Abstract
Chronic exposure to elevated levels of free fatty acids (FFA) causes β-cell dysfunction and may induce β-cell apoptosis in type 2 diabetes. The execution of β-cell apoptosis occurs through activation of mitogen-activated protein kinases (MAPKs). Ginsenoside Rg3 (Rg3), one of the active ingredients of ginseng saponins, has not been known about the effects on β-cell apoptosis mediated with FFA. The aims of this study were to investigate the in vitro protective effects of Rg3 on MIN6N8 mouse insulinoma β-cells against FFA-induced apoptosis, as well as the modulating effects on p44/42 MAPK activation. Our results showed that Rg3 inhibited the palmitate-induced apoptosis through modulating p44/42 MAPK activation. We conclude that Rg3 has the potential role in suppressing the progression of type 2 diabetes by inhibiting FFA-mediated loss of β-cells.
Keywords: ginsenoside Rg3, MIN6N8 cells, palmitate, apoptosis, p44/42 MAPK
Introduction
Increased free fatty acids (FFA), alone or in conjunction with hyperglycemia, have been proposed to trigger β-cell loss in type 2 diabetes [1] and moreover, in vitro studies have demonstrated that long-term exposure to FFA can induce β-cell death in culture and in isolated islets [2]. The death was mainly apoptotic with cytochrome c release, caspase-3 activation, and DNA fragmentation [2–4]. Especially, saturated fatty acids like palmitic and stearic acids, were found to be generally cytotoxic to β-cells, whereas unsaturated fatty acids like linoleic, oleic, and palmitoleic acids, were not, and even protected cells from saturated FFA-induced apoptosis [5].
Cells recognize and respond to extracellular stimuli by engaging specific intracellular programs, such as the signaling cascade that leads to activation of the mitogen-activated protein kinases (MAPKs) [6]. P44/42 MAPK or extracellular-regulated protein kinases (ERK), one of MAPK family, is generally activated by mitogens and survival factors [7]. Therefore, selective activation of p44/42 can prevent apoptosis and ensures cell survival in several cell systems [7]. It should be emphasized, however, that the anti-apoptotic role of p44/42 MAPK is not absolute.
Ginseng (the root of Panax ginseng C.A. Meyer, family Aralianceae) has been used clinically to treat type 2 diabetes [8–9] and has also been used as a tonic, often taken for years without evidence of adverse effects or toxicity [10–11]. In recent years, accumulating evidence in vitro and in vivo has shown that ginseng and its extracts possess anti-diabetic activities [12–16]. The effects of ginseng might be attributable to its major ginsenoside constituents. However, little information is available about the effect of ginsenoside Rg3 (20-S-protopanaxadiol-3-[O-β-D-glucopyranosyl (1→2)-β-glucopyranoside]) (Rg3) on FFA-induced apoptosis in β-cells.
This study was designed to investigate whether Rg3 could mediate protective effects against palmitate-induced apoptosis, and whether Rg3 could modulate p44/42 MAPK activation in MIN6N8 mouse insulinoma β-cells.
Materials and Methods
Cell cultures and palmitate treatment
As a model of pancreatic β-cells, SV40 T-transformed insulinoma cells derived from nonobese diabetic (NOD) mice were used (MIN6N8). The cells were kindly provided by Prof. Myung-Shik Lee (Sungkyunkwan University School of Medicine, Seoul, Korea) under the permission of Prof. Junichi Miyazaki (Osaka University, Osaka, Japan) [17]. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 15% fetal bovine serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin. Reagents were obtained from the following sources: high glucose DMEM, FBS, trypsin-EDTA 100 IU/ml, penicillin and 100 µg/ml streptomycin from Gibco (Grand Island, NY). Sodium carbonate, β-mercaptoethanol, and sodium palmitate from Sigma-Aldrich Corp. (St. Louis, MO). Palmitate-mediated apoptosis was induced by incubation with 500 µM palmitate for 48 h. The choice of palmitate concentration and incubation time was based on the preliminary study.
Rg3 administration
Rg3 was purchased from Fleton Reference Substance Co., Ltd (Huaishu, Chengdu, China). Rg3 was administrated simultaneously with palmitate.
Cell viability assay
Cell viability was determined by the reduction of yellow 3-(4,5-dimethylthaizol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) into a purple formazane product by mitochondrial dehydrogenase of metabolically active cells. Briefly, cells were seeded at a density of 2 × 106 cells/well into 96-well plate. After overnight growth, cells were treated with palmitate and Rg3 for 48 h. The final concentrations of DMSO in the culture medium were <0.1%. At the end of treatment, 30 µl of MTT was added, and cells were incubated for a further 4 h. Cell viability was obtained by scanning with an Enzyme-Linked Immunosorbent Assay (ELISA) reader with a 570 nm filter [18].
