Abstract
Background:
Evidence that continuous positive airway pressure (CPAP) reduces cardiovascular morbidity comes largely from observational studies. This association may be confounded if CPAP adherents are healthier in ways not measured by investigators. We assessed whether patients adhering to lipid-lowering medications were more adherent to CPAP.
Methods:
This was a retrospective cohort study undertaken at the Philadelphia Veterans Affairs (VA) Medical Center (2005-2006) of consecutive patients on lipid-lowering therapy newly initiating CPAP for obstructive sleep apnea. Adherence to medications dispensed via the VA closed-pharmacy system was measured as the proportion of days covered (≥80% vs < 80%) in the year prior to CPAP initiation. CPAP adherence was defined as ≥ 4 h/d of “mask-on” time, measured electronically daily during the first week of CPAP. We examined the association between medication adherence and CPAP adherence using multivariable logistic regression.
Results:
Complete data were available for 117 of 142 (81.5%) subjects. After adjustment for age, race, medical comorbidity, and sleep apnea-related clinical factors, subjects with low medication adherence demonstrated a 40.1% (95% CI, 30.0-51.0) probability of using CPAP ≥ 4 h/d compared with 55.2% (95% CI, 46.9-63.1) for subjects with adequate (≥80%) medication adherence (adjusted for comparison, odds ratio (OR) = 1.8 [95% CI, 1.0-3.3], P = .04). Married patients were more adherent to medications and CPAP; inclusion of this factor reduced to nonsignificance the association of medication and CPAP adherence (OR = 1.6 [95% CI, 0.9-2.8], P = .12).
Conclusion:
Patients consistently refilling lipid-lowering medications were more adherent to CPAP, suggesting that differences in medication adherence or other health-promoting behaviors should be investigated in future nonrandomized, observational studies linking CPAP adherence and cardiovascular outcomes.
Obstructive sleep apnea (OSA), a disorder affecting 2% to 7% of adults,1-3 is characterized by recurrent collapse of the pharyngeal airway during sleep and results in episodes of oxygen desaturation and arousal. Experimental data suggest that OSA predisposes patients to increased rates of cardiovascular disease through such mechanisms as increased sympathetic activation, endothelial dysfunction, and oxidative stress.4-6
Nevertheless, the epidemiologic evidence supporting a direct, causal link between OSA and cardiovascular outcomes remains incomplete. OSA has been shown to be an independent risk factor for hypertension in population-based cohort studies,7,8 but prospective trials examining the efficacy of continuous positive airway pressure (CPAP) therapy on diurnal blood pressure provide somewhat conflicting results.9-15 Overall, a metaanalysis of trials has demonstrated a modest (2 mm Hg) improvement in diurnal systolic blood pressure associated with CPAP use.16 Two recent population-based studies have linked moderate-to-severe OSA17 and severe OSA18 with increased risk of death, but these studies may have been confounded by the high rates of comorbid hypertension, obesity, and cardiovascular disease in subjects with more severe sleep apnea.19 Long-term, randomized trials assessing the efficacy of CPAP in reducing cardiovascular outcomes have not been conducted due to ethical concerns about randomizing OSA subjects to sham treatment.20
Absent trial data for mortality, predominantly single-center, observational studies report that patients with OSA who refuse or do not adhere to CPAP therapy experience higher rates of myocardial infarction, stroke, and death compared with CPAP adherents.21-25 These nonrandomized, observational studies support the notion that CPAP reduces cardiovascular risk in patients with OSA, but they fail to control for the possibility that CPAP-adherent patients may also be healthier than patients who refuse or do not adhere to CPAP in ways not typically measured. For example, if CPAP adherence is associated with other health-promoting behaviors, then the magnitude of prior estimates of the impact of CPAP adherence on cardiovascular risk may be inflated.
The purpose of our study was to test whether medication adherence would be associated with CPAP adherence, independent of age, race, and clinical factors. We compared adherence to lipid-lowering medications and adherence to CPAP in a Veterans Affairs (VA) cohort of patients with OSA newly initiated on CPAP therapy.
