Abstract
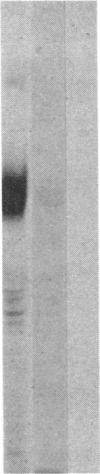
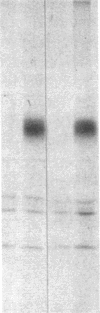
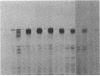
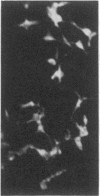
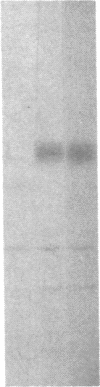
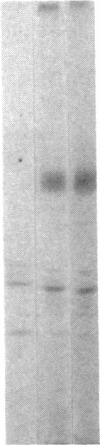
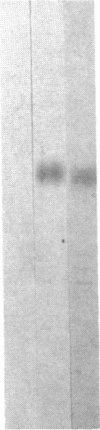
We have examined the rate of synthesis and degradation of tyrosinase (monophenol, 3,4-dihydroxyphenylalanine:oxygen oxidoreductase, EC 1.14.18.1), the critical enzyme involved in mammalian pigmentation, using pulse-chase metabolic labeling of murine melanoma cells and immunoprecipitation of protein extracts with antibodies directed specifically against the enzyme. We have found that tyrosinase is synthesized and glycosylated within melanocytes rapidly, since significant quantities of pulse-labeled enzyme could be detected within 30 min. The maximum amount of enzyme was processed within 4 hr, and the t1/2 of tyrosinase in vivo was 10 hr (compared to 120 hr with purified enzyme), suggesting that tyrosinase activity in melanocytes is at least in part regulated by rapid synthesis and active degradation. We also have examined the melanogenic stimulation caused by melanocyte-stimulating hormone, using metabolic labeling, radiometric assays, and immunofluorescence techniques; responding cells increased their melanogenic potential more than 7-fold within 4 days without increasing their levels of tyrosinase synthesis. The results demonstrate that a pool of inactive tyrosinase exists in melanocytes and that rapid increases in enzyme activity elicited by melanocyte-stimulating hormone reflect an alteration in the activity of a preexisting pool of intracellular tyrosinase.
Full text
PDF
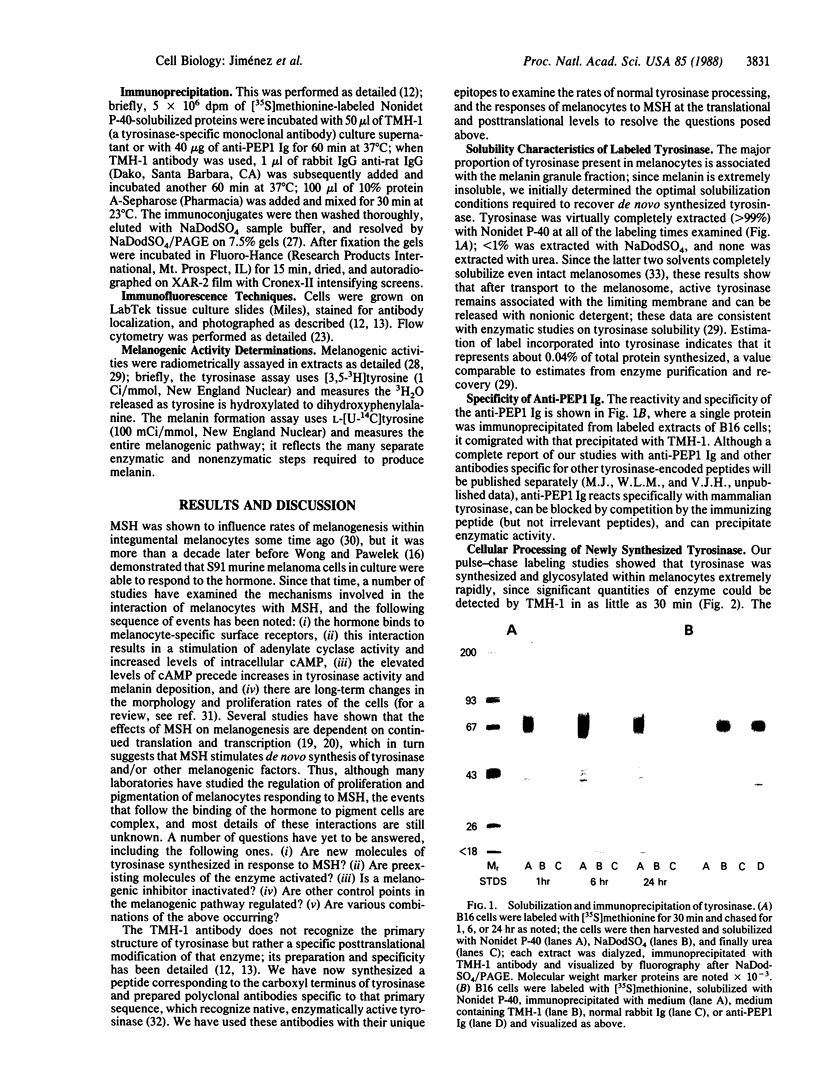
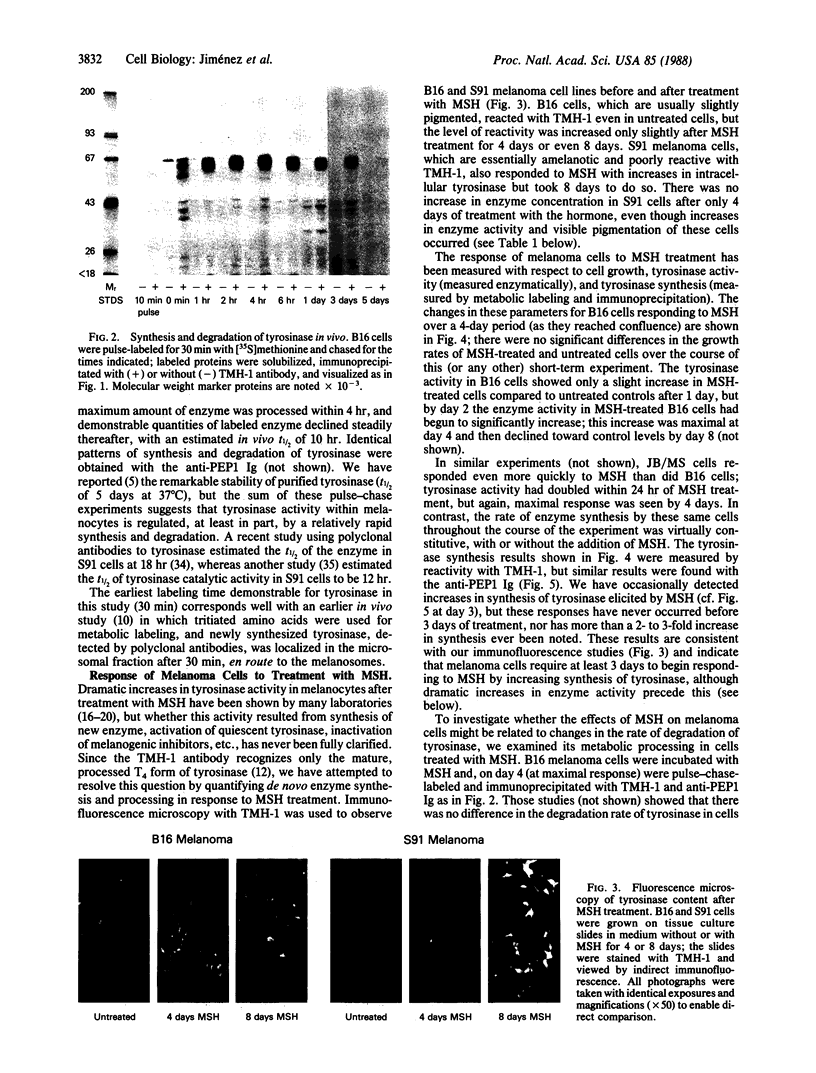
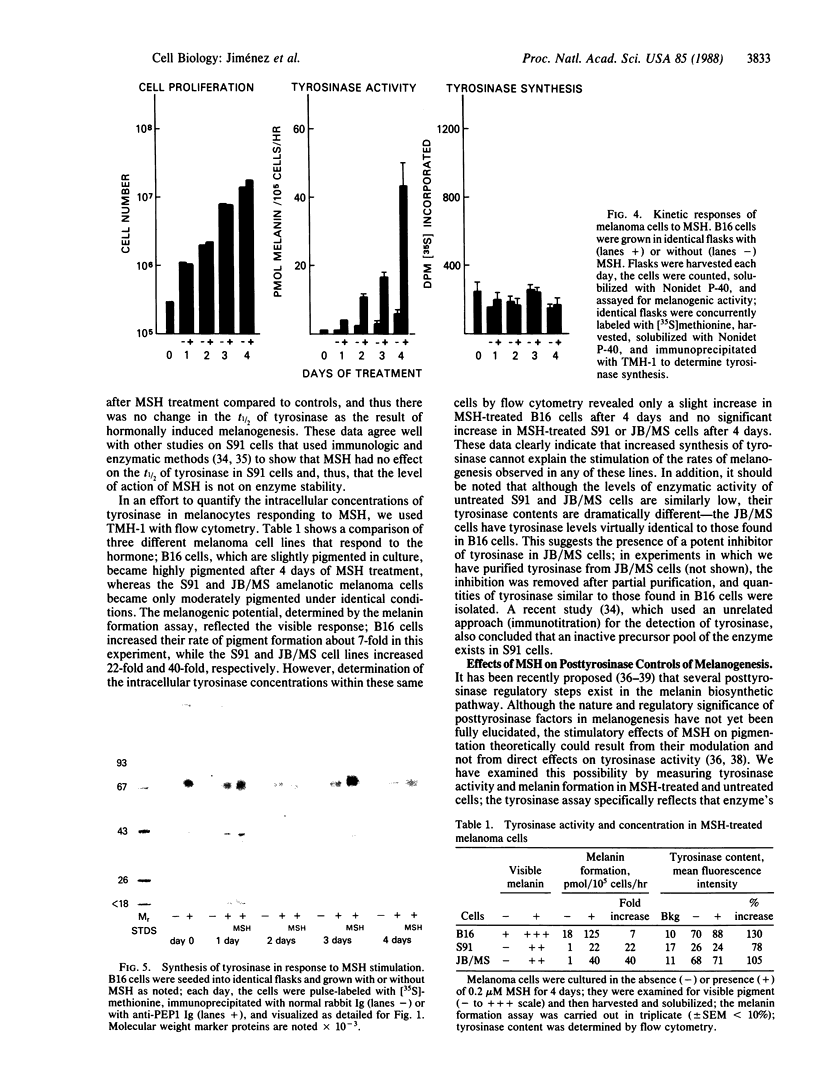

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. I., Townsend D., Olds D. P., King R. A. Dopachrome oxidoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984 Aug;83(2):145–149. doi: 10.1111/1523-1747.ep12263381. [DOI] [PubMed] [Google Scholar]
