Abstract
Background:
Sleep disorders are increasingly associated with insulin resistance, glucose intolerance, and type 2 diabetes mellitus. Whether the metabolic toll imposed by sleep-related disorders is caused by poor-quality sleep or due to other confounding factors is not known. The objective of this study was to examine whether experimental sleep fragmentation across all sleep stages would alter glucose metabolism, adrenocortical function, and sympathovagal balance.
Methods:
Sleep was experimentally fragmented across all stages in 11 healthy, normal volunteers for two nights using auditory and mechanical stimuli. Primary outcomes included insulin sensitivity (SI), glucose effectiveness (SG), and insulin secretion, as determined by the intravenous glucose tolerance test. Secondary outcomes included measures of sympathovagal balance and serum levels of inflammatory markers, adipokines, and cortisol.
Results:
Following two nights of sleep fragmentation, SI decreased from 5.02 to 3.76 (mU/L)−1min−1 (P < .0001). SG, which is the ability of glucose to mobilize itself independent of an insulin response, also decreased from 2.73 × 10−2 min−1 to 2.16 × 10−2 min−1 (P < .01). Sleep fragmentation led to an increase in morning cortisol levels and a shift in sympathovagal balance toward an increase in sympathetic nervous system activity. Markers of systemic inflammation and serum adipokines were unchanged with sleep fragmentation.
Conclusions:
Fragmentation of sleep across all stages is associated with a decrease in SI and SG. Increases in sympathetic nervous system and adrenocortical activity likely mediate the adverse metabolic effects of poor sleep quality.
Normal sleep is exceedingly important, as judged by the fact that even a modest reduction in sleep duration can lead to impairments in alertness and cognitive performance.1 Aside from its significance for normal daytime function, sleep has profound effects on several biologic processes, including memory consolidation, immunologic responses, and neuroendocrine function.2 There is also a substantial body of empirical evidence indicating that sleep can influence glucose metabolism.3 Research conducted almost a decade ago has shown that even a modest reduction in sleep duration impairs glucose disposal.4 Thus, it is becoming increasingly evident that sleep has multiple functions and that its recuperative value is, in part, determined by sleep duration.
In addition to sleep duration, sleep continuity is essential for normal daytime function. Sleep disorders such as obstructive sleep apnea, which is characterized by fragmented sleep, can have clinical consequences despite normal sleep duration.5 Clinical and epidemiologic studies have shown an independent association between disorders of sleep and altered glucose metabolism.6-8 However, much of the evidence linking poor sleep quality to metabolic dysfunction is based on observational research that provides limited insight into pathophysiologic mechanisms. Moreover, causal inferences on sleep quality and altered glucose metabolism are hindered by confounding factors that are inherent in epidemiologic research. Finally, conditions that fragment sleep often elicit other physiologic changes that can affect glucose metabolism. For example, obstructive sleep apnea is characterized by recurrent episodes of intermittent hypoxemia that terminate with brief arousals from sleep. Thus, at least for obstructive sleep apnea, it is virtually impossible to distinguish the pathophysiologic effects of poor sleep quality from intermittent hypoxemia. Only by experimentally inducing one of the two components in the absence of the other can the independent impact of each be elucidated.
Given the increasing prevalence of obesity in the general population and the associated burden of metabolic and sleep-related disorders, the overarching goal of the current study was to determine whether poor sleep quality could adversely affect glucose metabolism. Specifically, we sought to investigate whether nonspecific fragmentation across all sleep stages, while maintaining normal sleep duration, can alter glucose metabolism in normal people. As a secondary objective, the effects of sleep fragmentation on sympathovagal balance, adrenocortical function, levels of circulating adipokines, and markers of low-grade systemic inflammation were also assessed. The focus on these secondary outcomes was motivated by the need to define intermediate mechanisms through which sleep fragmentation and poor sleep quality could alter glucose metabolism.