Methods
Design and Patient Population
We collected data on consecutive patients aged 18 years and older who were diagnosed with OSA (apnea-hypopnea index [AHI] ≥5 events per hour) at the Philadelphia VA Medical Center and newly initiated on CPAP therapy from January 1, 2005 to December 31, 2006. We included patients prescribed chronic lipid-lowering medications ordered via the VA pharmacy system at least 6 months prior to CPAP initiation and excluded subjects who had a history of prior CPAP use. The chronic lipid-lowering medications included 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, or statins (simvastatin, atorvastatin, rosuvastatin, fluvastatin, lovastatin), and non-statin medications (gemfibrozil, colestipol, and ezetimibe). The Institutional Review Board at the Philadelphia VA Medical Center approved the study and waived informed consent because of the retrospective, observational study design. Ethical standards were used in the research.
Medication Adherence
Adherence to lipid-lowering medications was assessed by using the log of medication refills in the VA electronic pharmacy database to determine the total number of days that a candidate medication was supplied during the exposure window.26,27 The exposure window was defined a priori as a maximum of 365 days and a minimum of 180 days before the initiation of CPAP therapy. The proportion of days covered (PDC) was calculated as the ratio of the total number of days that a candidate medication was supplied divided by the number of days in the exposure window.26,27 Patients were designated as adherent on a given day during the exposure period if, at a minimum, the patient had at least one lipid-lowering medication of any dosage available on that day. Subjects were then classified dichotomously as having adequate vs low medication adherence based on a generally accepted threshold of 80% or more days covered during the exposure period.28,29
Covariates
We collected data on individual demographic characteristics and medical comorbidities (International Classification of Diseases, 9th ed [ICD-9] codes) and Charlson Index30 from the VA electronic medical record. We reviewed sleep study interpretations and provider notes to confirm the diagnosis of OSA, record BMI, the AHI on the diagnostic sleep study, the Epworth Sleepiness Scale31 score before initiation of CPAP therapy, and the type of diagnostic sleep study used to confirm the diagnosis of OSA (in-laboratory polysomnogram vs unattended, in-home study).
Sleep Studies
Patients were diagnosed with OSA at the Philadelphia VA according to a traditional in-laboratory, attended polysomnogram or an unattended, in-home sleep study. The in-laboratory polysomnogram was performed by an experienced technician in attendance using Sandman software (Sandman Diagnostic Systems; Nellcor-Puritan Bennett Inc.; Kanata, ON, Canada) as a full-night diagnostic polysomnogram generally followed by a full-night CPAP titration polysomnogram or as a single split-night study. For in-home testing, patients were provided with a type 3 portable monitor (Suzanne Portable Recording System; Nellcor Puritan Bennett; or Embletta; Embla; Broomfield, CO) that recorded nasal airflow, snoring, respiratory effort, body position, oxygen saturation, and heart rate. In-home diagnostic studies were generally followed by an unattended in-home automatic CPAP titration study (AutoSet Spirit; ResMed; Poway, CA) over 4 to 5 days in the home. Both the in-laboratory and in-home diagnostic sleep studies were recorded and scored according to the policies and procedures of the American Academy of Sleep Medicine.32,33
CPAP Initiation
All subjects underwent an education program on OSA and use of CPAP therapy prior to initiating treatment, including face-to-face information provided by the treating physicians as well as by the respiratory therapists and nurses. In addition, patients were shown a video and given written pamphlets specifically made to educate them about OSA and CPAP therapy. After the sleep testing was completed, a board-certified sleep specialist prescribed fixed CPAP to each patient. All patients received a CPAP unit (RemStar Pro; Phillips; Murrayville, PA) that recorded daily mask-on time (ie, the time the CPAP circuit was pressurized at the prescribed level) on a data card. The data card was collected approximately 1 week after delivery of the CPAP device. The first day of CPAP use was defined as the initial day during which any pressurized mask-on time was recorded by the CPAP electronic card monitor after delivery of the CPAP device to the patient.
