Abstract
Background:
The exact role of neutrophils in the pathogenesis of TB is poorly understood. Recent evidence suggests that neutrophils are not simply scavenging phagocytes in Mycobacterium tuberculosis (Mtb) infection.
Methods:
Three different types of clinical specimens from patients with active pulmonary TB who underwent lung surgery were examined: sputum, BAL fluid, and cavity contents. Differential cell separation and quantification were performed for intracellular and extracellular bacteria, and bacterial length was measured using microscopy.
Results:
Neutrophils were more abundant than macrophages in sputum (86.6% ± 2.2% vs 8.4% ± 1.3%) and in BAL fluid (78.8% ± 5.8% vs 11.8% ± 4.1%). Inside the cavity, lymphocytes (41.3% ± 11.2%) were the most abundant cell type, followed by neutrophils (38.8% ± 9.4%) and macrophages (19.5% ± 7.5%). More intracellular bacilli were found in neutrophils than macrophages in sputum (67.6% ± 5.6% vs 25.2% ± 6.5%), in BAL fluid (65.1% ± 14.4% vs 28.3% ± 11.6%), and in cavities (61.8% ± 13.3% vs 23.9% ± 9.3%). The lengths of Mtb were shortest in cavities (1.9± 0.1 μ m), followed by in sputum (2.9 ± 0.1 μm) and in BAL fluid (3.6 ± 0.2 μm).
Conclusions:
Our results show that neutrophils are the predominant cell types infected with Mtb in patients with TB and that these intracellular bacteria appear to replicate rapidly. These results are consistent with a role for neutrophils in providing a permissive site for a final burst of active replication of the bacilli prior to transmission.
Immunocompetent humans exposed to Mycobacterium tuberculosis (Mtb) mount an early innate and late adaptive immune response that ultimately destroys most Mtb bacilli and usually prevents the development of clinical disease.1,2 Whereas the late response is dependent on the acquisition of CD4= T-cell-mediated immunity and is characterized by granuloma formation involving epithelioid macrophages and multinucleated giant cells,3 the early response is characterized by an influx of phagocytic cells. The early host response to Mtb infection involves primarily resident alveolar macrophages and recruited neutrophils and provides the first line of defense against mycobacterial infection. The role of alveolar macrophages in innate as well as adaptive immunity against Mtb infection is very well documented.4-6
Neutrophils are professional phagocytes that play important roles in many infections, and abundant neutrophils are observed in the BAL fluid of patients with pulmonary TB.7 Some animal studies have also described acute and chronic neutrophil accumulation during mycobacterial infection,8,9 leading to speculation on various roles for these cells in the development of TB.10,11 Nevertheless, the exact role of neutrophils in the pathogenesis of Mtb infection in humans is still not completely defined. There are few reports describing neutrophil populations and their phagocytic activity in patients with active pulmonary TB.