Materials and Methods
Study Sample
Normal volunteers were recruited from the general community. To participate, volunteers had to be less than 40 years in age, have a BMI < 30 kg/m2, consume less than two alcoholic or three caffeinated beverages per day, habitually sleep for at least 7 h/night, have a usual bedtime before midnight, and not work at night or on a rotating shift schedule. Additional exclusion criteria included cigarette smoking and the use of any prescription medications or nonprescription antiinflammatory agents. Finally, eligibility for participation also required the absence of the following conditions: type 2 diabetes mellitus, angina, myocardial infarction, coronary revascularization, congestive heart failure, stroke, obstructive lung disease, renal or hepatic dysfunction, or neurologic disease. After an initial telephone screening, eligible volunteers were required to complete a serologic screen and an overnight polysomnogram to rule out obstructive sleep apnea, as previously described.9 Usual sleep habits were also objectively assessed with a wrist activity monitor that was worn for at least 5 days, including one weekend. A normal polysomnogram, a demonstration of habitual bedtime by midnight, an average of at least 7 h of sleep on actigraphy, and normal serum chemistry test results were required for enrollment.
After enrollment, multiple contacts were made to counsel each volunteer on maintaining at least 7 h of sleep per night. Ambulatory monitoring of sleep habits was repeated for three nights prior to the baseline metabolic evaluation to confirm that habitual sleep patterns remain unchanged. Volunteers were excluded from participating in the study if sleep duration on any night was less than 6 h or the average sleep duration was less than 7 h preceding admission to the clinical research unit (CRU). Female volunteers were scheduled for the study protocol during the follicular phase (days 4-10) of the menstrual cycle. Dietary records were collected from all volunteers for 3 days prior to the study to assess the average daily intake of carbohydrates, protein, and fat from any source (eg, fruits, vegetables), as instructed by a certified dietician. Informed consent was obtained from each volunteer, and the study protocol was approved by the local institutional review board.
Study Protocol
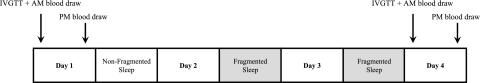
Figure 1 displays the timeline for the study protocol. Each eligible participant was admitted to the CRU (~8:00 am) after an overnight fast for the insulin-modified frequently sampled intravenous glucose tolerance test (IVGTT).10 One night of uninterrupted and two nights of fragmented sleep then followed. The IVGTT was repeated after two nights of sleep fragmentation. Venous blood samples were obtained at 8:00 am and 4:00 am on the day of admission and after two nights of sleep fragmentation. Recordings of the electrocardiogram were acquired at midday (~2:00 pm) before and after sleep fragmentation for heart rate variability analysis to assess whether sleep fragmentation was associated with changes in sympathovagal balance. Finally, anthropometric measures including height, weight, and total body-fat mass and soft lean-tissue mass (by whole-body dual-energy x-ray absorptiometry scan) were also obtained before and after sleep fragmentation.
Figure 1.
Study protocol timeline. IVGTT = intravenous glucose tolerance test.
Sleep Fragmentation
Continuous polysomnographic monitoring was performed on each of the three nights in the CRU. Lights out and morning wake times for each subject were matched to their usual bedtimes and wake times and kept constant throughout the three-night CRU stay. During the day, subjects were ambulatory in the CRU but were not allowed to sleep. Sleep fragmentation was achieved by auditory and mechanical stimuli in anticipation of habituation that may occur with a single repeated auditory stimulus type. Auditory tones were broadcast through two speakers placed 12 inches from the head of the bed. Mechanical stimuli were administered using a commercially available mechanical vibrator. Four such devices were placed underneath the mattress. EEG microarousals (≥ 3 s), as defined according to standard criteria,11 were elicited at a frequency of 30 or more events per hour using the following guidelines. Following lights out, 2 min of continuous stage 2 sleep (or higher) were observed before applying the first auditory stimulus, a sine-wave auditory tone of 500 ms duration and 57 dB. If an EEG microarousal was not elicited, subsequent stimuli were varied by increasing tone volume in 5 to 10 dB increments, up to a maximum of 100 dB, modulating the frequency of the auditory tone and applying the mechanical stimulus alone or in combination with the auditory tone. Once an arousal was elicited, an interval of at least 30 s of stimulus-free sleep was required before applying a subsequent stimulus. Arousals were elicited irrespective of sleep stage.