Apoptosis assay
For quantitative determination of apoptotic cell death, cytoplasmic histone-associated DNA fragments were measured with the Cell Death Detection ELISA kit from Roche (Mannheim, Germany) according to the manufacture’s instructions. This assay is based on a quantitative sandwich enzyme immunoassay principle, using mouse monoclonal antibodies directed against DNA and histones. This allows the specific determination of mono- and oligonucleosomes but not free histone or DNA that may generate during nonapoptotic cell death [19] in the cytoplasmic fraction of cell lysates. At the end of the culture period, cells were washed with phosphate-buffered saline (PBS), lysed according to the manufacturer’s protocol, centrifuged (200 × g, 10 min), placed in a streptavidin-coated microtiter plate, and incubated with a mixture of antihistone (biotin-labeled) and anti-DNA (conjugated with peroxidase) antibodies. After removal of the unbound antibodies by a washing step, the amount of nucleosomes was quantified photometrically by the peroxidase retained in the immunocomplex.
Preparation of cell lysates and immunoblotting
Treated cells were harvested after centrifugation at 4°C, then washed with ice-cold PBS, and immediately lysed in lysis buffer (cellLytic MT Mammalian Tissue lysis/extraction reagent, Sigma) for 30-min vortex at 4°C. After centrifugation at 13,000 × g at 4°C for 20 min to separate the cellular debris, the supernatant was collected and stored at −70°C until use. The protein concentrations were determined using an assay kit for protein determination (Quick Start Bradford Dye Reagent; Bio-Rad Laboratories, Richmond, CA) according to the manufacturer’s instructions. SDS-PAGE was performed in 10% polyacrylamide gels loading equal amount of proteins per lane. Proteins were then transferred to immunoblot PVDF membranes (Bio-Rad) in Tris-borate-EDTA buffer at 100 V for 1 h. The membrane was blocked with 5% non-fat milk in TBST buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.01% Tween 20) for 1 h. Membranes were incubated for 4 h at room temperature with primary antibodies (diluted 1:200–1:1000). The antibodies against polyclonal p-44/42 MAPK and phosphorylated p-44/42 MAPK was purchased from Cell Signaling Technology (Beverly, MA). The antibodies against cleaved PARP and β-actin was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Secondary antibodies were obtained from Vector Laboratories (Burlingame, CA); horseradish peroxidase-conjugated anti goat or anti rabbit, and exposed to an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech., Piscataway, NJ). Immunoblots were analyzed with a GS-800 calibrated densitometer (Bio-Rad).
Statistical analyses
Experiments were performed in triplicate and replicated three times. All values were expressed as mean and standard deviations (S.D.). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test were used for statistical analyses (SAS software, SAS Inc., Cary, NC).
Results
Ginsenoside Rg3 attenuated palmitate-induced cytotoxicity in MIN6N8 cells
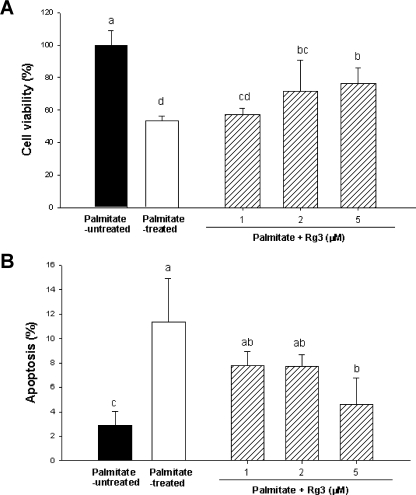
We first examined the effects of Rg3 on the cell viability of MIN6N8 cells using the MTT assay. Cells treated with 500 µM palmitate for 48 h reduced cell viability by approximately 50% relative to the palmitate-untreated cells. This cytotoxic effect was attenuated by co-treatment with Rg3 in 1–5 µM in part dose-dependently (Fig. 1A). Palmitate-untreated cells administered with 1–5 µM Rg3 showed no difference on cell viability from that of the palmitate-untreated control cells (not shown in data).
Fig. 1.