The metabolism of Mtb is highly adaptable to the precise microenvironment in which the bacterium is residing.12 Current efforts to develop new chemotherapies to shorten the course of TB treatment are dependent upon understanding the metabolism of Mtb in patients with uncontrolled disease.13 Although there is no doubt of the importance of infected macrophages in successfully controlling initial infection with Mtb, the role of these cells in a patient with uncontrolled disease when initiating chemotherapy is far less clear. Nonetheless, new chemotherapeutic candidates with in vitro activity are often prioritized based on their activity in Mtb-infected macrophages.14-16 The aim of this study was therefore to quantify the location of Mtb bacilli in various clinical specimens, including samples from sputum, BAL fluid, and cavities, reflecting the bacterial populations in patients with active TB. We examined whether the bacilli were intracellular or extracellular and which cell types were primarily infected. We also measured the lengths of bacilli in each specimen to investigate the metabolic status of Mtb bacilli in this environment.17
Materials and Methods
Study Subjects
Human tissue collection from adult patients undergoing lung resection for the management of multidrug-resistant TB was approved by the National Masan Tuberculosis Hospital institutional review board and was granted an exemption by the US National Institutes of Health, Office of Human Subject Research. All subjects gave written informed consent for the collection and use of their specimens for research. Sputum was provided by 15 outpatients who visited National Masan Tuberculosis Hospital and received a diagnosis of active TB based on clinical findings and radiologic examination. Sputum was obtained for routine examination, and the remainder was used for this study. The mean age of the subjects was 47.5 ± 4 years (range 29-47), and the average number of treatment episodes and average period of treatment history of the subjects were 2.5 ± 0.9 episodes (range 0-9) and 24.5 ± 8.2 months (range 0-87). All but one subject had cavitary lesions. Observed comorbidities included two subjects with diabetes mellitus, two with hepatitis, and one with hypertension. Sputa from 11 of the 15 subjects were acid-fast bacilli (AFB) smear positive. BAL fluid was obtained from 10 hospitalized subjects with TB who were undergoing bronchoscopy and BAL for medical indications. The mean age was 38.3 ± 4.7 years (range 17-60), and the average number of previous treatment episodes in these subjects was 2.1 ± 0.3 (range 0-4). Seven subjects had cavitary lesions, and among them three subjects had taken no medication before BAL. The average duration of therapy for these subjects before BAL was 13.9 ± 3.7 months (range 6-45). Obser ved complications included two cases of diabetes mellitus, one hepatitis case, and one case of COPD. Five of the 10 subjects who underwent BAL were AFB negative. Necrotic contents from nine cavities were collected from waste lung tissue that had been surgically resected for the debulking of multidrug-resistant disease in four patients. The mean age was 36 ± 3.9 years (range 29-47), and the average number of previous treatments for these patients was 1.5 ± 0.6 (range 0-3). The average duration of therapy prior to surgery for these patients was 11.1 ± 1.5 months (range 9.5-15.5). No other comorbidities were observed in these patients.
Preparation of Clinical Specimens
Sputum specimens were diluted 1:1 using RPMI 1640 medium (Gibco; Gaithersburg, MD), and collagenase type 4 (2 mg/mL) was added to digest the adhesive materials. After slight vortexing, the sputum was incubated in a shaking water bath at 37°C for 1 h and filtered through sterile gauze. The filtered sputum was overlaid carefully on Ficoll-Hypaque and centrifuged at 624g for 20 min at room temperature. After removing the supernatant, the buffy coat, ficoll, and pellet layers were recovered and transferred to three separate 50-mL conical tubes and washed with HBSS (SIGMA H2387; St. Louis, MO) at room temperature. The cell pellet of each tube was resuspended, and cytospin slides were prepared from each tube. All the cytospin slides in this study were stained with Ziehl-Neelsen and Hemacolor (Merck; Darmstadt, Germany). Few cells were observed among the sticky debris in the buffy coat and pellet layers, so only the cytospin from the ficoll layer was used in this study.
BAL fluid was obtained by bronchoscopy. The bronchoscope was inserted near a region of affected lung and about 15 to 30 mL of sterile 0.9% saline was infused and recovered through the aspiration port. The collected BAL fluid was filtered through sterilized gauze and centrifuged at 699g for 10 min at room temperature. After removing the supernatants, the pellets were resuspended and washed with HBSS (SIGMA H2387). Cytospin slides were prepared according to the manufacturer’s instructions.
Cavity caseum was carefully removed using a 10-mL disposable syringe and spatula while dissecting the lung tissue. The caseum was transferred into a 50-mL conical tube, mixed with 10 mL of RPMI 1640 medium (Gibco), overlaid on Ficoll-Hypaque (GE Healthcare; Uppsala, Sweden), and centrifuged onto cytospin slides, as above. Because only dead cell debris was found in the pellet layer, the slides from the buffy coat and ficoll layers were counted and then pooled and evaluated.