Frequently Sampled Insulin-Modified IVGTT
The IVGTT was performed as previously described.8,9 An intravenous line was placed in the right and left antecubital veins for blood sampling and kept patent with a continuous infusion of 0.9% saline. The intravenous line in the dominant arm was used for blood sampling, while glucose and insulin were administered through the contralateral intravenous line. Basal sampling occurred at 15, 10, 5 and 1 min before glucose administration. At time 0 min, a weight-adjusted dose of glucose (50% dextrose, 0.3 g/kg) was administered intravenously over 1 min, followed by infusion of normal saline. Twenty minutes after the glucose dose, a weight-adjusted dose of regular insulin (0.03 units/kg) was administered. Blood samples were collected after the glucose infusion at the following times: 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min. Glucose was measured enzymatically in duplicate using a Glucose Analyzer II (Beckman Instruments; Fullerton, CA). Insulin concentrations were determined in duplicate by radioimmunoassay using standard commercial kits (Linco Research; St Charles, MO). The resulting glucose and insulin values were subjected to minimal model analysis10,12 for determination of insulin sensitivity (SI), glucose effectiveness (SG), and glucose effectiveness at zero insulin (GEZI).8,9
SI quantifies the effect of insulin in enhancing glucose disposal. SG quantifies the effects of glucose on its own disposal independent of any insulin response. SG is divided into two components: (a) the contribution of hyperglycemia per se to increase glucose disposal, and (b) the effect of basal insulin (Ib) levels. The basal component of SG is referred to as the Ib effect and is the product of Ib and SI. The contribution of non-insulin-dependent glucose uptake (GEZI) to glucose disposal is the difference between the total SG and the basal insulin effect: GEZI = SG−(Ib × SI). In addition, the acute insulin response to glucose (AIRg), an index of pancreatic β-cell response, was also determined as the area under the insulin curve between 0 and 10 min. Finally, the disposition index, an integrated measure of pancreatic β-cell function, was calculated as the cross-product of SI and AIRg.
Heart Rate Variability Analysis
Indices of heart rate variability were used as a measure of sympathovagal balance during sleep and wakefulness.13 A single-lead ECG was continuously recorded during the entire sleep period on all nights. ECG data were also collected for at least 5 min at midday (~2:00 pm), before and after sleep fragmentation. Each heart rate trace was reviewed, and regions of artifact were visually removed. The following frequency domain indices were computed using a computerized algorithm in 5-min epochs of the ECG: very-low-frequency power (VLF) (0.0033-0.04Hz), low-frequency power (LF) (0.04-0.15Hz), high-frequency power (HF) (0.15-0.4Hz), and the ratio of low to high-frequency power (LF/HF). LF power reflects a combination of sympathetic and parasympathetic activity, whereas HF power reflects the activity of parasympathetic nervous system activity.14
Biochemical Assays
Serum samples were also analyzed for cortisol, high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), leptin, adiponectin, and resistin using commercially available kits. Radioimmunoassay methods were used to measure serum cortisol (Diagnostic Systems Laboratories, Inc.; Webster, TX) and leptin (Linco Research). Enzyme-linked immunosorbent assay techniques were used to measure hs-CRP (Alpco Diagnostics; Salem, NH), IL-6 (MSD; Gaithersburg, MD), adiponectin (Linco Research), and resistin (R&D Systems; Minneapolis, MN).
Statistical Analysis
Data were analyzed with the SAS 9.0 software package (SAS Institute; Cary, NC). All results are presented as means along with the SEM. The sign-rank test was used to compare metabolic parameters before and after sleep fragmentation. For outcomes with multiple measurements, regression methods for repeated events employing the framework of generalized estimating equations were used. A P value of .05 was used as the threshold for statistical significance.