Outcome and Statistical Analysis
Daily CPAP adherence during the first 7 days of therapy was the primary outcome for our analyses, and was defined a priori as ≥4 h/d of mask-on time.34,35 The first week of CPAP closely correlates with subsequent adherence.34,36,37 Daily CPAP adherence was modeled as a dependent, dichotomous repeated outcome variable using multivariable logistic regression STATA 9.0 (STATA Corporation; College Station, TX). We accounted for the within-subject correlation of the repeated outcome of daily CPAP use using general estimating equations with an exchangeable correlation matrix structure.38 We included a within-patient factor indicating whether the night observed was the first night vs the remaining nights, as CPAP adherence on the first night was observed to be shorter than that on subsequent nights.
Results
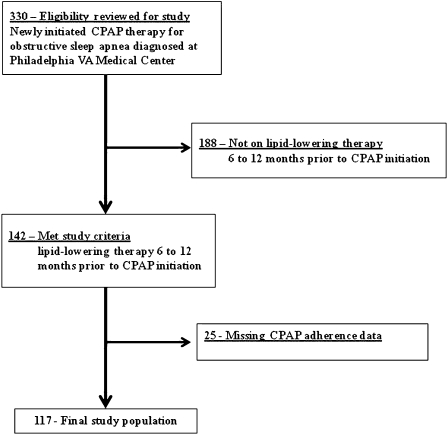
Of 330 subjects who were diagnosed with OSA and newly initiated on CPAP therapy from 2005 to 2006, 142 subjects met study criteria for exposure to lipid-lowering therapy prior to OSA diagnosis. Complete clinical and CPAP data were available for analysis for 117 (82%) of the 142 subjects (Fig 1). The study cohort was 97% male and had a high level of OSA disease severity (mean AHI 40.2 ± 25.5 events/h) and comorbid medical disease (Table 1). There were no significant differences in baseline demographic or clinical characteristics or medication adherence to lipid-lowering therapy among those excluded because of missing data (n = 25) compared with those included in the study (n = 117).
Figure 1.
Philadelphia VA study cohort (2005-2006). CPAP = continuous positive airway pressure; VA = Veterans Affairs.
Table 1.
—Baseline Patient Characteristics (n = 117)
| Variable | Value |
| Age, y, mean (SD) | 59.7 (10.4) |
| Male, % | 96.6 |
| Race, % | |
| Black | 47.9 |
| White | 44.4 |
| Othera | 7.7 |
| Married,b % | 47.9 |
| Medical comorbiditiesc | |
| Charlson Index, median (IQR) | 2 (1-4) |
| Hypertension, % | 86.3 |
| Diabetes mellitus, % | 69.2 |
| Coronary artery disease, % | 49.6 |
| Hyperlipidemia | 91.5 |
| Chronic obstructive lung disease, % | 34.2 |
| BMI, kg/m2, mean (SD) | 35.1 (6.7) |
| Epworth Sleepiness Scale, mean (SD) | 11.9 (5.5) |
| Apnea-hypopnea index, mean (SD) | 40.2 (25.5) |
| Type of diagnostic sleep study, in-laboratory (vs home, unattended), % | 53.9 |
| Total number of d in lipid-lowering drug exposure window, y before CPAP initiation, median (range)d | 365 (208-365) |
| Total patient-d of CPAP observation, number | 787 |
| Number of CPAP patient-d observed per subject,e median (range) | 7 (1-7) |
| Median h of CPAP use per d, across all patient-d, median (IQR) | 4.1 (0.1-6.4) |
CPAP = continuous positive airway pressure; ICD-9 = International Classification of Disease, 9th ed.; IQR = interquartile range.
Other race category consists of Pacific Islander, Asian, and Indian American (n = 5), and race classification missing (n = 4).
Nonmarried patients: separated/divorced (26.5%), never married (19.7%), widowed (6.0%).
Medical comorbidities measured according to ICD-9 classification. Of the 10 subjects on lipid-lowering therapy without an ICD-9 diagnosis of hyperlipidemia, seven had comorbid diagnoses of diabetes mellitus and one had comorbid coronary artery disease.
Drug ascertainment exposure window is defined as a minimum 180 and a maximum 365 d before CPAP initiation.
A full 7 patient-d on CPAP were observed for 103 (88.0%) of subjects; 6 patient-d were observed for eight (6.8%); and ≤5 patient-d on CPAP for six (5.1%) of the 117 subjects.