Counting Infected Cells and Determination of Bacterial Length
Cells and Mtb bacilli were counted using light microscopy. First, differential cell counts were performed using a set of standardized morphometric appearance criteria (magnification ×400), and the percentage of each cell population was recorded after counting more than 200 cells. Second, the percentage of extracellular and intracellular Mtb bacilli were counted (magnification ×1,000) in more than 100 fields from the same slides. Finally, the number of cells containing bacilli was counted (magnification ×1,000) in at least 100 fields, and the percentage of each phagocytic cell type was calculated. Differential cell counting, intracellular and extracellular localization, and determination of the number of cells with bacilli were all done at least twice by two independent, blinded readers.
The length of at least 200 Mtb bacilli within each sample was measured (magnification ×1,000) independently by two different persons using the AxioImage A1 and AxioVison Rel. 4.5 software (Zeiss; Jena, Germany). For statistical analysis, the precise lengths were used. For histographic representation, individual bacilli were regrouped based on a range of lengths (eg, ≤ 1 μm, ≤1.5 μm), calculated as a percentage.
Statistical Analysis
The statistical analysis was performed using GraphPad PRISM (Graph Pad Software, Inc.; La Jolla, CA), version 4.0. The data among groups were analyzed using one-way analysis of variance, and the differences between two groups were tested using the unpaired t test. Differences were considered statistically significant when P ≤ .05.
Results
Differential Cell Counts in Sputum, BAL Fluid, and Cavity Caseum
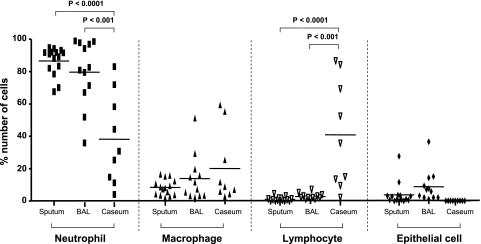
Differential cell counts were done using cytospins obtained from sputum (Fig 1A), BAL fluid (Fig 1B), and cavity caseum (Fig 1C) from patients who were either AFB positive or AFB negative (for all samples from 15 subjects). In sputum and BAL, the most abundant cells observed were neutrophils, which comprised 86.6% ± 2.2% and 78.8% ± 5.8% of the total cells in each sample, respectively (Fig 2). Macrophages were much less numerous than neutrophils in both of these samples (8.4% ± 1.3% in sputum and 11.8% ± 4.1% in BAL), and lymphocytes comprised only about 1% of the total cells found in both sputum and BAL. The remaining cells were epithelial cells and amounted to 3.9% ± 1.9% and 7.8% ± 2.9% in sputum and BAL, respectively (Fig 2). In the cavity caseum, however, 41% of the cells observed were lymphocytes, similar to the number of neutrophils (38.8% ± 9.4%), whereas macrophages comprised only about 19.5% ± 7.5% of the identifiable cells (Fig 2).
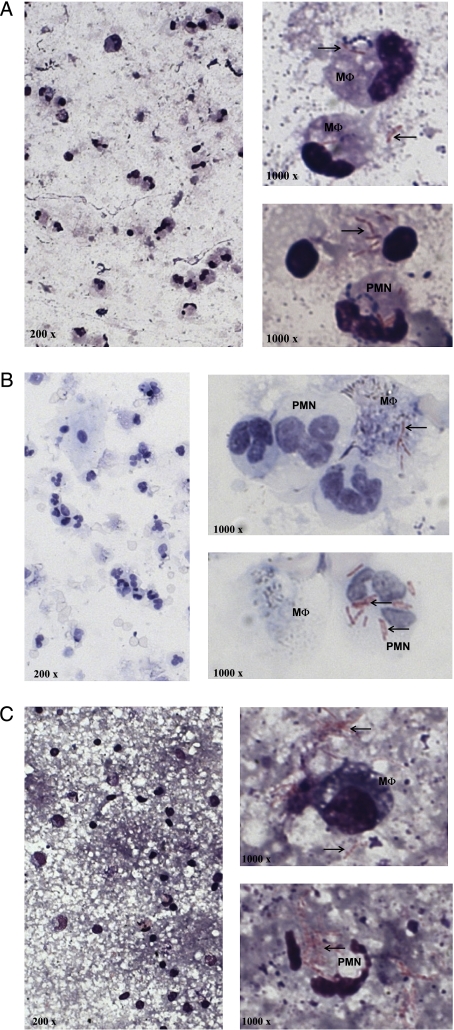
Figure 1.
