Abstract
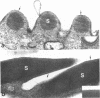
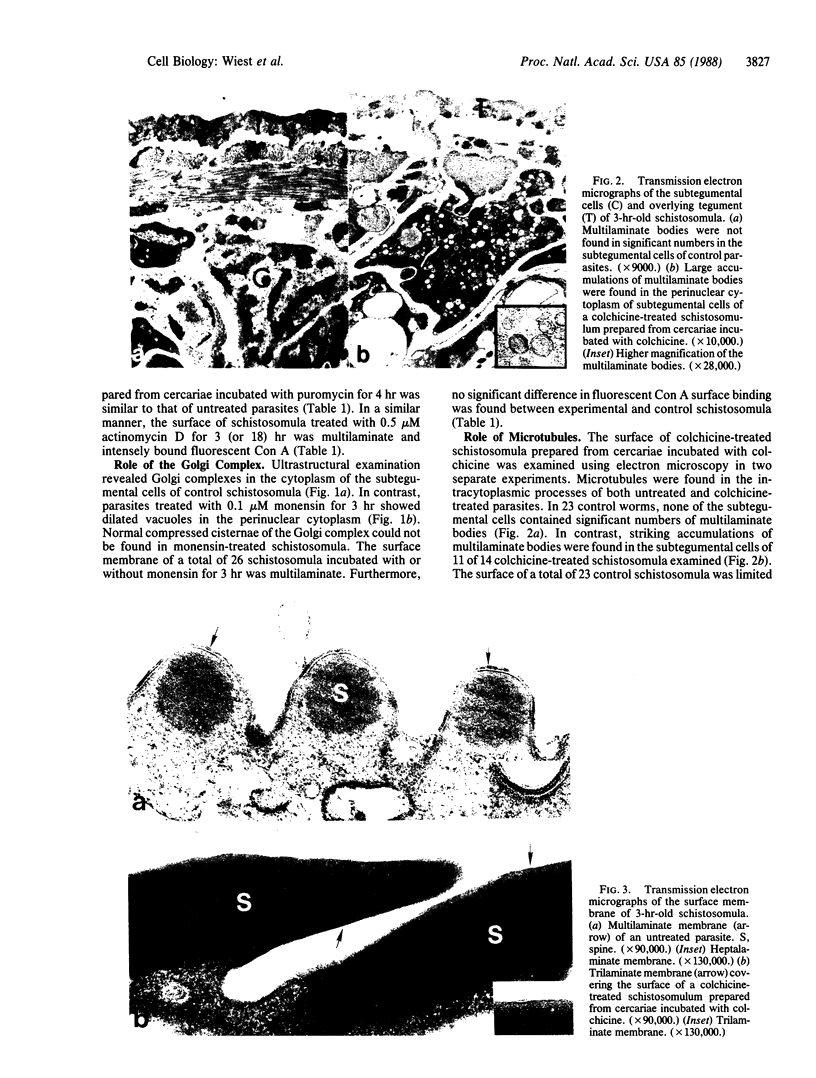
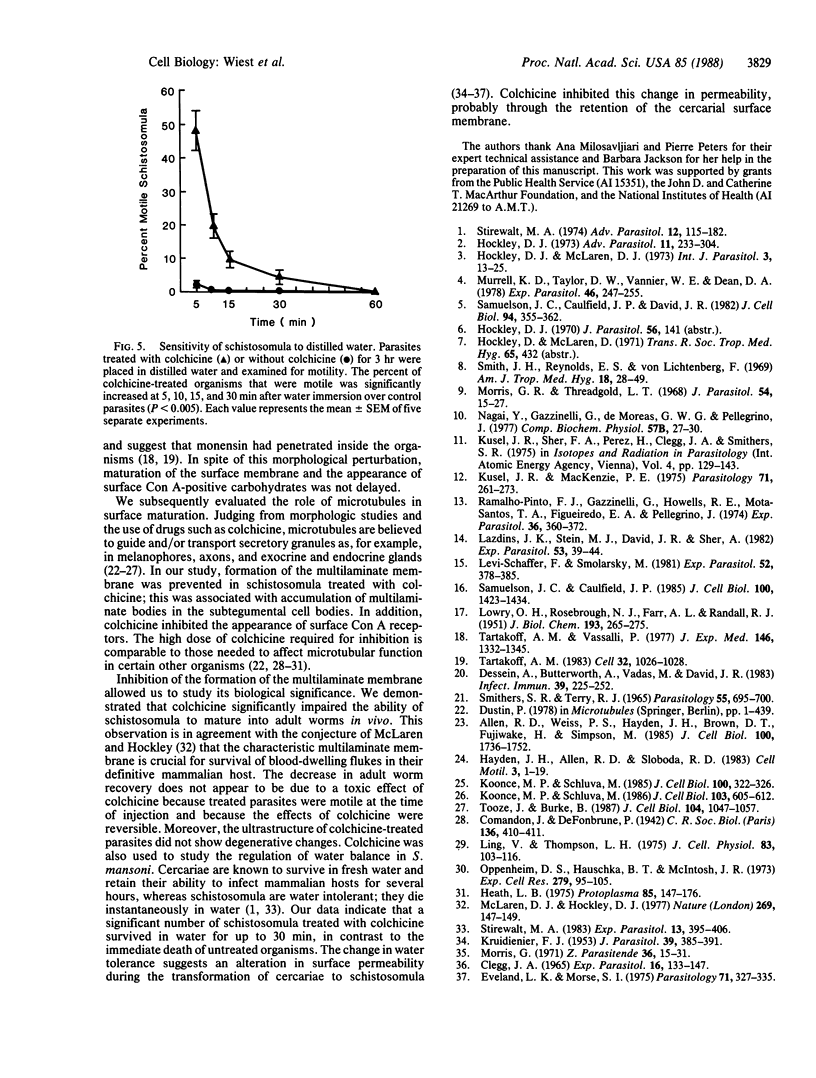
The surface membrane of the multicellular parasite Schistosoma mansoni is radically reorganized during the transformation of cercariae into schistosomula. The current study investigates factors involved in maturation of the surface from a trilaminate to a multilaminate membrane. When maturation was induced in the presence of puromycin (900 microM), the acquisition of a multilaminate surface and stainability with fluorescein-conjugated Con A were similar to that of control parasites. Similarly, although organisms treated with monensin (0.1 microM) for 3 hr showed large vacuoles in the perinuclear cytoplasm of the subtegumental cells, the surface membrane became multilaminate. In contrast, microtubule-active drugs interfered with maturation: the surface remained largely trilaminate and the percentage of organisms binding Con A to their surface was significantly reduced. Furthermore, large accumulations of multilaminate bodies were found in the subtegumental cells of colchicine-treated parasites, whereas few were seen in the controls. Colchicine-treated schistosomula failed to mature to adult worms upon injection into mice and, like cercariae, they were water tolerant. We therefore conclude that the components that constitute the schistosomula surface preexist in cercariae and suggest that they are stored in multilaminate bodies before being transported to the surface with the help of microtubules. The acquisition of the multilaminate membrane may be essential for survival of the parasites in vivo and in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Weiss D. G., Hayden J. H., Brown D. T., Fujiwake H., Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985 May;100(5):1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEGG J. A. IN VITRO CULTIVATION OF SCHISTOSOMA MANSONI. Exp Parasitol. 1965 Apr;16:133–147. doi: 10.1016/0014-4894(65)90037-8. [DOI] [PubMed] [Google Scholar]
- Dessein A., Butterworth A. E., Vadas M. A., David J. R. Maturation in vivo of Schistosoma mansoni schistosomula after culture in vitro with granulocytes and antibody. Infect Immun. 1983 Jan;39(1):225–232. doi: 10.1128/iai.39.1.225-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland L. K., Morse S. I. Schistosoma mansoni: in vitro conversion of cercariae to schistosomula. Parasitology. 1975 Oct;71(2):327–335. doi: 10.1017/s003118200004676x. [DOI] [PubMed] [Google Scholar]