Results
The study sample consisted of 11 healthy volunteers (nine men and two women) with an average age of 23.2 years (range 18-29 years). The average BMI was 24.3 kg/m2 (SEM 0.9), and the average percentage of body fat was 20.5% (SEM 1.8). The average glucose and insulin levels for the study sample were 85.5 mg/dL (SEM 3.5) and 7.5 μU/mL (SEM 0.6), respectively. Dietary records for 3 days prior to enrollment showed an average caloric intake of 2,547 kcal/d (SEM 236). The average caloric intake in the CRU was 2,637 kcal/d (SEM 191), and no significant differences were noted in the daily intake of carbohydrates, fat, and protein comparing the home and CRU environments. Actigraphy monitoring for the week prior to the study protocol revealed an average time in bed of 8.1 h (SEM 0.2; range 7.4-9.8 h).
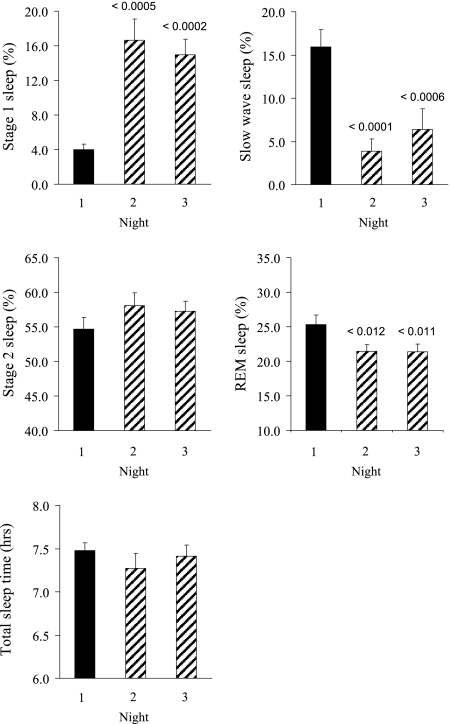
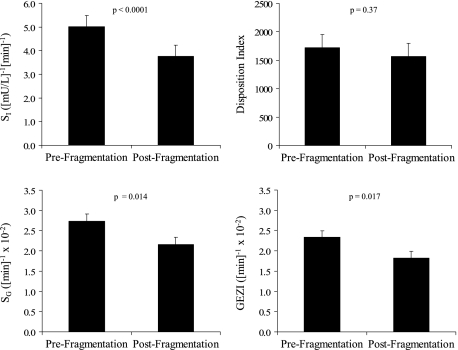
Figure 2 displays the sleep architecture variables for each of the CRU nights. Total sleep time with fragmented sleep remained constant and within normal limits (range 7.3-7.5 h). The arousal frequency was 5.5 events/h (SEM 0.7) on the nonfragmented night compared with 31.4 events/h (SEM 5.1) and 28.8 events/h (SEM 3.3) on the two fragmented nights. Not surprisingly, sleep fragmentation altered sleep stage distribution and was most notably associated with an increase in stage 1 sleep and a decrease in slow wave and rapid eye movement sleep (Fig 2). Results from the IVGTT are shown in Figure 3. Fragmented sleep was associated with a decrease in SI as well as SG. Compared with the baseline value, mean SI decreased by 25.2% after sleep fragmentation, from 5.02 to 3.76 (mU/L)–1 min–1 (P < .0001). Moreover, SG decreased by 20.9% with sleep fragmentation, from 2.73 × 10−2 min−1 to 2.16 × 10−2 min−1 (P = .014). GEZI was also lower with fragmented sleep (2.3 × 10−2 min−1 vs 1.8 × 10−2 min−1, P = .017). The AIRg increased with sleep fragmentation (368.3 vs 481.2 [mU/L-min]; P = .08). Because SI decreased and AIRg increased with fragmented sleep, the resulting disposition index was lower but not statistically different comparing the prefragmentation and postfragmentation values (1717.8 vs 1568.4; P = .37). Body weight remained unchanged over the course of the study protocol (74.9 kg before and 74.6 kg after sleep fragmentation; P = .10).
Figure 2.
Sleep stage parameters with sleep fragmentation. Black and hashed bars represent nonfragmented and fragmented nights. REM = rapid eye movement.
Figure 3.
Insulin sensitivity, disposition index, glucose effectiveness, and glucose effectiveness at zero insulin before and sleep fragmentation. GEZI = glucose effectiveness at zero insulin; SG = glucose effectiveness; SI = insulin sensitivity.