The PDC for lipid-lowering medications during the observation window in the year before CPAP initiation averaged 80.1% (SD, 22.4) (Figs 1 and 2 in the online supplement). Nearly all subjects (114 of 117) were taking statin medications (Table 2). Mean adherence was similar among subjects receiving statin medications, non-statin lipid-lowering medications, or combinations of the two medication classes.
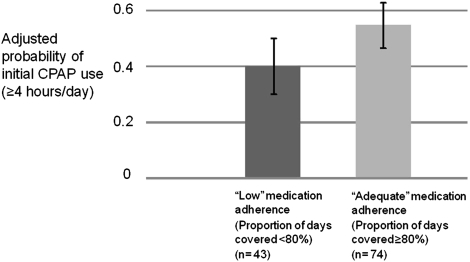
Figure 2.
Adjusted probability of initial CPAP use ≥4 h, by lipid-adherence subgroup (n = 117). Medication adherence is measured as the PDC, calculated as the number of d with medication available from the Veterans Affairs pharmacy divided by the total number of d exposed in the year before CPAP therapy. Estimates are adjusted for age, race, BMI, Epworth Sleepiness Scale, apnea-hypopnea index, in-laboratory vs unattended sleep study, Charlson Index, and CPAP use on first night vs subsequent nights. PDC = proportion of days covered. See Figure 1 for expansion of other abbreviation.
Table 2.
—Review of Pharmacy Refill Adherence to Lipid-Lowering Therapy, by Medication Type
| Subjects, No. | PDC, Mean (SD) | |
| Adherence to lipid-lowering therapy | 117 | 80.1 (22.4) |
| Prescribed simvastatin alone | 81 | 79.2 (23.0) |
| Prescribed atorvastatin, rosuvastatin, lovastatin, or fluvastatin | 18 | 79.3 (21.3) |
| Prescription switched among statin drugs (simvastatin, atorvastatin, rosuvastatin, lovastatin, fluvastatin) | 5 | 78.8 (31.6) |
| Prescribed a statin and a non-statina lipid-lowering drug | 10 | 89.3 (16.7) |
| Prescription of a non-statina lipid-lowering medication exclusively | 3 | 83.7 (12.4) |
Pharmacy refill adherence is measured as the PDC or the percent of d with a lipid-lowering medication available divided by the total number of d on drug therapy during the total d on therapy in the 1-y exposure window before CPAP initiation. Analysis of variance for difference among groups, P = .75. A statin is defined as a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. PDC = proportion of days covered. See Table 1 for expansion of other abbreviation.
Colestipol, gemfibrozil, or ezetimibe.
Across the 787 total patient-days observed during the first week of CPAP therapy for the 117 subjects, median CPAP use = 4.1 h/d (interquartile range [IQR], 0.1-6.4). Patients used CPAP for ≥ 4 h on 50.8% of nights (see Figs 3 and 4 in the online supplement).
Association of Medication and CPAP Adherence
Subjects with a history of adequate adherence to lipid-lowering therapy (PDC 80% or greater in the year before CPAP initiation) had substantially higher odds of adhering daily to CPAP therapy in unadjusted analysis (crude odds ratio [OR] = 1.7, [95% CI, 1.0-2.9], P = .04) and in adjusted analysis (adjusted OR = 1.8 [CI, 1.0-3.3], P = .04) after accounting for the following potential demographic and clinical confounders: age, race, BMI, Epworth Sleepiness Scale, AHI, in-laboratory vs home unattended diagnostic sleep study, Charlson Index, and the first day of CPAP use compared with subsequent days (Table 3).
Table 3.
—Adjusted Odds Ratios for Adequate Initial Adherence to CPAP (n = 117)
| Variable | Adjusted OR (95% CI) | P Value |
| Lipid medication adherence ≥ 80% ;(vs < 80%)a | 1.8 (1.0-3.3) | .04 |
| Age (per 10-y increase) | 1.3 (0.9-1.6) | .13 |
| Race, black (vs white/other)b | 0.8 (0.5-1.5) | .55 |
| BMI (per SD change) | 0.8 (0.6-1.0) | .10 |
| Epworth Sleepiness Score (per SD change) | 1.1 (0.9-1.5) | .41 |
| Apnea-hypopnea index (per SD change) | 1.0 (0.8-1.3) | .91 |
| Diagnostic sleep study, unattended (vs in-laboratory) | 1.2 (0.7-2.1) | .44 |
| First night of CPAP use (vs subsequent nights) | 0.4 (0.3-0.6) | < .001 |
OR = odds ratio. See Table 1 for expansion of other abbreviation.