- Hayden J. H., Allen R. D., Goldman R. D. Cytoplasmic transport in keratocytes: direct visualization of particle translocation along microtubules. Cell Motil. 1983;3(1):1–19. doi: 10.1002/cm.970030102. [DOI] [PubMed] [Google Scholar]
- Heath I. B. The effect of antimicrotubule agents on the growth and ultrastructure of the fungus Saprolegnia ferax and their ineffectiveness in disrupting hyphal microtubules. Protoplasma. 1975;85(2-4):147–176. doi: 10.1007/BF01567943. [DOI] [PubMed] [Google Scholar]
- Hockley D. J., McLaren D. J. Schistosoma mansoni: changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973 Jan;3(1):13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Hockley D. J. Ultrastructure of the tegument of Schistosoma. Adv Parasitol. 1973;11(0):233–305. doi: 10.1016/s0065-308x(08)60188-8. [DOI] [PubMed] [Google Scholar]
- Hockley D., McLaren D. The outer membrane of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1971;65(4):432–432. doi: 10.1016/0035-9203(71)90136-2. [DOI] [PubMed] [Google Scholar]
- KRUIDENIER F. J. Studies on the formation and function of mucoid glands in cercariae; opisthorchoid cercariae. J Parasitol. 1953 Aug;39(41):385–391. [PubMed] [Google Scholar]
- Koonce M. P., Schliwa M. Bidirectional organelle transport can occur in cell processes that contain single microtubules. J Cell Biol. 1985 Jan;100(1):322–326. doi: 10.1083/jcb.100.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce M. P., Schliwa M. Reactivation of organelle movements along the cytoskeletal framework of a giant freshwater ameba. J Cell Biol. 1986 Aug;103(2):605–612. doi: 10.1083/jcb.103.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusel J. R., Mackenzie P. E. The measurement of the relative turnover rates of proteins of the surface membranes and other fractions of Schistosoma mansoni in culture. Parasitology. 1975 Oct;71(2):261–273. doi: 10.1017/s0031182000046709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazdins J. K., Stein M. J., David J. R., Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982 Feb;53(1):39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F., Smolarsky M. Schistosoma mansoni: effect of insulin and a low-molecular-weight fraction of serum on schistosomula in chemically defined media. Exp Parasitol. 1981 Dec;52(3):378–385. doi: 10.1016/0014-4894(81)90096-5. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Mclaren D. J., Hockley D. J. Blood flukes have a double outer membrane. Nature. 1977 Sep 8;269(5624):147–149. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Threadgold L. T. Ultrastructure of the tegument of adult Schistosoma mansoni. J Parasitol. 1968 Feb;54(1):15–27. [PubMed] [Google Scholar]