Localization of Mtb bacilli in sputum (A), in BAL (B), and in cavity caseum (C). Cytospin slides of each sample were prepared and stained for acid-fast bacilli (Ziehl-Neelsen, original magnification ×200) and human cell morphology (Hemacolor, original magnification ×1,000). The bacilli (arrows) collocate with macrophages as well as neutrophils and the extracellular matrix. M ɸ = macrophages; Mtb = Mycobacterium tuberculosis; PMN = neutrophils.
Figure 2.
Differential cell counts in sputum, in BAL, and in cavity caseum. The percentage of each cell type was calculated when the counted total cell numbers were more than 200 and represented as neutrophil, macrophage, lymphocyte, and epithelial cells. Horizontal bars indicate mean value. The data among groups were analyzed using one-way analysis of variance, and the differences between two groups were tested using the unpaired t test; P ≤.05 was considered significant.
Intracellular vs Extracellular Localization of Mtb Bacilli in Sputum, BAL, and Cavity Caseum
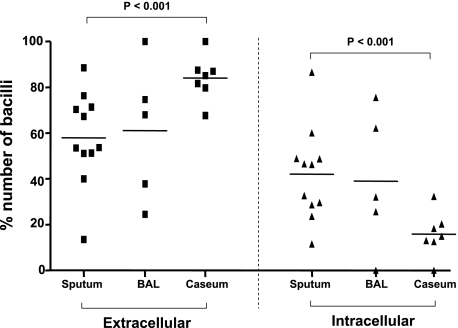
For these analyses, only the AFB-positive cytospins (11 of the initial 15) from sputum, BAL fluid, and cavity caseum were counted. The number of bacilli was individually tallied based on whether they were intracellular or extracellular by manually scoring at least 100 fields of the AFB-stained cytospin preparation. In both sputum and BAL, the bacilli were evenly distributed between extracellular and intracellular (Fig 3). Strikingly, in the cavity caseum, more than 80% of bacilli were extracellular, which was statistically significant compared with both sputum and BAL (P ≤.001) (Fig 3).
Figure 3.
Localization of Mtb bacilli in sputum, in BAL, and in cavity caseum. Cytospin slides were prepared and stained by the Ziehl-Neelsen method followed by Hemacolor staining. The number of bacilli was counted both inside and outside of the cells in more than 100 fields (original magnification ×1,000) under a light microscope, and the total number ranged from 5 to 855. The percentage of extracellular and intracellular bacilli was calculated and represented in the extracellular (bacilli in extracellular space) and intracellular (bacilli in intracellular space) columns. Horizontal bars indicate mean value. The data among groups were analyzed using one-way analysis of variance, and the differences between two groups were tested using the unpaired t test; P ≤.05 was significant. See Figure 1 legend for expansion of abbreviation.
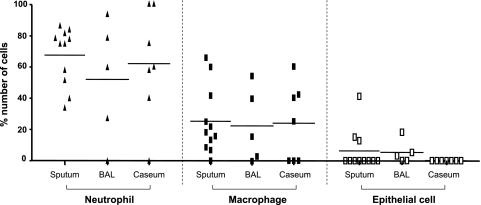
The type of cells engulfing bacilli was also quantified from each source. Neutrophils were more likely to have phagocytosed bacilli than macrophages in sputum (67.6% ± 5.6% vs 25.2% ± 6.5%), in BAL (65.1% ± 14.4% vs 28.3% ± 11.6%), and in cavity caseum (61.8% ± 13.3% vs 23.9% ± 9.3%) (Fig 4). Some bacilli were also found inside of epithelial cells in sputum and BAL (6.3% ± 3.9% and 6.6% ± 4.0%, respectively) (Fig 4). No epithelial cells were found in the cavity caseum.
