Abstract
Nitric oxide (NO), produced by inducible NO synthase (iNOS) during infection, plays a crucial role in host defense mechanisms. Salmonella typhimurium infection in mice is associated with excessive production of NO from iNOS as a host defense response. An important cytoprotective and antimicrobial function of NO is mediated by induction of heme oxygenase (HO)-1. The signaling mechanism of NO-dependent HO-1 induction has remained unclear, however. We recently discovered a nitrated cyclic nucleotide, 8-nitroguanosine 3',5'-cyclic monophosphate (8-nitro-cGMP), which is formed via guanine nitration with NO and reactive oxygen species. iNOS-dependent 8-nitro-cGMP formation and HO-1 induction were identified in Salmonella-infected mice. Extensive apoptosis observed with iNOS-deficient macrophages infected with Salmonella was remarkably suppressed via HO-1 induced by 8-nitro-cGMP formed in cells. This cytoprotective signaling appears to be mediated by the reaction of 8-nitro-cGMP with protein sulfhydryls to generate a novel post-translational modification named protein S-guanylation. We also found that 8-nitro-cGMP specifically S-guanylates Keap1, a negative regulator of transcription factor Nrf2, which in turn up-regulates transcription of HO-1. Here, we discuss the unique mechanism of NO-mediated host defense that operates via formation of a novel signaling molecule - 8-nitro-cGMP - during microbial infections.
Keywords: nitric oxide, host defense, 8-nitro-cGMP, heme oxygenase-1, protein S-guanylation
Introduction
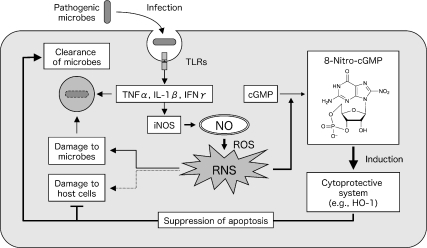
Nitric oxide (NO) plays a crucial role in innate host defense mechanisms against microbial infection. Regardless of the type of pathogen, whether bacteria, viruses, or fungi, an inducible NO synthase (iNOS) is induced almost universally during the infection process. This induction occurs in various cells after recognition by host cells of microbial structural components (e.g., lipopolysaccharides, lipoteichoic acid, peptidoglycans, and fungal polysaccharides) and nucleic acid components (such as dsRNA, ssRNA, and CpG DNA) via pattern recognition receptors including Toll-like receptors [1]. iNOS induction is synergistically enhanced by inflammatory cytokines and interferon produced during infection [2]. NO produced by iNOS reportedly reacts with simultaneously generated reactive oxygen species (ROS), is converted to reactive nitrogen species (RNS), such as peroxynitrite (ONOO−) and nitrogen dioxide (NO2), and may demonstrate direct antimicrobial activities (Fig. 1) [3–5]. In fact, infection with Salmonella typhimurium, a facultative intracellular bacterium, causes excessive production of NO via iNOS induction, along with microabscess formation, in the liver in mice. A comparative experiment with iNOS-deficient (iNOS−/−) and wild-type mice demonstrated that the bacterial growth in the liver and mortality in iNOS−/− mice were significantly higher than those in wild-type mice. This finding indicates that NO from iNOS participates in host defense against infection, possibly by means of antimicrobial activity [3].
Fig. 1.
iNOS induction and NO-mediated host defense mechanism against microbial infection. NO overproduced by iNOS induced during microbial infection is converted to reactive nitrogen species (RNS) via reaction with reactive oxygen species (ROS). RNS have two opposite biological effects: potent bactericidal activity contributing to host defense and damage of cells and tissues of the host. 8-Nitro-cGMP may function as a signaling molecule mediating oxidative stress responses, such as HO-1 induction, and it may play a crucial role in the innate host immunity.
In contrast, NO and ROS also reportedly damage host cells and tissues, which causes oxidative stress. In a murine influenza virus-infected pneumonia model, iNOS expression increased in infected lungs, especially in the respiratory epithelium and alveolar macrophages, with resultant excessive production of NO [6, 7]. However, unlike progression of Salmonella infection, progression of pneumonia is well correlated with iNOS induction; pneumonia was less severe and mortality was lower in iNOS−/− mice than in wild-type mice [6, 8]. In general, NO and ROS show antibacterial activity, but because they have no effective antiviral activity, they cause nonspecific damage of host cells and tissues. Therefore, the role of NO in the pathogenesis of infection is known as a double-edged sword (Fig. 1) [6, 8].