- Murrell K. D., Taylor D. W., Vannier W. E., Dean D. A. Schistosoma mansoni: analysis of surface membrane carbohydrates using lectins. Exp Parasitol. 1978 Dec;46(2):247–255. doi: 10.1016/0014-4894(78)90138-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Gazzinelli G., de Moraes G. W., Pellegrino J. Protein synthesis during cercaria-schistosomulum transformation and early development of the Schistosoma mansoni larvae. Comp Biochem Physiol B. 1977;57(1):27–30. doi: 10.1016/0305-0491(77)90077-3. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Hauschka B. T., McIntosh J. R. Anaphase motions in dilute colchicine. Evidence of two phases in chromosome segregation. Exp Eye Res. 1973 Apr;79(1):95–105. [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- STIREWALT M. A. Cercaria vs. schistosomule (Schistosoma mansoni): absence of the pericercarial envelope in vivo and the early physiological and histological metamorphosis of the parasite. Exp Parasitol. 1963 Jun;13:395–406. doi: 10.1016/0014-4894(63)90090-0. [DOI] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P., David J. R. Schistosomula of Schistosoma mansoni clear concanavalin A from their surface by sloughing. J Cell Biol. 1982 Aug;94(2):355–362. doi: 10.1083/jcb.94.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P. The cercarial glycocalyx of Schistosoma mansoni. J Cell Biol. 1985 May;100(5):1423–1434. doi: 10.1083/jcb.100.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H., Reynolds E. S., Von Lichtenberg F. The integument of Schistosoma mansoni. Am J Trop Med Hyg. 1969 Jan;18(1):28–49. [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Stirewalt M. A. Schistosoma mansoni: cercaria to schistosomule. Adv Parasitol. 1974;12:115–182. doi: 10.1016/s0065-308x(08)60388-7. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Burke B. Accumulation of adrenocorticotropin secretory granules in the midbody of telophase AtT20 cells: evidence that secretory granules move anterogradely along microtubules. J Cell Biol. 1987 Apr;104(4):1047–1057. doi: 10.1083/jcb.104.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]