- Burnett J. B., Seiler H., Brown I. V. Separation and characterization of multiple forms of tyrosinase from mouse melanoma. Cancer Res. 1967 May;27(5):880–889. [PubMed] [Google Scholar]
- Ferrini U., Mileo A. M., Hearing V. J. Microheterogeneity of melanosome-bound tyrosinase from the Harding-Passey murine melanoma. Int J Biochem. 1987;19(3):227–234. doi: 10.1016/0020-711x(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Fuller B. B., Lunsford J. B., Iman D. S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987 Mar 25;262(9):4024–4033. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Halaban R., Pomerantz S. H., Marshall S., Lerner A. B. Tyrosinase activity and abundance in Cloudman melanoma cells. Arch Biochem Biophys. 1984 Apr;230(1):383–387. doi: 10.1016/0003-9861(84)90121-8. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M. Mammalian tyrosinase. A comparison of tyrosine hydroxylation and melanin formation. Biochem J. 1976 Sep 1;157(3):549–557. doi: 10.1042/bj1570549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M., Montague P. M. Mammalian tyrosinase: isozymic forms of the enzyme. Int J Biochem. 1981;13(1):99–103. doi: 10.1016/0020-711x(81)90141-5. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jr Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods Enzymol. 1987;142:154–165. doi: 10.1016/s0076-6879(87)42024-7. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Korner A. M., Pawelek J. M. New regulators of melanogenesis are associated with purified tyrosinase isozymes. J Invest Dermatol. 1982 Jul;79(1):16–18. doi: 10.1111/1523-1747.ep12510422. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Lutzner M. A. Mammalian melanosomal proteins: characterization by polyacrylamide gel electrophoresis. Yale J Biol Med. 1973 Dec;46(5):553–559. [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Nicholson J. M., Montague P. M., Ekel T. M., Tomecki K. J. Mammalian tyrosinase. Structural and functional interraltionship of isozymes. Biochim Biophys Acta. 1978 Feb 10;522(2):327–339. doi: 10.1016/0005-2744(78)90067-0. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Vieira W. D., Law L. W. Malignant melanoma: cross-reacting (common) tumor rejection antigens. Int J Cancer. 1985 Mar 15;35(3):403–409. doi: 10.1002/ijc.2910350317. [DOI] [PubMed] [Google Scholar]
