Abstract
Sustained virologic response with peg-interferon and ribavirin combination therapy for 48 weeks is still inadequate. Our study examined whether short-term administration of retinol clinically influences the anti-viral activity of interferon early during interferon and ribavirin combination therapy. The control group received 6 MIU of interferon α-2b every day for two weeks and then 3 times a week for 22 weeks intramuscularly plus 600 mg or 800 mg per day of ribavirin orally for 24 weeks. The retinol group, in addition to above treatment, received retinol 30,000 units per day orally for 3 weeks from one week before the start of interferon α-2b plus ribavirin combination therapy. The hepatitis C virus (HCV) RNA negativity rate at 1 week after the end of interferon α-2b and ribavirin combination therapy was 46.7% (28/60) for the retinol group and 31.7% (19/60) for the control group, which was significantly higher for the retinol group. The level of serum HCV RNA in the retinol group was significantly lower at 1 week after beginning treatment as compared to the control group (p<0.01). Furthermore, serum 2,5'AS protein at 1 week after beginning treatment was significantly higher in the retinol group (p = 0.0002). The results suggest that retinol supplement increases the antiviral effect of interferon α-2b plus ribavirin only during the administration of IFN α-2b, ribavirin and retinol in patients with chronic hepatitis C.
Keywords: hepatitis C, retinol, ribavirin, chronic hepatitis, virological response
Introduction
Higher sustained virologic response is achieved with interferon (IFN) and ribavirin combination therapy compared to IFN monotherapy in patients with chronic hepatitis C. However, the effect of peg-interferon (PEG-IFN) and ribavirin combination therapy for 48 weeks, the most effective treatment for chronic hepatitis C currently available, is still inadequate [1–6].
Viral load and hepatitis C virus (HCV) genotype are commonly known as factors predictive of antiviral treatment outcome in patients with chronic hepatitis C [7–9]. In addition, our group proposed that increased IFN receptors in the liver enhance the effect of IFN treatment of chronic hepatitis C. The manifestation of IFN-receptors on hepatocytes and lymphocytes influences the effect of IFN treatment [10].
Retinoic acid is a derivative of vitamin A. All-trans-retinoic acid (ATRA) is common and there are some optical isomers such as 9-cis retinoic acid. Retinoic acid binding to retinoic acid receptor (RAR) and retinol X receptor (RXR) leads to some transcription activities [10–12]. Our group previously reported that some retinoic acids raise IFN activity via increasing IFN receptors on hepatoma cells in vitro [13]. However, no study to date has examined clinically the effect of retinoic acids on IFN treatment. Our study was conducted to determine whether short-term administration of retinol clinically influences the anti-viral activity of IFN early during IFN plus ribavirin combination therapy.
Materials and Methods
Patients and treatment regimen
The ethical review board of the hospital approved this study design. One-hundred and twenty adult patients with chronic hepatitis C (60 patients/group) who gave informed consent and who met the inclusion criteria were enrolled. Excluded from the study were: immunocompromised or human immunodeficiency virus-positive patients, patients with severe psychiatric disease, patients with poorly controlled diabetes mellitus or hypertension, pregnant patients, patients with liver cirrhosis, hypersensitivity to IFN or ribavirin, poorly controlled heart diseases, renal failure or with creatinine clearance of less than 50 ml/min, past history of cerebrovascular attack, patients with hepatoma or other malignancies, and HBs antigen-positive patients.
The control group received 6 MIU of IFN α-2b by intramuscular injection every day for two weeks and then 3 times a week for 22 weeks plus ribavirin at the dose of 600 mg (for patients weighing <60 kg) or 800 mg (for patients weighing ≥60 kg) per day orally for 24 weeks. The retinol group, in addition to above treatment, received vitamin A (Chocora A®, Eisai Co. Ltd., Tokyo, Japan) 30,000 units per day orally for three weeks (from one week before to two weeks after the start of IFN α-2b plus ribavirin combination therapy).
Determination of serum HCV RNA and HCV genotype
Blood samples were obtained from all patients at baseline, 3 days, one week, 2 weeks after the start of combination therapy for measurement to be conducted by a central laboratory. Serum HCV RNA levels were determined by Cobas Amplicor HCV Monitor test, version 2 (Amplicor M: Roche Diagnostics, Tokyo, Japan). HCV genotype at baseline was determined using a commercially available probe which can distinguish HCV genotype 1a, 1b, 2a, 2b, and 3a (Monitor genotype: Roche Diagnostics).
Determination of serum retinol, 2,5'-oligoadenylate synthetase and ribavirin concentration
Serum retinol concentration was determined by high-speed liquid chromatography (HPLC 800 series, Wako Junyaku Co., Ltd., Kyoto, Japan). Serum 2,5'-oligoadenylate synthetase (2,5'AS) protein was measured using a radioimmunoassay kit (Eiken Chemical Co., Tokyo, Japan). Serum ribavirin concentration was determined by HPLC (Hitachi L-7000, Hitachi Ltd., Tokyo, Japan).