Recently, much attention has focused on the signaling functions of NO and ROS. NO can suppress apoptosis of host cells caused by infection, and it is involved, together with ROS, in responses to oxidative stress [9–11] (Fig. 1). Here, we reexamine the role of NO and ROS in host defense against infection, with a focus on a unique signaling function of the nitrated cyclic nucleotide 8-nitroguanosine 3',5'-cyclic monophosphate (8-nitro-cGMP), which mediates cytoprotective oxidative stress responses occurring during infections.
NO- and ROS-dependent Formation of 8-Nitro-cGMP
NO was initially discovered as a signaling molecule regulating vascular tone and neuronal systems [12, 13]. These functions are mainly mediated through a guanosine 3',5'-cyclic monophosphate (cGMP)-dependent mechanism, but other pathways that are not directly related to cGMP appear to be responsible for many aspects of NO signaling [14–16]. Although NO has diverse pathophysiological functions, NO itself is an inert molecule. Much of its chemical reactivity depends on RNS generated through the reaction with ROS produced together with in various cells. The reaction of NO with O2 and superoxide (O2−), and the reaction of nitrite (NO2−) with the H2O2-peroxidase system lead to the generation of RNS [17–19]. NO- and ROS-derived RNS have strong nitration potentials for various biological molecules such as proteins, lipids, and nucleic acids, and they possess cGMP-independent signaling functions, as mentioned above.
In fact, nitrated guanine derivatives, such as 8-nitroguanine and 8-nitroguanosine, are known to be formed by RNS, and their formation was identified in various cultured cells and in tissues from influenza virus-infected mice with viral pneumonia and humans with lung disease [8, 20, 21]. We recently clarified the NO- and ROS-dependent formation of a nitrated cyclic nucleotide, 8-nitro-cGMP, as a completely new derivative of cGMP (Fig. 1) [22]. In vitro experiments showed that reaction of cGMP with authentic peroxynitrite and with the nitrite/H2O2-peroxidase system generated 8-nitro-cGMP, but NO alone did not, which indicates that RNS are involved in nitration of cGMP (unpublished observation). We developed an anti-8-nitro-cGMP monoclonal antibody and immunostained cytokine-stimulated macrophage-like cells (RAW264.7 cells) to demonstrate that 8-nitro-cGMP formation depended on production of both NO and ROS [22]. Moreover, Salmonella infection in cultured murine macrophages generated 8-nitro-cGMP in wild-type mice but not in iNOS−/− mice. Furthermore, immunostaining analysis of liver tissue from Salmonella-infected mice demonstrated abundant 8-nitro-cGMP generation in wild-type mice but not in iNOS−/− mice [23]. This NO-dependent 8-nitro-cGMP formation was also observed in lung tissue from influenza virus-infected mice (unpublished data), which indicated that 8-nitro-cGMP was produced in an iNOS-dependent manner in vivo.
NO-mediated Induction of the Cytoprotective Molecule HO-1
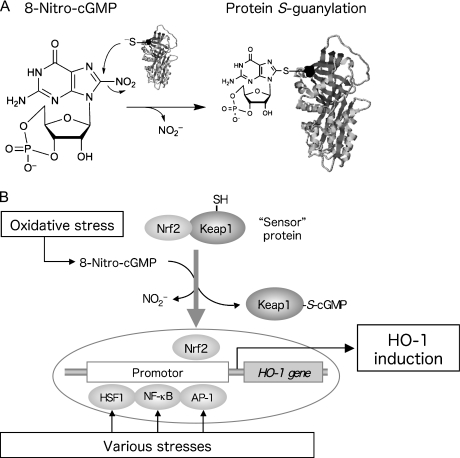
During infection, various cytoprotective and antioxidant systems are induced to protect cells and tissues from pathogenic microbes [24–26]. Heme oxygenase (HO)-1 is known as a factor that is rapidly induced during infection and that contributes to the host defense mechanism [27]. HO-1 degrades free heme, used as a substrate, into biliverdin, iron ions, and carbon monoxide (CO). Both biliverdin and iron ions carry out antioxidant activity by means of reduction to bilirubin and production of ferritin, respectively [28, 29]. CO is known to exhibit cytoprotection via suppressing production of inflammatory cytokines and apoptosis [30]. HO-1 is reportedly induced by various stresses and by multiple transcription factors, e.g., heat shock factor 1 (HSF1), nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and nuclear factor-erythroid 2-related factor 2 (Nrf2) (Fig. 2) [27]. Modulators that upregulate these transcription factors include heat shock or intracellular accumulation of abnormal proteins (HSF1), infection or inflammatory responses (NF-κB), abnormal cell growth (AP-1), and electrophiles or oxidants (Nrf2).