- Herrmann W. P., Uhlenbruck G. Demonstration of carbohydrate structures in malignant melanoma tyrosinase. Klin Wochenschr. 1976 Jan 15;54(2):95–96. doi: 10.1007/BF01468775. [DOI] [PubMed] [Google Scholar]
- Hirobe T., Takeuchi T. Induction of melanogenesis in the epidermal melanoblasts of newborn mouse skin by MSH. J Embryol Exp Morphol. 1977 Feb;37(1):79–90. [PubMed] [Google Scholar]
- Holstein T. J., Burnett J. B., Quevedo W. C., Jr Genetic regulation of multiple forms of tyrosinase in mice: action of a an b loci. Proc Soc Exp Biol Med. 1967 Nov;126(2):415–418. doi: 10.3181/00379727-126-32463. [DOI] [PubMed] [Google Scholar]
- Kageshita T., Ono T., Arao T., Tomita Y. [Immunohistochemical demonstration of tyrosinase 4 in normal skin and melanocytic tumors]. Nihon Hifuka Gakkai Zasshi. 1987 May;97(6):685–690. [PubMed] [Google Scholar]
- Körner A., Pawelek J. Activation of melanoma tyrosinase by a cyclic AMP-dependent protein kinase in a cell-free system. Nature. 1977 Jun 2;267(5610):444–447. doi: 10.1038/267444a0. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., MCGUIRE J. S. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961 Jan 21;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law L. W., Vieira W. D., Kameyama K., Hearing V. J. A unique tumor rejection antigen from the S91 murine malignant melanoma. Cancer Res. 1987 Nov 15;47(22):5841–5845. [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E., Barra Y., Jay G. Detection of a secreted form of the murine H-2 class I antigen with an antibody against its predicted carboxyl terminus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1216–1220. doi: 10.1073/pnas.81.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marglin A., Merrifield R. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Brumbaugh J. A. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J Cell Biol. 1971 Jan;48(1):41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K. Conversion of particulate tyrosinase to soluble form and to desialylated tyrosinase in human malignant melanoma. FEBS Lett. 1977 Aug 1;80(1):225–228. doi: 10.1016/0014-5793(77)80445-6. [DOI] [PubMed] [Google Scholar]