Figure 4.
Differential counts of the cells ingesting Mtb bacilli in sputum, in BAL, and in cavity caseum. Cytospin slides were prepared and stained by the Ziehl-Neelsen method followed by Hemacolor staining. The number of cells engulfing bacilli was counted in more than 100 fields (original magnification ×1,000) under a light microscope and ranged from 4 to 47. The percentage of each cell type was calculated and represented as Neutrophil, Macrophage, and Epithelial Cell. Horizontal bars indicate mean value. The data among groups were analyzed using one-way analysis of variance, the differences between two groups were tested using the unpaired t test, and no significance was found among groups. See Figure 1 legend for expansion of the abbreviation.
Bacterial Length Within Samples
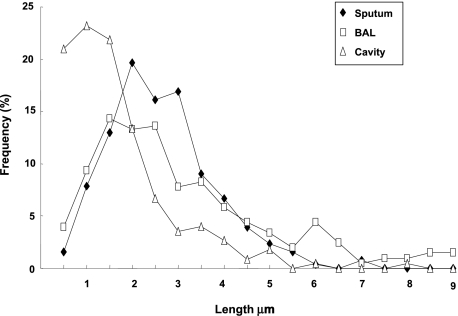
Bacterial cell length was directly measured to the nearest tenth of a micron in each specimen. Figure 5 shows the frequency of Mtb bacilli in each 0.5-μm increment. A broad distribution of Mtb lengths was observed, and the average value of Mtb cell lengths was 2.9 ± 0.1 μm, 3.6 ± 0.2 μm, and 1.9 ± 0.1 μm for sputum, BAL, and cavity caseum, respectively. A statistically significant difference between the primarily extracellular cavitary organisms and both BAL and sputum organisms (which contain primarily organisms within neutrophils) was observed (P <.001).
Figure 5.
Frequency of Mtb bacilli lengths measured in sputum, in BAL, and in cavity caseum. More than 200 bacilli in each specimen were measured, grouped (as described in the “Materials and Methods ” section), and plotted. Individual measurements were used for the calculation of average length and other statistical analyses. Extensive heterogeneity was observed in cell length in different specimens. The average values of Mtb cell lengths were 2.9 ± 0.1 μm, 3.6 ± 0.2 μm, and 1.9 ± 0.1 μm for sputum, BAL and cavity caseum, respectively. A statistically significant difference between cavity caseum and both BAL and sputum was observed (P ≤.001). See Figure 1 legend for expansion of the abbreviation.
Discussion
Neutrophils are the first defensive cells recruited to tissue following infection, where their role has been thought to involve eliminating invading pathogens via mechanisms such as the generation of reactive oxygen species18 and the release of preformed oxidants and proteolytic enzymes from granules.19,20 However, the proteolytic enzymes released by degranulation may also cause the destruction of neighboring cells and the dissolution of tissue.21,22 This neutrophil-dependent tissue damage is known as the “neutrophil paradox, ” in which the defending cells become an enemy.23 Thus, a strict regulation of neutrophil influx and their turnover in infected tissues is essential. Neutrophils also have immunomodulatory function; when stimulated by Mtb, they release an array of cytokines and chemokines that attracts other inflammatory cells.24,25 In addition, the interaction of neutrophils with Mtb triggers apoptosis of neutrophils,26,27 and phagocytosis of apoptotic neutrophils by macrophages results in the decreased viability of intracellular Mtb,28 suggesting a cooperative role of neutrophils in the host’s defensive strategy against Mtb infection.
