final approval.
Mr Schroeder: assisted in data collection; performed statistical analyses; and assisted in manuscript preparation, revision, and final approval.
Dr St. Sauver: performed statistical analyses and assisted in manuscript revision and final approval.
Dr Hartman: worked on radiologic case characterization and assisted in manuscript preparation, revision, and final approval.
Dr Bartholmai: worked on radiologic case characterization and assisted in manuscript revision and final approval.
Dr Yi: worked on pathologic case characterization and assisted in manuscript revision and final approval.
Dr Ryu: worked on clinical, radiologic, and pathologic case characterization and assisted in manuscript preparation, revision, and final approval.
Financial/nonfinancial disclosures: The authors have reported to the CHEST the following conflict of interest: Dr. Bartholmai has received some research funding from GlaxoSmithKline. The other authors have reported no potential conflicts with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Aaron Bungum for his help with the study coordination and data collection.
Abbreviations
- ATS/ERS
American Thoracic Society and European Respiratory Society
- HRCT
high-resolution computed tomography
- IPF
idiopathic pulmonary fibrosis
- REP
Rochester Epidemiology Project
- UIP
usual interstitial pneumonia
Footnotes
An abstract of this study was presented at the American College of Chest Physicians 74th Congress, October 25-30, 2008, Philadelphia, Pennsylvania.
Funding/support: This study was made possible by the Rochester Epidemiology Project (Grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Roelandt M, Demedts M, Callebaut W, et al. Epidemiology of interstitial lung disease (ILD) in Flanders: registration by pneumologists in 1992-1994. Working group on ILD, VRGT. Vereniging voor Respiratoire Gezondheidszorg en Tuberculosebestrijding. Acta Clin Belg. 1995;50(5):260–268. doi: 10.1080/17843286.1995.11718459. [DOI] [PubMed] [Google Scholar]
- 2.Schweisfurth H, Kieslich C, Satake N, et al. How are interstitial lung diseases diagnosed in Germany? Results of the scientific registry for the exploration of interstitial lung diseases (“Fibrosis Registry”) of the WATL [in German] Pneumologie. 2003;57(7):373–382. doi: 10.1055/s-2003-40557. [DOI] [PubMed] [Google Scholar]
- 3.Thomeer M, Demedts M, Vandeurzen K VRGT Working Group on Interstitial Lung Diseases. Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta Clin Belg. 2001;56(3):163–172. doi: 10.1179/acb.2001.026. [DOI] [PubMed] [Google Scholar]
- 4.von Plessen C, Grinde O, Gulsvik A. Incidence and prevalence of cryptogenic fibrosing alveolitis in a Norwegian community. Respir Med. 2003;97(4):428–435. doi: 10.1053/rmed.2002.1466. [DOI] [PubMed] [Google Scholar]
- 5.Xaubet A, Ancochea J, Morell F, et al. Spanish Group on Interstitial Lung Diseases, SEPAR. Report on the incidence of interstitial lung diseases in Spain. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(1):64–70. [PubMed] [Google Scholar]
- 6.Kolek V. Epidemiology of cryptogenic fibrosing alveolitis in Moravia and Silesia. Acta Univ Palacki Olomuc Fac Med. 1994;137:49–50. [PubMed] [Google Scholar]
- 7.Agostini C, Albera C, Bariffi F, et al. Registro Italiano Pneumopatie Infiltrative Diffuse. First report of the Italian register for diffuse infiltrative lung disorders (RIPID) Monaldi Arch Chest Dis. 2001;56(4):364–368. [PubMed] [Google Scholar]
- 8.Demedts M, Wells AU, Antó JM, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J Suppl. 2001;32:2s–16s. [PubMed] [Google Scholar]
- 9.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 11.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30(4):819–834. doi: 10.1016/j.rdc.2004.07.010. vii. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Census Bureau. US Census Bureau Population Projections. http//:www.census.gov/population/www/projections/usinterimproj/ . Accessed April 28, 2009.
- 16.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 17.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 18.Mapel DW, Hunt WC, Utton R, Baumgartner KB, Samet JM, Coultas DB. Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax. 1998;53(6):469–476. doi: 10.1136/thx.53.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadrous HF, Pellikka PA, Krowka MJ, et al. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(6 Suppl):616S–617S. doi: 10.1378/chest.128.6_suppl.616S-a. [DOI] [PubMed] [Google Scholar]
- 20.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175(9):875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 21.Kizer JR, Zisman DA, Blumenthal NP, et al. Association between pulmonary fibrosis and coronary artery disease. Arch Intern Med. 2004;164(5):551–556. doi: 10.1001/archinte.164.5.551. [DOI] [PubMed] [Google Scholar]
- 22.Tobin RW, Pope CE, II, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(6):1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 23.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]