Fig. 2.
New post-translational modification of proteins and HO-1 induction by 8-nitro-cGMP. (A) 8-Nitro-cGMP, a novel nitrated cyclic nucleotide generated from NO and ROS in cells, possesses an electrophilic property and causes S-guanylation of proteins, i.e., adduction of cGMP to protein sulfhydryls. (B) 8-Nitro-cGMP activates the Nrf2 pathway via S-guanylation of a sensor protein, Keap1, which leads to HO-1 induction.
Analyses of HO-1 levels in Salmonella-infected mice demonstrated that both the amount of HO-1 protein and activity of HO-1 (as evidenced by blood CO levels) as well as its mRNA levels increased during infection, with induction seen mainly in macrophages [23]. Pharmacological inhibition of HO-1 activity increased bacterial growth (by approximately 10-fold) and apoptosis in liver tissues [23]. Therefore, HO-1 was suggested to function in host defense by suppressing macrophage apoptosis essential for elimination of Salmonella (Fig. 1). An important finding was lower levels of protein, mRNA, and activity of HO-1 in iNOS−/− mice compared with wild-type mice, which suggests the possible involvement of NO in HO-1 induction [3, 23]. NO- and ROS-mediated induction of HO-1 has been reported from other groups [10, 31].
The Unique Signaling Function of 8-Nitro-cGMP via Protein S-Guanylation
To further clarify the mechanisms of NO-mediated induction of HO-1, we used cultured macrophages from iNOS−/− mice to analyze the effect of authentic 8-nitro-cGMP. Treatment of iNOS−/− macrophages with 8-nitro-cGMP resulted in increased HO-1 levels in a manner that depended on time and concentration of 8-nitro-cGMP. In addition, HO-1 levels in Salmonella-infected macrophages were lower in iNOS−/− cells than in wild-type cells, but addition of 8-nitro-cGMP restored these lower levels to values comparable to those found in wild-type macrophages. Moreover, 8-nitro-cGMP treatment also markedly suppressed apoptosis associated with infection [23]. These findings suggest the possible involvement of 8-nitro-cGMP that is generated during infection in the signaling pathway of HO-1 induction (Fig. 1).
We further analyzed the molecular mechanisms governing 8-nitro-cGMP signaling functions. Because of its electrophilicity, 8-nitro-cGMP adducted with a sulfhydryl group of proteins via nucleophilic substitution with the nitro moiety of 8-nitro-cGMP to form a protein-S-cGMP adduct, which is a novel post-translational modification called protein S-guanylation (Fig. 2A) [22]. This protein adduct formation accompanies the denitration of 8-nitro-cGMP, with release of nitrite. Because 8-nitro-cGMP loses electrophilicity after S-guanylation, this electrophilic modification seems to be irreversible, at least under physiological conditions without specific catalysts yet to be identified. We found that one of the most important target proteins for S-guanylation is Kelch-like ECH-associated protein 1 (Keap1), which is now increasingly recognized as a potent redox-sensing protein. Keap1 is a negative regulator of Nrf2, which is a transcription factor regulating antioxidant enzymes for electrophiles and ROS [32, 33]. Binding of Keap1 to Nrf2 inhibits Nrf2 transcriptional activity via sustaining rapid degradation of Nrf2 by proteasomes in the cytosolic compartment of cells. Keap1 is proposed to be a sensor protein for oxidative stress (Fig. 2B). Because Keap1 has highly reactive Cys residues, chemical modification of the Cys residue sulfhydryl groups by electrophiles and ROS is considered to trigger dissociation of Nrf2. Activated Nrf2 then translocates to nuclei to induce expression of various antioxidant and cytoprotective enzymes including HO-1, which contributes to the adaptive response to oxidative stress [34–36].