Heart rate variability analysis was conducted to assess the role of the sympathovagal balance in mediating the adverse metabolic effects of sleep fragmentation. Compared with the baseline night, LF power was 29.7% (P < .001) and 34.4% (P < .001) higher on the first and second nights of sleep fragmentation, respectively (Table 1). HF power, which reflects vagal tone, decreased by 11.0% (P < .03) and 12.8% (P < .001), respectively, on the first and second nights of fragmented sleep. The corresponding normalized LF power (LF/LF+HF), which reflects sympathetic modulation, increased by 14.7% (P < .001) and 16.8% (P < .001) on the two fragmented nights. Analysis of daytime heart rate data showed a similar shift in sympathovagal balance comparing baseline and postfragmentation days, with an increase in LF and a decrease in HF power (Table 2). In addition, the average beat-to-beat interval at rest decreased after sleep fragmentation, from 846.0 ms (SEM 32.8) to 750.7 ms (SEM 37.9), with a corresponding increase in resting average heart rate from 70.9 beats/min to 79.9 beats/min providing additional support for an increase in sympathetic nervous activity.
Table 1.
—Power Spectral Measures of Heart Rate Variability With Sleep Fragmentation
| HRV Parameter | Night 1, Nonfragmented | Night 2, Fragmented | Night 3, Fragmented |
| VLF power, ms2 | 4758.5 (466.5) | 5722.5 (393.3)a | 5537.5 (585.7)b |
| LF power, ms2 | 4548.5 (276.5) | 6098.8 (343.1)a | 6356.3 (277.6)a |
| HF power, ms2 | 3346.0 (460.1) | 2950.2 (344.2)b | 2882.8 (319.3)a |
| LF/(LF+HF) | 0.59 (0.04) | 0.68 (0.03)a | 0.69 (0.03)a |
Values reported are mean and (SEM). HF = high frequency; HRV = heart rate variability; LF = low frequency; VLF = very low frequency.
P < .001 comparing fragmented with nonfragmented sleep.
P < .03 comparing fragmented with nonfragmented sleep.
Table 2.
—Parameters of Daytime HRV Before and After Sleep Fragmentation
| HRV Parameter | Prefragmentation | Postfragmentation |
| VLF power, ms2 | 2741.5 (374.2) | 2621.0 (308.4) |
| LF power, ms2 | 2215.3 (365.2) | 3045.5 (373.7)a |
| HF power, ms2 | 1751.2 (226.9) | 1334.0 (351.1)b |
| LF/(HF+LF) | 0.55 (0.07) | 0.70 (0.07)a |
Values reported are mean and (SEM). See Table 1 for expansion of abbreviations.
P < .001 comparing sleep prefragmentation and postfragmentation.
P < .03 comparing sleep prefragmentation and postfragmentation.
Mean serum values for inflammatory cytokines and adipokines, including hs-CRP, IL-6, leptin, adiponectin, and resistin, before and after fragmented sleep were unchanged (Table 3). Inspection of intraindividual changes in these biomarkers also showed no consistent effect of sleep fragmentation. However, morning cortisol values were 12.5% higher after 2 days of fragmented sleep (11.8 μg/dL before and 13.8 μg/dL after sleep fragmentation, P < .015).
Table 3.
—Serum Measurements Before and After Sleep Fragmentation
| Variable | Prefragmentation | Postfragmentation |
| hs-CRP, μg/mL | ||
| am | 1.15 (0.33) | 0.98 (0.29) |
| pm | 1.40 (0.47) | 1.00 (0.30) |
| IL-6, pg/mL | ||
| am | 0.62 (0.16) | 0.54 (0.11) |
| pm | 1.20 (0.59) | 2.07 (0.64) |
| Leptin, ng/mL | ||
| am | 4.38 (0.99) | 4.63 (1.01) |
| pm | 2.85 (0.51) | 3.14 (0.56) |
| Adiponectin, μg/L | ||
| am | 8.76 (1.32) | 9.09 (1.23) |
| pm | 9.26 (1.27) | 9.27 (1.19) |
| Resistin, ng/mL | ||
| am | 1.10 (0.10) | 1.12 (0.10) |
| pm | 1.23 (0.12) | 1.28 (0.17) |
Values reported are mean and (SEM). hs-CRP = high sensitivity C-reactive protein; IL-6 = interleukin-6.