Ultrasound-guided liver biopsy
Ultrasound-guided liver biopsy was conducted using Majima needles in 115 patients within 2 weeks before the start of IFN α-2b plus ribavirin combination therapy. Two patients in the retinol group and 3 patients in the control group did not agree to have a liver biopsy. Liver specimens were assessed histologically using Knodell’s score after hematoxylin-eosin and Azan staining.
Statistical analysis
Data are expressed as the mean and standard error. Statistical significance between groups was assessed with 1-way analysis of variance, Mann Whitney-U test and χ-square test.
Results
Baseline characteristics
The retinol group and control group consisted of 60 patients each. There was no significant difference between the groups in age, sex, body weight, alanine aminotransferase (ALT), platelet count, WBC count, hemoglobin level, HCV RNA level, HCV genotype, Knodell score, or ribavirin dose. Demographic characteristics in both groups are shown in Table 1.
Table 1.
Baseline characteristics
| Retinol group (n = 60) | Control group (n = 60) | p value | |
|---|---|---|---|
| Age (yr) | 55.1 ± 10.3 | 55.5 ± 10.3 | 0.65 |
| Males (%) | 36 (60) | 40 (67) | 0.45 |
| Body weight (kg) | 60.6 ± 13.3 | 59.6 ± 8.3 | 0.49 |
| ALT (IU/l) | 80.7 ± 46.2 | 125.0 ± 117.7 | 0.05 |
| Platelet (×104/µl) | 15.2 ± 5.3 | 15.9 ± 4.7 | 0.94 |
| WBC (/µl) | 4863 ± 1541 | 5252 ± 1343 | 0.14 |
| Hemoglobin(g/dl) | 14.1 ± 1.1 | 14.3 ± 1.3 | 0.26 |
| HCV-RNA (KIU/ml) | 529 ± 334 | 519 ± 273 | 0.86 |
| HCV genotype 2a | 30 | 27 | 0.73 |
| 2b | 3 | 2 | |
| 1b | 27 | 31 | |
| Fibrosing score (F-factor) | 1.4 ± 1.2 | 1.7 ± 1.0 | 0.14 |
| Ribavirin dose (mg/kg) | 11.6 ± 1.7 | 11.8 ± 1.80 | 0.55 |
Data are expressed as the mean ± SD.
Serum retinol concentration during retinol administration
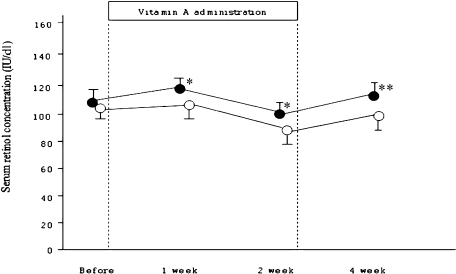
Serum retinol concentration was increased 1, 2, and 4 weeks after the start of IFN and ribavirin combination therapy compared with baseline, and a significant difference was observed after 1, 2 and 4 weeks (Fig. 1).
Fig. 1.
Serum retinol concentration before and 1, 2, and 4 weeks after the start of retinol administration. Open circle shows serum concentration with control group, and closed circle shows that with retinol group. *p<0.05, **p = 0.002.
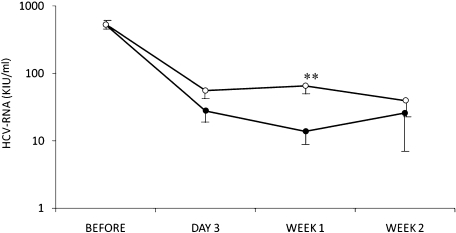
Time-dependent changes in serum HCV-RNA levels during retinol administration
In both the retinol and control groups, serum HCV-RNA was decreased. However, the HCV-RNA viral load in the retinol group was lower than that in the control group at all time points. At 1 week after the start of combination therapy, the serum HCV-RNA level in the retinol group was significantly lower than that in the control group (p<0.01) (Fig. 2).
Fig. 2.
Changes in serum HCV-RNA levels. Closed circles showed HCV-RNA levels in the retinol group and open circles those in the control group. Data are expressed as the mean ± standard error (SE). **p<0.01; Mann-Whitney U test.
HCV RNA negativity rate at time points during retinol administration
In the retinol group, HCV RNA negativity rate was 28.3% (17/60) 3 days after the start of IFN α-2b plus ribavirin combination therapy, 46.7% (28/60) after 1 week, and 60.0% (36/60) after 2 weeks. In the control group, HCV RNA negativity rate was 16.6% (10/60) 3 days after the start of IFN α-2b plus ribavirin combination therapy, 31.7% (19/60) after 1 week, and 48.3% (36/60) after 2 weeks. At one week after the start of combination therapy, the HCV RNA negativity rate in the retinol group was significantly higher than that in the control group (p<0.01). The HCV RNA negativity rate in the retinol group tended to be higher than in the control group up to two weeks of combination therapy. This tendency was not observed at 4 weeks after the start of combination therapy (Table 2).
Table 2.