(A) Effect of ginsenoside Rg3 on cell viability in palmitate-treated MIN6N8 cells. The cells were treated with 500 µM palmitate in the presence or absence of 1–5 µM Rg3 for 48 h. Cell viability was determined by MTT assay. Values are means ± SD (n = 9). Means with different letters differ significantly among groups (p<0.05). (B) Effect of Rg3 on palmitate-induced apoptosis in MIN6N8 cells. The cells were treated with Rg3 simultaneously with palmitate for 48 h. Apoptosis was determined as the amount of cytosolic histone-associated DNA fragments. Values are means ± SD (n = 9). Means with different letters differ significantly among groups (p<0.05).
Ginsenoside Rg3 reduced palmitate-induced apoptosis
Next, we measured nucleosomal release as an early biochemical feature and quantitative marker of apoptosis [20]. Incubating the cells with 500 µM palmitate resulted in an enrichment of mono- and oligonucleosomes in the cytoplasm due to the DNA degradation by 4.3 fold compared to palmitate-untreated control (Fig. 1B). The palmitate-mediated nucleosomal release was significantly inhibited by the co-treatment with Rg3 at the concentration of 1–5 µM in part dose-dependently (Fig. 1B).
Ginsenoside Rg3 suppressed palmitate-induced cleavage of PARP
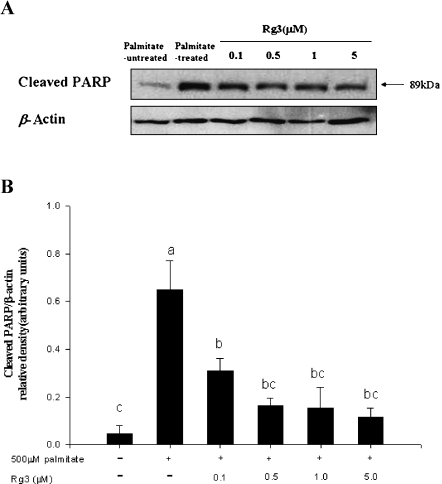
To investigate the underlying mechanisms for protection by Rg3 against palmitate-induced apoptosis, we analyzed whether poly (ADP-ribose) polymerase (PARP) were involved in this process. PARP (116 kDa) is cleaved to produce an 89 kDa fragment during apoptosis. When MIN6N8 cells were incubated with 500 µM palmitate for 48 h, PARP cleavage, as evidenced by accumulation of 89 kDa species, was noted (Fig. 2A). The activation of PARP was significantly recovered by co-treatment with Rg3 at 0.1–5.0 µM (Fig. 2).
Fig. 2.
Effect of ginsenoside Rg3 on palmitate-induced cleavage of PARP in MIN6N8 cells. Cells were incubated with 500 µM palmitate in the presence or absence of Rg3 (0.1–5.0 µM) for 48 h. Cell lysates were subjected to Western blot analysis for cleaved PARP and β-actin. (A) Results are representative of three independent experiments. (B) Density ratio of cleaved PARP/β-actin. Means with different letters differ significantly among groups (p<0.05).
Ginsenoside Rg3 suppressed palmitate-induced activation of p44/42 MAPK
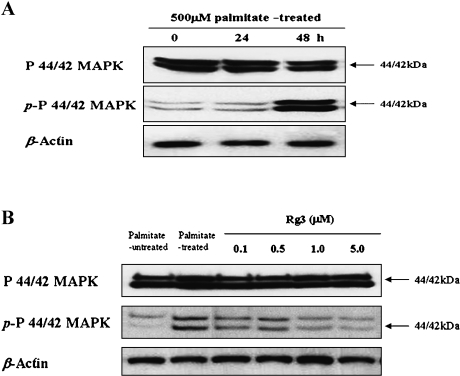
When MIN6N8 cells were incubated with 500 µM palmitate and analyzed over a 48-h, p44/42 MAPK activation, as evidenced by phosphorylation of what appeared to be two isoforms, was noted at 48 h after administration with palmitate (Fig. 3A). To explore the involvement of p44/42 MAPK in the mechanism of protection by Rg3, we examined the effects of Rg3 on palmitate-mediated p44/42 MAPK activation. Based on the results of Western blot, it was found that co-treatment of the cells with Rg3 (0.1–5.0 µM) suppressed palmitate-induced phosphorylation of p44/42 MAPK (Fig. 3B).
Fig. 3.
Effect of ginsenoside Rg3 on palmitate-induced activation of p44/42 MAPK in MIN6N8 cells. (A) Time course of p44/42 activation with palmitate exposure to MIN6N8 cells. The cells were administrated with 500 µM palmitate for the indicated time points and harvested for whole-cell lysates to be used in Western blots. Total p44/42 was probed as well. All data are representative of three independent experiments. (B) Effect of Rg3 on palmitate-induced phosphorylation of p44/42 in the cells. The cells were treated with palmitate in the presence or absence of Rg3 for 48 h, and Western blot analysis was performed. All data are representative of three independent experiments.