Discussion
The primary objective of this study was to investigate whether fragmented sleep had adverse effects on glucose metabolism. Sleep was nonspecifically disrupted across all stages for two consecutive nights, and EEG microarousals were induced at a frequency of approximately 30 events/h on each night. Despite the moderate degree of disruption, sleep duration was preserved and was characterized by an increase in the amount of stage 1 sleep and a concomitant decrease in the amounts of slow wave and rapid eye movement sleep. Sleep fragmentation had a negative impact on glucose metabolism, as evidenced by a 20% to 25% reduction in SI and SG. Sleep fragmentation also caused alterations in sympathovagal balance, with a shift toward increased sympathetic nervous system activity during sleep and wakefulness. In addition, morning levels of serum cortisol were higher with fragmented sleep, whereas markers of low-grade systemic inflammation and serum adipokines remained unchanged. Taken together, these findings support the hypothesis that, independent of sleep duration, nonspecific fragmentation of sleep can alter glucose homeostasis.
An emerging body of epidemiologic literature indicates that habitually short sleep duration is associated with altered glucose metabolism.3,15 The potentially causal nature of this link is supported by experimental evidence indicating that short-term sleep restriction in normal subjects impairs glucose tolerance, augments sympathetic nervous system activity, and increases levels of circulating cortisol.4 While the neurobehavioral consequences of sleep fragmentation are perhaps indistinguishable from that seen with sleep deprivation,5 its effect on glucose homeostasis and other clinical sequelae are largely unknown. Conditions that disrupt sleep continuity such as obstructive sleep apnea have been implicated in the pathogenesis of insulin resistance, glucose intolerance, and type 2 diabetes.7 The challenge, however, in interpreting the available data on the metabolic implications of obstructive sleep apnea is in segregating whether the excess risk can be attributed to sleep fragmentation or intermittent hypoxemia. The results of this study support the hypothesis that poor sleep quality in conditions such as obstructive sleep apnea may potentially alter SI and glucose disposal. This notion was recently corroborated by the report from Tasali et al16 that showed that selective suppression of slow wave sleep can also worsen insulin resistance in normal people. The current study expands the relatively limited body of available literature by demonstrating that nonspecific disruption of sleep continuity can also adversely affect insulin and glucose kinetics.
The findings of higher morning cortisol levels coupled with the shift in sympathovagal balance in the current study provide potential mechanisms through which sleep fragmentation could alter glucose metabolism. It is well established that elevations of cortisol, even within the normal physiologic range, can decrease SI, enhance hepatic gluconeogenesis, and inhibit insulin secretion.17 Sustained awakenings or even brief arousals from sleep can abruptly increase adrenocortical activity and increase circulating cortisol.18,19 There is also substantial evidence that activation of the sympathetic nervous system promotes insulin resistance and is associated with abnormalities in glucose metabolism.20 Increased sympathetic activity decreases insulin-mediated glucose uptake, inhibits pancreatic insulin secretion, decreases SG, and stimulates hepatic glucose release.21-24 Although we had hypothesized that sleep fragmentation would also be associated with alterations in systemic inflammation and circulating adipokines, no such changes were observed. It is possible that fragmentation of sleep for two nights is insufficient to alter these markers, all of which have been associated with insulin resistance and type 2 diabetes.25,26 Alternatively, it is also possible that sleep continuity may exert little influence on these physiologic systems.
The current study has several strengths that merit discussion. These include the use of healthy volunteers and the strict pre-enrollment screening criteria that were imposed to minimize potential confounding from factors such as habitually short sleep duration, disorders of the sleep-wake cycle, and chronic medical conditions. The identification of heightened adrenocortical function and increase in daytime sympathetic nervous system activity also represent important strengths. There are also some limitations with the current study. First, characterizing the metabolic effects of sleep fragmentation for two nights provides evidence on the acute, but not chronic, effects of poor sleep quality. Experimental disruption of sleep for prolonged periods is not possible in normal subjects due to the participant burden and the possibility of untoward effects. Nonetheless, while acute manipulations of sleep may not be generalizable for chronic disease, they do highlight the potential significance of sleep quality for normal glucose homeostasis. Second, the restriction of the study sample to younger, predominantly male subjects limits the generalizability of our results.