Pharmacy refill adherence is measured as the percent of d covered with a lipid-lowering medication, calculated as the number of d covered divided by the total number of d on drug therapy during the exposure window in the year before CPAP initiation.
Other race category consists of Pacific Islander, Asian, and Indian American (n = 5), and race classification missing (n = 4).
To provide a clinically meaningful estimate of the association of higher medication refill adherence (PDC ≥ 80%) and CPAP adherence based on the model presented in Table 3, we converted the odds ratios of initial daily CPAP use ≥ 4 h into estimated probabilities. After adjustment for all factors in Table 3, the probability of CPAP use ≥ 4 h = 40.1% (CI, 30.0-51.0) for subjects with a history of low adherence to lipid-lowering medications (PDC < 80%) compared with 55.2% (95% CI, 46.9-63.1) for subjects with a history of high adherence to lipid-lowering therapy (PDC ≥ 80%) (Fig 2).
To assess what other patient-specific factors might codetermine adherence to lipid-lowering medications and CPAP adherence, we examined marital status. We found that married patients (n = 56) had higher medication adherence than nonmarried patients (n = 61) (84.9% [SD, 19.3] vs 75.8% [SD, 24.3], P = .03) and higher CPAP adherence across all patient days (4.6 h per day [SD, 3.1] vs 3.2 [SD, 3.1], P < .001). Inclusion of marital status in the final model reduced the adjusted association of medication and CPAP adherence to a statistically nonsignificant P value (OR = 1.6 [CI, 0.9-2.8], P = .12).
Sensitivity Analyses
In sensitivity analyses, adjusting for differences in number of days on lipid-lowering therapy prior to CPAP initiation did not affect the association of higher medication and CPAP adherence (adjusted OR = 1.9 [CI, 1.1-3.4] P = .03). Changing the threshold for medication adherence from the mean (≥ 80%) to the median (≥ 88%) also did not affect the association of medication and CPAP adherence (adjusted OR = 2.0 [CI, 1.1-3.4], P = .02). Limiting our analyses to the subset of subjects with a full 7-day CPAP window of observation (n = 103) resulted in a similar point estimate of the association of medication and CPAP adherence, albeit with a loss of power in this subset analysis (adjusted OR = 1.8 [CI, 1.0-3.2], P = .06). Varying the outcome definition of CPAP adherence over a 1-h range did not affect the results: CPAP adherence ≥ 3.5 h (vs < 3.5 h), adjusted OR = 1.8 (CI, 1.0-3.3), P = .04; CPAP adherence ≥ 4.5 h (vs < 4.5 h), adjusted OR = 1.8 (CI, 1.0-3.2), P = .04.
Discussion
In this retrospective cohort of 117 veterans newly initiated on CPAP therapy for OSA who had been exposed to lipid-lowering therapy, we found that patients who had higher rates of medication adherence (PDC ≥ 80%) were significantly more likely to adhere ≥ 4 h to CPAP therapy on a daily basis. Consistent with several prior studies, clinical factors of disease severity, daytime sleepiness, or medical comorbidity did not predict daily CPAP use.39,40
These results suggest that a so-called “healthy-user” effect could be present in observational studies of CPAP use, such that subjects who adhere well to medications for chronic conditions, such as hyperlipidemia, are also more likely to adhere regularly to CPAP. If confirmed in other studies, these results suggest that in nonrandomized studies, adherence to CPAP may be a marker for a collection of health-promoting behaviors that together could reduce cardiovascular morbidity. Simply put, those who adhere to CPAP may also be taking their statins and other medications, and it may be adherence to statins, other types of medications, and other health-promoting behaviors that explain some of the observed reduction in cardiovascular disease associated with CPAP adherence.