We thus examined whether Keap1 can indeed be modified by 8-nitro-cGMP produced via NO in cells. We infected cultured murine macrophages with Salmonella, and we found clear evidence of Keap1 S-guanylation by means of Western blotting using anti-S-guanylation antibody after isolation by immunoprecipitation [22, 23]. We interpret these results to mean that 8-nitro-cGMP is involved in the major NO signaling pathway for cytoprotection and adaptive responses to ROS and oxidative stress through S-guanylation of Keap1. Strong support for this interpretation comes from the finding that cytoprotection and host defense conferred by 8-nitro-cGMP were clearly associated with increased HO-1 expression during Salmonella infection in macrophages and in vivo, as mentioned above [23] (Fig. 2B).
Beneficial and Pathological Effects of HO-1 in Various Microbial Infections
Analysis of the Salmonella infection model demonstrated that the NO-dependent and 8-nitro-cGMP-mediated signal pathway leads to HO-1 expression, and suppression of apoptosis of infected macrophages potentiates microbial clearance—a host defense function against infection. However, some types of intracellular pathogens survive and multiply in macrophages because they escape the macrophage bactericidal system. One possible survival mechanism of these bacteria is, however, suppression of apoptosis of infected macrophages [37]. That is to say, HO-1 overexpression and resultant suppression of apoptosis are presumed to provide pathogens with a favorable intracellular environment so that they survive and grow. Similarly, inhibition of virus elimination by suppression of apoptosis of infected respiratory epithelial cells has been suggested as a potential pathogenic mechanism for the harmful effects of NO observed in a murine influenza virus-infected pneumonia model [6, 8]. Although HO-1 induction via 8-nitro-cGMP is believed to contribute to host defense against infection [23, 38, 39], at the same time it may promote survival of particular pathogens (typically viruses). Various factors, such as the type of cells infected and the timing of HO-1 expression during infections, may lead to completely opposite biological effects of HO-1.
Conclusions
In summary, we have here clarified NO-dependent formation and cell signaling functions of 8-nitro-cGMP, which lead to expression of HO-1 and consequent cytoprotection in infected hosts. The 8-nitro-cGMP-mediated signaling pathway may protect host cells from apoptosis and support the antimicrobial effects of macrophages that are critically involved in innate immunity. Now in progress in our laboratory are further investigations of the antimicrobial functions of NO and 8-nitro-cGMP, with a special focus on potential target proteins of S-guanylation that is mediated by 8-nitro-cGMP, which may help establish a new understanding of host defense and microbial pathogenesis.
Acknowledgments
We thank Judith B. Gandy for her excellent editing of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research (B and C) and Grants-in-Aid for Scientific Research on Innovative Areas (Research in a Proposed Area) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Grants-in-Aid from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- 8-nitro-cGMP
8-nitroguanosine 3',5'-cyclic monophosphate
- AP-1
activator protein-1
- cGMP
guanosine 3',5'-cyclic monophosphate
- HSF1
heat shock factor 1
- HO
heme oxygenase
- iNOS
inducible nitric oxide synthase
- iNOS−/−
iNOS-deficient
- Keap1
Kelch-like ECH-associated protein 1
- Nrf2
nuclear factor-erythroid 2-related factor 2
- NF-κB
nuclear factor-κB
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
References
- 1.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Saura M., Zaragoza C., Bao C., McMillan A., Lowenstein C.J. Interaction of interferon regulatory factor-1 and nuclear factor κB during activation of inducible nitric oxide synthase transcription. J. Mol. Biol. 1999;289:459–471. doi: 10.1006/jmbi.1999.2752. [DOI] [PubMed] [Google Scholar]