- Nishioka K. Particulate tyrosinase of human malignant melanoma. Solubilization, purification following trypsin treatment, and characterization. Eur J Biochem. 1978 Apr;85(1):137–146. doi: 10.1111/j.1432-1033.1978.tb12221.x. [DOI] [PubMed] [Google Scholar]
- POMERANTZ S. H. Separation, purification, and properties of two tyrosinases from hamster melanoma. J Biol Chem. 1963 Jul;238:2351–2357. [PubMed] [Google Scholar]
- Pawelek J. M. Studies on the Cloudman melanoma cell line as a model for the action of MSH. Yale J Biol Med. 1985 Nov-Dec;58(6):571–578. [PMC free article] [PubMed] [Google Scholar]
- Pawelek J., Körner A., Bergstrom A., Bologna J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature. 1980 Aug 7;286(5773):617–619. doi: 10.1038/286617a0. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y., Hariu A., Kato C., Seiji M. Transfer of tyrosinase to melanosomes in Harding-Passey mouse melanoma. Arch Biochem Biophys. 1983 Aug;225(1):75–85. doi: 10.1016/0003-9861(83)90008-5. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Hearing V. J. Monoclonal antibodies produced against murine tyrosinase identify pigmented human melanocytes. Diagn Immunol. 1986;4(3):149–154. [PubMed] [Google Scholar]
- Tomita Y., Montague P. M., Hearing V. J. Anti-T4-tyrosinase monoclonal antibodies--specific markers for pigmented melanocytes. J Invest Dermatol. 1985 Nov;85(5):426–430. doi: 10.1111/1523-1747.ep12277121. [DOI] [PubMed] [Google Scholar]
- Weatherhead B., Logan A. Interaction of alpha-melanocyte-stimulating hormone, melatonin, cyclic AMP and cyclic GMP in the control of melanogenesis in hair follicle melanocytes in vitro. J Endocrinol. 1981 Jul;90(1):89–96. doi: 10.1677/joe.0.0900089. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat New Biol. 1973 Feb 14;241(111):213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975 Jun 19;255(5510):644–646. doi: 10.1038/255644a0. [DOI] [PubMed] [Google Scholar]