To date, the only similar study in the literature exploring a healthy-user effect in CPAP adherence, to our knowledge, was conducted by Villar et al.41 In their investigation, Villar and colleagues studied medication-refill-patterns data among those subjects who accepted vs those who declined CPAP and found that CPAP adherence was unrelated to medication adherence. The difference in the study results of Villar and colleagues and our own may be possibly explained by our more precise monitoring of initial daily CPAP therapy, differences in the population studied, or differences in the metric used to track medication adherence.42
Notwithstanding the negative findings of Villar and colleagues,41 the impact of the healthy-user effect has been noted in numerous other clinical settings, including the association of hormone replacement therapy and improved cardiovascular outcomes (documented in observational studies but subsequently refuted by randomized, controlled trials);43 the improved survival found in large-scale cardiovascular trials among placebo-adherent subjects compared with placebo-nonadherent subjects;44-47 and the higher use of preventive health services, such as immunization, among elderly patients who adhere regularly to lipid-lowering therapy vs medication nonadherent subjects.48 In these examples, adherence to treatment is a marker for other unmeasured behaviors or characteristics that presumably help to explain the improved outcomes.
What might explain a healthy-user effect? It is certainly plausible that some patients are generally more adherent to medical advice or social norms than others. There may also be differences in psychologic factors, such as active coping styles or higher perceived self-efficacy, among adherers and nonadherents.49,50 It is also possible that other factors might codetermine adherence to CPAP and lipid-lowering medications. In our sample, we found that married patients had higher medication adherence and higher CPAP adherence, and inclusion of this variable in the analysis attenuated the observed association between medication and CPAP to a nonsignificant P value (OR = 1.6 [CI, 0.9-2.8], P = .12). Indeed, it seems plausible that married patients have more internal and external cues that prompt adherence to medications and CPAP. As a future direction, health-promoting behaviors, such as medication adherence, primary care follow-up, or immunization records, as well as marital status or other important sociodemographic factors, might simultaneously be assessed in observational studies of CPAP use to obtain a less biased estimate of the association of CPAP use and improved cardiovascular morbidity.
Limitations
Several important limitations should be considered. First, our study was performed in a predominantly male, veteran cohort, which might limit generalizability. Our study findings should be assessed in other populations of patients with OSA. Second, although prescription-refill adherence has been well validated as a method for measuring medication adherence and is associated with important clinical outcomes, such as glycosylated hemoglobin, low-density lipid levels, diastolic blood pressure, hospitalization, and mortality,27 this method of measuring medication adherence does not directly assess actual daily pill-taking and might therefore result in some degree of exposure misclassification. Prospective assessment via electronic pill cap monitoring might allow a more direct, daily comparison of medication and CPAP adherence. A third potential limitation is outcome misclassification arising from our having measured CPAP adherence daily over the first week of use and not for longer periods of time. We are reassured, given that numerous studies have demonstrated that CPAP adherence during the first week interval closely predicts subsequent adherence.26,36,37 Last, given that our study is observational, additional unmeasured or unobserved confounders could explain the observed association of medication and CPAP nonadherence.
In summary, in a retrospective cohort of veterans with OSA newly started on CPAP therapy, we have demonstrated an association of adherence to lipid-lowering medications and initial daily CPAP adherence, independent of age, race, and clinical factors, but not of individual marital status. If cardiovascular medication adherence and CPAP adherence are linked in other observational study populations, then the magnitude of prior estimates of the impact of CPAP adherence on cardiovascular risk among patients with OSA might be inflated by such unmeasured health-promoting behaviors. Our findings argue for better assessment of potential confounding factors in future observational studies of CPAP adherence and, where ethically feasible, for prospective, controlled evaluations of the potential benefits of CPAP.
Supplementary Material
Acknowledgments
Author contributions: Dr Platt had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Platt: contributed to study design, data collection and analysis, and manuscript preparation and review.
Dr Kuna: contributed to study design, data analysis, and manuscript preparation and review.
Dr Field: contributed to study design, data analysis, and manuscript preparation and review.
Dr Chen: contributed to study design, data analysis, and manuscript preparation and review.