- 3.Alam M.S., Akaike T., Okamoto S., Kubota T., Yoshitake J., Sawa T., Miyamoto Y., Tamura F., Maeda H. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect. Immun. 2002;70:3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doi T., Ando M., Akaike T., Suga M., Sato K., Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect. Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umezawa K., Akaike T., Fujii S., Suga M., Setoguchi K., Ozawa A., Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect. Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akaike T., Noguchi Y., Ijiri S., Setoguchi K., Suga M., Zheng Y.M., Dietzschold B., Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karupiah G., Chen J.H., Mahalingam S., Nathan C.F., MacMicking J.D. Rapid interferon γ-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akaike T., Okamoto S., Sawa T., Yoshitake J., Tamura F., Ichimori K., Miyazaki K., Sasamoto K., Maeda H. 8-Nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:685–690. doi: 10.1073/pnas.0235623100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brune B., von Knethen A., Sandau K.B. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 1998;351:261–272. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 10.Sandau K., Pfeilschifter J., Brune B. Nitrosative and oxidative stress induced heme oxygenase-1 accumulation in rat mesangial cells. Eur. J. Pharmacol. 1998;342:77–84. doi: 10.1016/s0014-2999(97)01321-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.M., Bergonia H., Lancaster J.R. Jr. Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS Lett. 1995;374:228–232. doi: 10.1016/0014-5793(95)01115-u. [DOI] [PubMed] [Google Scholar]
- 12.Bredt D.S., Hwang P.M., Snyder S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 13.Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J. Clin. Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Ruiz A., Lamas S. Two decades of new concepts in nitric oxide signaling: from the discovery of a gas messenger to the mediation of nonenzymatic posttranslational modifications. IUBMB Life. 2009;61:91–98. doi: 10.1002/iub.144. [DOI] [PubMed] [Google Scholar]
- 15.Freeman B.A., Baker P.R., Schopfer F.J., Woodcock S.R., Napolitano A., d’Ischia M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamler J.S., Lamas S., Fang F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 17.Eiserich J.P., Hristova M., Cross C.E., Jones A.D., Freeman B.A., Halliwell B., van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 18.Schopfer F.J., Baker P.R., Freeman B.A. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terasaki Y., Akuta T., Terasaki M., Sawa T., Mori T., Okamoto T., Ozaki M., Takeya M., Akaike T. Guanine nitration in idiopathic pulmonary fibrosis and its implication for carcinogenesis. Am. J. Respir. Crit. Care Med. 2006;174:665–673. doi: 10.1164/rccm.200510-1580OC. [DOI] [PubMed] [Google Scholar]
- 21.Yoshitake J., Akaike T., Akuta T., Tamura F., Ogura T., Esumi H., Maeda H. Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity. J. Virol. 2004;78:8709–8719. doi: 10.1128/JVI.78.16.8709-8719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawa T., Zaki M.H., Okamoto T., Akuta T., Tokutomi Y., Kim-Mitsuyama S., Ihara H., Kobayashi A., Yamamoto M., Fujii S., Arimoto H., Akaike T. Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat. Chem. Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 23.Zaki M.H., Fujii S., Okamoto T., Islam S., Khan S., Ahmed K.A., Sawa T., Akaike T. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J. Immunol. 2009;182:3746–3756. doi: 10.4049/jimmunol.0803363. [DOI] [PubMed] [Google Scholar]
- 24.D’Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 25.Bove P.F., van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic. Biol. Med. 2006;41:515–527. doi: 10.1016/j.freeradbiomed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardolo F.L., Di Stefano A., Sabatini F., Folkerts G. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 2006;533:240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Alam J., Cook J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 28.Balla G., Jacob H.S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J.W., Vercellotti G.M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 29.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 30.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 31.Srisook K., Kim C., Cha Y.N. Role of NO in enhancing the expression of HO-1 in LPS-stimulated macrophages. Methods Enzymol. 2005;396:368–377. doi: 10.1016/S0076-6879(05)96031-X. [DOI] [PubMed] [Google Scholar]
- 32.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova A.T., Holtzclaw W.D., Kensler T.W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 34.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 37.Takaya A., Suzuki A., Kikuchi Y., Eguchi M., Isogai E., Tomoyasu T., Yamamoto T. Derepression of Salmonella pathogenicity island 1 genes within macrophages leads to rapid apoptosis via caspase-1- and caspase-3-dependent pathways. Cell. Microbiol. 2005;7:79–90. doi: 10.1111/j.1462-5822.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 38.Wiesel P., Patel A.P., DiFonzo N., Marria P.B., Sim C.U., Pellacani A., Maemura K., LeBlanc B.W., Marino K., Doerschuk C.M., Yet S.F., Lee M.E., Perrella M.A. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- 39.Poss K.D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]