Many reports have demonstrated the presence of neutrophils in the sputum of patients suffering from mycobacterial infections (in fact, large numbers of neutrophils are considered a hallmark of high-quality sputum).29,30 Previous reports in which researchers observed fewer neutrophils than we report here show a greater proportion of epithelial cells, indicating less productive sputum (the epithelial cells presumably originating from saliva).31 Neutrophils are also persistently recruited to the sites of chronic mycobacterial infection.32,33
Of the many cell types present in the naive human lung that may mediate control of Mtb, most investigators have focused on alveolar macrophages or monocyte-derived macrophages. Yet the ability of human macrophages to kill Mtb in culture has never been convincing and consistent, suggesting that other cell types present at the site may also play important roles in innate resistance. Neutrophils are also essential for granuloma formation during chronic Mtb infection.34 However, the ability of human neutrophils to solely mediate the outcome of pulmonary infection with Mtb is likewise questionable. Neutrophil depletion of mice was shown to have no impact on mycobacterial infections,35 but reconstitution of mice with neutrophils increased resistance to Mycobacterium avium.36 The ability of human neutrophils to kill virulent Mtb in vitro has been inconsistent at best,37-40 but there have been consistent reports that circulating neutrophils from patients with active TB are highly activated upon stimulation with Mtb.41,42
The unit cell length of mycobacteria changes depending on the growth state in vitro, increasing during logarithmic growth and decreasing in the stationary phase.17 In both sputum and BAL fluid, Mtb were found to be significantly longer than typical stationary phase cells. However, bacilli isolated from the cavities of resected lung tissue were significantly shorter than those from either of these two sources, averaging about 1 μm in length (Fig 5). Thus, cavitary organisms appear to resemble in length stationary-phase organisms in vitro, whereas organisms in sputum (and BAL) appear to resemble logarithmically replicating organisms in vitro.
The caseum of a liquefying cavity has long been thought to be the final site of the rapid replication of bacilli prior to their exit into the airways and subsequent transmission.43 Surprisingly, our results suggest that this final burst of replication prior to transmission does not happen in the liquifying cavity, but rather upon the exit of the bacilli from that cavity into the sputum, and most likely in the context of an infected neutrophil.
There are, of course, several limitations to the current study, including the fact that the bacilli observed in the three different specimen types were not obtained from the same patient nor from patients with the same extent of disease. These patients have different histories of TB and treatment that could also affect the comparison. Nonetheless, the striking consistency in cell lengths and cell types observed across these samples suggests that this difference is meaningful.
Neutrophils may therefore play a much more profound role in the life cycle of this pathogen than has been previously appreciated. Neutrophils have been shown to participate in the transport of live mycobacteria from peripheral tissue to the lymphoid organ in mice,44 and tissue neutrophils from TB-susceptible mouse strains have a significantly higher migration capacity, survive longer, and contain more intracellular mycobacteria.45 There is also the fact that patients with noncavitary TB nonetheless produce sputum laden with AFB. Thus, neutrophils may act as transporters for bacilli from lesions to the pulmonary surface. Neutrophils may be short-lived, but they nonetheless appear to be an important microenvironment occupied by Mtb during two critical events in the life of a patient with TB: transmission and chemotherapy. Understanding the bacterial adaptations that occur during replication in neutrophils may suggest novel ways to interfere with transmission that could become an important adjunct to conventional chemotherapy.
Acknowledgments
Author contributions: All of the authors have read and approved the final manuscript.
Dr Eum: contributed to the research conception, data acquisition and analysis, and drafting of the manuscript.
Ms Kong: performed the isolation of the cells from the specimens and the cytologic examinations.
Ms Hong: performed the isolation of the cells from the specimens and the cytologic examinations.
Ms Lee: performed the isolation of the cells from the specimens and the cytologic examinations.