The above limitations notwithstanding, the present study has important clinical and public health implications. The 20% to 25% reduction in SI and SG represents a clinically relevant shift. Data from the Insulin Resistance Atherosclerosis Study showed that SI among healthy adults decreases by approximately 30% for every successive step across categories, from normal to overweight and obese.27 Thus, our findings support the hypothesis that sleep fragmentation in conditions such as obstructive sleep apnea can induce clinically significant alterations in glucose metabolism.
Acknowledgments
Author contributions: Dr Stamatakis: participated in data collection, data analysis, interpretation of results, and preparation of the manuscript, and has seen and approved the final version.
Dr Punjabi: participated in data collection, data analysis, interpretation of results, and preparation of the manuscript, and has seen and approved the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Punjabi has received honoraria and travel support for continuing medical education lectures or symposia sponsored by Respironics and Resmed Inc. Dr Stamatakis has no potential conflicts with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We express our appreciation to the research volunteers who participated in these studies, to Kelly Devine, RN, for her efforts as the research nurse and coordinator, to Melissa Minotti, RPSGT, for her assistance with polysomnography, and to the CRU sleep technicians, nurses, and nutritionists who helped implement and execute the study protocol.
Abbreviations
- AIRg
acute insulin response to glucose
- CRU
clinical research unit
- GEZI
glucose effectiveness at zero insulin
- HF
high-frequency power
- hs-CRP
high-sensitivity C-reactive protein
- Ib
basal insulin
- IL-6
interleukin-6
- IVGTT
intravenous glucose tolerance test
- LF
low-frequency power
- SG
glucose effectiveness
- SI
insulin sensitivity
- VLF
very-low-frequency power
Footnotes
Funding/Support: This study was supported by the Johns Hopkins University Bayview Medical Center General Clinical Research Center [5M01RR00056] and was principally funded by the National Institute of Heart, Lung, and Blood Institute [Grants HL075078 and HL086862].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Dinges DF, Rogers NL, Baynard MD. Chronic sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier/Saunders; 2005. pp. 67–76. [Google Scholar]
- 2.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64(10):1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7(4):297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99(5):1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179(3):235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106(5):1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68(6):1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 12.Bergman RN. Minimal model: perspective from 2005. Horm Res. 2005;64(Suppl 3):8–15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- 13.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71(1):1–2. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 16.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96(5):513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 18.Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J. Nocturnal cortisol release in relation to sleep structure. Sleep. 1992;15(1):21–27. doi: 10.1093/sleep/15.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29(6):575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 20.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 21.Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65(3):717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin IK, Weber KM, Boston RC, Alford FP, Best JD. Effects of epinephrine infusion on determinants of intravenous glucose tolerance in dogs. Am J Physiol. 1988;255(5 Pt 1):E668–E673. doi: 10.1152/ajpendo.1988.255.5.E668. [DOI] [PubMed] [Google Scholar]
- 23.Lembo G, Capaldo B, Rendina V, et al. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol. 1994;266(2 Pt 1):E242–E247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- 24.Avogaro A, Toffolo G, Valerio A, Cobelli C. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion. A stable label intravenous glucose tolerance test minimal model study. Diabetes. 1996;45(10):1373–1378. doi: 10.2337/diab.45.10.1373. [DOI] [PubMed] [Google Scholar]
- 25.Sjöholm A, Nyström T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev. 2006;22(1):4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 26.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karter AJ, D’Agostino RB, Jr, Mayer-Davis EJ, et al. IRAS investigators. Abdominal obesity predicts declining insulin sensitivity in non-obese normoglycaemics: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Obes Metab. 2005;7(3):230–238. doi: 10.1111/j.1463-1326.2004.00441.x. [DOI] [PubMed] [Google Scholar]