Discussion
FFA have been shown to promote a pro-apoptotic effect on β-cells [21] that may explain, at least partially, the elevated rates of β-cell apoptosis and reduction in β-cell mass seen in diabetes [22]. FFA can affect β-cell apoptosis directly through the influence on modulating multiple intracellular signaling pathways, or remotely through cytokines that are secreted by adipose tissues-adipokines [23]. Apoptosis is a highly regulated death process which is predominant mode of cell death induced by palmitate in pancreatic β-cells [24]. The data that we obtained with MIN6N8 cells indicating that palmitate inhibited the proliferation of the cells, induced an increase of the histone-associated DNA fragments, and cleaved PARP, are in agreement with an earlier study using the MIN6N8a cells [24]. In the present study, we demonstrated for the first time that Rg3 protected MIN6N8 pancreatic β-cells against palmitate-induced apoptotic cell death.
PARP is a DNA repair enzyme that can be activated by DNA strand breaks [25]. The cleavage of full-length PARP (116 kDa) to cleaved PARP (89 kDa) serves as a marker of cell apoptosis [26]. Our study indicated that Rg3-treatment blocked the cleavage of PARP caused by palmitate in MIN6N8 cells. Our results suggest that the anti-apoptotic effect of Rg3 involves the suppression of PARP cleavage.
In contrast to stress-activated protein kinase/c-Jun N-terminal kinases (SAPK/JNK) and p38, p44/42 MAPK is usually associated with differentiation and proliferation in many mammalian cells, including β-cells [27]. P44/42 activity, relative to SAPK/JNK and p38, was unaffected throughout the stressful procedure for pancreatic islet transplantation [28]. Meanwhile, several studies show that the cytotoxic effects of human islet amyloid polypeptide on rat insulinoma cell lines RINm5F and INS-1E were mediated by activation of multiple MAPK pathways including p44/42 [29-31]. Interleukin (IL)-1β, an inflammatory cytokine, induces cell death via activating MAPKs, including SAPK/JNK, p38 and p44/42 [32]. Interestingly, we showed that palmitate activated p44/42 MAPK in MIN6N8 cells, and that co-treatment of Rg3 with palmitate suppressed p44/42 MAPK activation. Our result suggests that palmitate-induced apoptosis involves the activation of p44/42 MAPK and that the anti-apoptotic effect of Rg3 involves the suppression of p44/42 activation.
In conclusion, the present study suggests that Rg3 protects MIN6N8 pancreatic β-cells against palmitate-induced apoptosis in part through suppressing PARP cleavage and p44/42 activation. The results supports the potential role of Rg3 in suppressing the progression of type 2 diabetes by inhibiting FFA-mediated loss of pancreatic β-cells.
Acknowledgment
We gratefully acknowledge financial support from the Korea Research Foundation (Grant No. KRF-2006-531-F00017) and Korea Food Research Institute (Grant No. E090102).
References
- 1.Leonardi O., Mints G., Hussain M.A. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur. J. Endocrinol. 2003;149:99–102. doi: 10.1530/eje.0.1490099. [DOI] [PubMed] [Google Scholar]
- 2.Lupi R., Dotta F., Marselli L., Del Guerra S., Masini M., Santagelo C., Patane G., Boggi U., Piro S., Anello M., Bergamini E., Mosca F., Di Mario U., Del Prato S., Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 3.Maedler K., Spinas G.A., Dyntar D., Moritz W., Kaiser N., Donath M.Y. Distinct effects of saturated and monounsaturated fatty acids on beta cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 4.Wrede. C.E ., Dickson L.M., Lingohr M.K., Briaud I., Rhodes C.J. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J. Bio.l Chem. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 5.Maedler K., Oberholzer J., Bucher P., Spinas G.A., Donath M.Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 6.Roux P.P., Blenis J. ERK and p38 MAPK-activated protein kinases: a Family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whimarch A.J., Davis R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 8.Bensky D., Gamble A. Chinese Herbal Medicine Materia Medica. Eastland Press; Seattle, WA: 1993. [Google Scholar]
- 9.Huang K.C. In: Herbs with multiple actions, in The Pharmacology of Chinese Herbs. Second edition. Boca Raton FL., editor. CRC Press; 1999. p. 300. [Google Scholar]
- 10.Lee F.C. In: Facts about Ginseng: The Elixir of Life, Hollyn International Corporation. First edition. Elizabeth NJ., editor. 1992. p. 200. [Google Scholar]