Dr Kim: performed bronchial washings and surgeries and critically reviewed the manuscript.
Dr Hwang: contributed to the review and the final revision of the manuscript, and provided guidance.
Dr Cho: contributed to research conception and critically revised the manuscript.
Dr Via: contributed to research conception and analysis of the data and critically revised the manuscript.
Dr Barry: contributed to research conception and analysis of the data, and critically revised the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This work was performed at the International Tuberculosis Research Center. We thank Dr Seung-Kyu Park, the director of National Masan TB Hospital for his support for this study.
Abbreviations
- AFB
acid-fast bacilli
- Mtb
Mycobacterium tuberculosis
Footnotes
Funding/Support: This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases (Z01 AI000783-11), and in part by a grant from the Bill and Melinda Gates Foundation and the Wellcome Trust through the Grand Challenges in Global Health Initiative (C.E.B. and S.-N.C.) Grant No. 37882 (Douglas Young, Imperial College, Principal Investigator).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157(3 Pt 1):679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 2.van Crevel R, Ottenhoff THM, van der Meer JWM. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15(2):294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulrichs T, Kaufmann SHE. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208(2):261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 4.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 5.Wallis RS, Ellner JJ. Cytokines and tuberculosis. J Leukoc Biol. 1994;55(5):676–681. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Broser M, Cohen H, et al. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95(2):586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law KF, Jagirdar J, Weiden MD, Bodkin M, Rom WN. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 8.Antony VB, Sahn SA, Harada RN, Repine JE. Lung repair and granuloma formation. Chest. 1983;83(5 Suppl):95S. doi: 10.1378/chest.83.5.95s. [DOI] [PubMed] [Google Scholar]
- 9.Appelberg R, Silva MT. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989;78(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- 10.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68(2):577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawant KV, McMurray DN. Guinea pig neutrophils infected with Mycobacterium tuberculosis produce cytokines which activate alveolar macrophages in noncontact cultures. Infect Immun. 2007;75(4):1870–1877. doi: 10.1128/IAI.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boshoff HI, Barry CE., III A low-carb diet for a high-octane pathogen. Nat Med. 2005;11(6):599–600. doi: 10.1038/nm0605-599. [DOI] [PubMed] [Google Scholar]
- 13.Young DB, Perkins MD, Duncan K, Barry CE., III Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118(4):1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataprasad N, Ledger P, Ivanyi J. The effect of glucosaminylmuramyl dipeptide injection to mice on the course of tuberculous infection and in vitro superoxide anion production. Int Arch Allergy Immunol. 1997;114(1):23–29. doi: 10.1159/000237638. [DOI] [PubMed] [Google Scholar]
- 15.Boshoff HI, Xu X, Tahlan K, et al. Biosynthesis and recycling of nicotinamide cofactors in Mycobacterium tuberculosis. An essential role for NAD in nonreplicating bacilli. J Biol Chem. 2008;283(28):19329–19341. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspar MM, Cruz A, Fraga AG, Castro AG, Cruz ME, Pedrosa J. Developments on drug delivery systems for the treatment of mycobacterial infections. Curr Top Med Chem. 2008;8(7):579–591. doi: 10.2174/156802608783955629. [DOI] [PubMed] [Google Scholar]
- 17.Thanky NR, Young DB, Robertson BD. Unusual features of the cell cycle in mycobacteria: polar-restricted growth and the snapping-model of cell division. Tuberculosis (Edinb) 2007;87(3):231–236. doi: 10.1016/j.tube.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.May ME, Spagnuolo PJ. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect Immun. 1987;55(9):2304–2307. doi: 10.1128/iai.55.9.2304-2307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedek-Spät E, Di Felice R, Andersen E, Cimasoni G. In vitro release of elastase from human blood and gingival crevicular neutrophils. Arch Oral Biol. 1991;36(7):507–510. doi: 10.1016/0003-9969(91)90143-i. [DOI] [PubMed] [Google Scholar]