Mr Gupta: contributed to data collection and manuscript preparation and review.
Mr Roche: contributed to data collection and analysis and manuscript preparation and review.
Dr Christie: contributed to study design, data analysis, and manuscript preparation and review.
Dr Asch: contributed to study design, data analysis, and manuscript preparation and review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kuna receives grant funding support from Respironics, Inc, a manufacturer of continuous positive airway pressure appliances, unrelated to this work. Dr Platt, Dr Field, Dr Chen, Mr Gupta, Mr Roche, Dr Christie, and Dr Asch have reported no potential conflicts of interest with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Jacqueline Ferguson, CRT, Elaine Dynako, RN, Avery Anderson, RRT, and Susan McCloskey, CRNP for their dedication in monitoring patient adherence.The study was conducted at the Center for Health Equity Research and Promotion, Philadelphia VA Medical Center, Philadelphia, PA.
Role of Sponsors: The funding sources had no role in the design of the study, data collection, management, analysis and interpretation of the data, or preparation or review of the manuscript.
Abbreviations
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- ICD-9
International Classification of Disease, 9th ed.
- IQR
interquartile range
- OR
odds ratio
- OSA
obstructive sleep apnea
- PDC
proportion of days covered
- VA
Veterans Affairs
Footnotes
Funding/Support: This study was supported by grant funding to Dr Platt from Center for Health Equity Research and Promotion, Philadelphia VA Medical Center, Philadelphia, PA. Dr Platt was supported by a training grant from National Institutes of Health [Grant T-32 HL07713-14].
Dr Platt presented the results from this paper in abstract form on June 9, 2008, at the 22nd Annual Meeting of the Associated Professional Sleep Societies, Baltimore, MD.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNicholas WT, Bonsigore MR Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JW, Liu MD, Huang J. Physiological basis for a causal relationship of obstructive sleep apnoea to hypertension. Exp Physiol. 2007;92(1):21–26. doi: 10.1113/expphysiol.2006.035733. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 10.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 11.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 12.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 13.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107(1):68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 14.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 15.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 16.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 17.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 19.Pack AI, Platt AB, Pien GW. Does untreated obstructive sleep apnea lead to death? A commentary on Young et al. Sleep 2008;31:1071-8 and Marshall et al. Sleep 2008;31:1079-85. Sleep. 2008;31(8):1067–1068. [PMC free article] [PubMed] [Google Scholar]
- 20.Karlawish JH, Pack AI. Addressing the ethical problems of randomized and placebo-controlled trials of CPAP. Am J Respir Crit Care Med. 2001;163(4):809–810. doi: 10.1164/ajrccm.163.4.ed0201d. [DOI] [PubMed] [Google Scholar]
- 21.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 22.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 23.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 24.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 25.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 26.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 27.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 28.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120(1):26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165(10):1147–1152. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20(6):406–422. [PubMed] [Google Scholar]
- 33.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 34.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 35.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30(5):635–640. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 36.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 37.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229–240. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- 38.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 39.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui DS, Choy DK, Li TS, et al. Determinants of continuous positive airway pressure compliance in a group of Chinese patients with obstructive sleep apnea. Chest. 2001;120(1):170–176. doi: 10.1378/chest.120.1.170. [DOI] [PubMed] [Google Scholar]
- 41.Villar I, Izuel M, Carrizo S, Vicente E, Marin JM. Medication adherence and persistence in severe obstructive sleep apnea. Sleep. 2009;32(5):623–628. doi: 10.1093/sleep/32.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt AB, Kuna ST. To adhere or not to adhere—patients selectively decide. Sleep. 2009;32(5):583–584. doi: 10.1093/sleep/32.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 44.Influence of adherence to treatment and response of cholesterol on mortality in the Coronary Drug Project. N Engl J Med. 1980;303(18):1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 45.Horwitz RI, Viscoli CM, Berkman L, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336(8714):542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 46.Granger BB, Swedberg K, Ekman I, et al. CHARM investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 47.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 49.Stepnowsky CJ, Jr, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25(7):758–762. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 50.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. 2004;24(3):461–465. doi: 10.1183/09031936.04.00114603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.