- 11.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Han K.L., Jung M.H., Sohn J.H., Hwang J.K. Ginsenoside 20(S)-protopanaxatriol (PPT) activities peroxisome proliferators-activated receptor γ (PPARγ) in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2006;29:110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- 13.Dey L., Xie J.T., Wang A., Wu J., Maleckar S.A., Yuan C.S. Antihyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 14.Sotaniemi E.A., Haapakoski E., Rautio A. Ginseng therapy in noninsulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 15.Yeh G.Y., Eisenberg D.M., Kaptchuk T.J., Phillips R.S. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 16.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 17.Yagi N., Yokono K., Amano K., Nagata M., Tsukamoto K., Hasegawa Y., Yoneda R., Okamoto N., Moriyama H., Miki M., Tominaga Y., Miyazaki J.I., Yagita H., Okumura K., Mizoguchi A., Miki A., Ide C., Maeda S., Kasuga M. Expression of intercellular adhesion molecule 1 on pancreatic β-cell destruction by cytotoxic T-cells in murine autoimmune diabetes. Diabetes. 1995;44:744–752. doi: 10.2337/diab.44.7.744. [DOI] [PubMed] [Google Scholar]
- 18.Di Matteo M.A., Loweth A.C., Thomas S., Mabley J.G., Morgan N.G., Thorpe J.R., Green I.C. Superoxide, nitric oxide, peroxynitrite and cytokine combinations all cause functional impairment and morphological changes in rat islets of langerhans and insulin-secreting cell lines but dictate cell death by different mechanism. Apoptosis. 1997;2:164–177. doi: 10.1023/a:1026412414666. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R., Mandal M., Lipton A., Harvey H., Thompson C.B. Overexpression of HER2 modulates Bcl-2, Bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin. Cancer Res. 1996;2:1215–1219. [PubMed] [Google Scholar]
- 20.Allen R.T., Hunter W.J., Agrawal D.W. Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. 1997;37:21–28. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 21.Unger R.H., Zhou Y.T., Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2327–2332. doi: 10.1073/pnas.96.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 23.Eldor R., Raz I. Lipotoxicity versus adipotoxicity—The deleterious effects of adipose tissue on beta cells in the pathogenesis of type 2 diabetes. Diabetes Res. Clin. Pract. 2006;74:S3–S8. [Google Scholar]
- 24.Choi S.E., Kim H.E., Shin H.C., Jang H.J., Lee K.W., Kim Y.S., Kang S.S., Chun J.S., Kang Y. Involvement of Ca2+-mediated apoptotic signals in palmitate-induced MIN6N8a beta cell death. Mol. Cell Endocrinol. 2007;272:50–62. doi: 10.1016/j.mce.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber V., Dantzer F., Ame J.C., de Murcia G.. Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 26.Levine A.J. P53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 27.Briaud I., Lingohr M.K., Dickson L.M., Wrede C.E., Rhodes C.J. Differential activation mechanisms of Erk-1/2 and p70 (56K) by glucose in pancreatic beta-cells. Diabetes. 2003;52:974–983. doi: 10.2337/diabetes.52.4.974. [DOI] [PubMed] [Google Scholar]
- 28.Abdelli S., Ansite J., Roduit R., Borsello T., Matsumoto I., Sawada T., Allaman-Pillet N., Henry H., Beckmann J.S., Hering B.J., Bonny C. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53:2815–2823. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 29.Rumora L., Hadzija M., Barisic K., Maysinger D., Grubiic T.Z. Amylin-induced cytotoxicity is associated with activation of caspase-3 and MAP kinases. Biol. Chem. 2002;383:1751–1758. doi: 10.1515/BC.2002.196. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S., Liu J., Dragunow M., Cooper G.J. Fibrilogenic amylin evokes islet beta-cell apoptosis through linked activation of a cascade and JNK1. J. Biol. Chem. 2003;278:52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- 31.Li X.L., Xu G., Chen T., Wong Y.S., Zhao H.L., Fan R.R., Gu X.M., Tong P.C.Y., Chan J.C.N. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Intern. J. Biochem. Cell Biol. 2009;41:1526–1535. doi: 10.1016/j.biocel.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Larsen L., Storling J., Darville M., Eizirik D.L., Bonny C., Billestrup N., Mandrup-Poulsen T. Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor κB-mediated gene expression in insulin-producting INS-1E cells. Diabetologia. 2005;48:2582–2590. doi: 10.1007/s00125-005-0039-9. [DOI] [PubMed] [Google Scholar]