- 20.Witko-Sarsat V, Cramer EM, Hieblot C, et al. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94(7):2487–2496. [PubMed] [Google Scholar]
- 21.Fujie K, Shinguh Y, Inamura N, Yasumitsu R, Okamoto M, Okuhara M. Release of neutrophil elastase and its role in tissue injury in acute inflammation: effect of the elastase inhibitor, FR134043. Eur J Pharmacol. 1999;374(1):117–125. doi: 10.1016/s0014-2999(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7(1):1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 24.Petrofsky M, Bermudez LE. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clin Immunol. 1999;91(3):354–358. doi: 10.1006/clim.1999.4709. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178(1):127–137. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 26.Alemán M, García A, Saab MA, et al. Mycobacterium tuberculosis-induced activation accelerates apoptosis in peripheral blood neutrophils from patients with active tuberculosis. Am J Respir Cell Mol Biol. 2002;27(5):583–592. doi: 10.1165/rcmb.2002-0038OC. [DOI] [PubMed] [Google Scholar]
- 27.Alemán M, Schierloh P, de la Barrera SS, et al. Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect Immun. 2004;72(9):5150–5158. doi: 10.1128/IAI.72.9.5150-5158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177(3):1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 29.McCarter YS, Robinson A. Quality evaluation of sputum specimens for mycobacterial culture. Am J Clin Pathol. 1996;105(6):769–773. doi: 10.1093/ajcp/105.6.769. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro-Rodrigues R, Resende Co T, Johnson JL, et al. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9(4):818–823. doi: 10.1128/CDLI.9.4.818-823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shata AMA, Coulter JBS, Parry CM, Ching’ani G, Broadhead RL, Hart CA. Sputum induction for the diagnosis of tuberculosis. Arch Dis Child. 1996;74(6):535–537. doi: 10.1136/adc.74.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 33.Appelberg R, Pedrosa JM, Silva MT. Host and bacterial factors control the Mycobacterium avium-induced chronic peritoneal granulocytosis in mice. Clin Exp Immunol. 1991;83(2):231–236. doi: 10.1111/j.1365-2249.1991.tb05620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiler P, Aichele P, Bandermann S, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33(10):2676–2686. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- 35.Seiler P, Aichele P, Raupach B, Odermatt B, Steinhoff U, Kaufmann SH. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J Infect Dis. 2000;181(2):671–680. doi: 10.1086/315278. [DOI] [PubMed] [Google Scholar]
- 36.Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63(9):3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156(6):985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 38.Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162(3):700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 39.Denis M. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J Infect Dis. 1991;163(4):919–920. doi: 10.1093/infdis/163.4.919. [DOI] [PubMed] [Google Scholar]
- 40.Aston C, Rom WN, Talbot AT, Reibman J. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1943–1950. doi: 10.1164/ajrccm.157.6.9705028. [DOI] [PubMed] [Google Scholar]
- 41.Alemán M, Beigier-Bompadre M, Borghetti C, et al. Activation of peripheral blood neutrophils from patients with active advanced tuberculosis. Clin Immunol. 2001;100(1):87–95. doi: 10.1006/clim.2001.5044. [DOI] [PubMed] [Google Scholar]
- 42.Fiorenza G, Farroni MA, Bogué C, Selenscig D, Lamas DM, Dlugovitzky D. Functional characteristics of neutrophils and mononuclear cells from tuberculosis patients stimulated in vitro with heat killed M. tuberculosis. Arch Med Res. 2007;38(5):526–533. doi: 10.1016/j.arcmed.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Canetti G. The Tubercle Bacillus in the Pulmonary Lesion of Man: Histobacteriology and Its Bearing on the Therapy of Pulmonary Tuberculosis. New York, NY: Springer; 1955. [Google Scholar]
- 44.Abadie V, Badell E, Douillard P, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106(5):1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 45.Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73(3):1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]