HCV RNA negativity rate at time points during retinol administration
| Rate of HCV-RNA disappearance from peripheral blood after start of treatment (%) |
|||
|---|---|---|---|
| 3 days | 1 week | 2 weeks | |
| Retinol group (n = 60) | 17 (28.3) | 28 (46.7) | 36 (60.0) |
| Control group (n = 60) | 10 (16.6) | 19 (31.7) | 29 (48.3) |
*p<0.01
Serum 2,5'AS protein one week after IFN α-2b plus ribavirin combination therapy
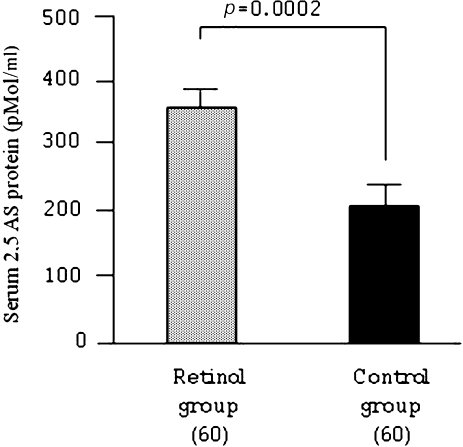
Serum 2,5'AS protein one week after IFN α-2b plus ribavirin combination therapy was higher in the retinol group at 346 ± 31 pMol/mL than in the control group at 201 ± 21 pMol/ml (p = 0.0002, Fig. 2).
Serum ribavirin concentration one weeks after IFN α-2b plus ribavirin combination therapy
Serum ribavirin concentration at one weeks after the start of IFN α-2b plus ribavirin combination therapy was 2397 ± 105 ng/ml in the retinol group and 2343 ± 105 ng/ml in control group (mean ± SE). There was no significant difference between the groups in serum ribavirin concentration at 2 weeks after the start of IFN α-2b plus ribavirin combination therapy.
Outcome
Sustained virological response (SVR), defined as HCV RNA undetectable at 24 weeks after the end treatment, was observed in 26 patients in the retinol group and 27 patients in the control group. Relapse, defined as HCV RNA undetectable at the end of treatment but detectable at 24 weeks after the end of treatment, was observed in 26 patients in the retinol group and 23 patients in the control group. No response, defined as HCV RNA detectable during treatment and at 24 weeks after the end of treatment, was observed in one patient in the retinol group and in 7 patients in the control group. Seven patients in the retinol group and 4 patients in the control group discontinued treatment. The dose of ribavirin was reduced in 12 patients in the retinol group and in 15 patients in the control group. There was no significant difference between groups in adverse reactions.
The cause of treatment discontinuation was depression in 5 patients, insulin-dependent diabetes mellitus in 2 patients, meningitis, bleeding of the ocular fundus, leukocytopenia, and idiopathic thrombocytopenia in one patient each.
Discussion
We have demonstrated clinically for the first time that retinoic acids increase the antiviral effect of IFN in patients with chronic hepatitis C. Our study showed a difference between the retinol and control groups not only in serum HCV RNA but also in serum 2,5'AS during vitamin A administration. There was no difference between the groups in serum ribavirin concentrations. Retinoids are a group of vitamin A-related compounds that influence proliferation of and induce differentiation in epithelial tissue and cells of the immune system [11, 12, 14]. Hamamoto et al. [13] reported that retinoic acids can enhance the anti-HCV replication effect of IFN-α through up-regulation of type I IFN receptors in a hepatoma cell-line. They showed that retinoic acids enhanced IFN-induced 2,5'AS augmentation. Our study did not directly prove the up-regulation of IFN receptors in hepatocytes in patients with chronic hepatitis C during the administration of retinol. However, retinol-enhanced anti-HCV replication accompanied with increased 2,5'AS was observed. Our clinical results therefore reflects Hamamoto’s study results. The double stranded RNA activated protein kinase (PKR) as well as 2-5 AS was initially discovered as an enzyme regulating protein synthesis in interferon-related cells, and play a critical role in the interferon-induced viral response [15]. Besides its antiviral effect, PKR is implicated in cell proliferation, transcriptional regulation, tumor suppression, and apoptosis. These actions are thought to be similar to those of retinol [16–19].
Unfortunately, anti-HCV effect of vitamin A in our study was transient and was not associated with increased sustained virological response. Recently, vitamin A is often being administered long-term to patients with liver cirrhosis and in patients at high risk of hepatocarcinogenesis to prevent the development of hepatocellular carcinoma [20, 21]. However, the subjects in our study had chronic hepatitis C, not liver cirrhosis, and the aim was not to prevent hepatocarcinogenesis. Vitamin A accumulates, and safety of long-term use is not established. Some chronic side effects of carotenoids have been reported, such as liver fibrosis with activated Ito-cell hyperplasia, hepatomegaly, musculoskeletal symptoms, and hydrocephalus [22–26]. On the other hand, even in the presence of normal β-carotene serum levels, patients with cirrhosis have extremely low hepatic levels and β-carotene supplementation may be justified [27, 28]. Patients with chronic hepatitis have mildly low hepatic levels of β-carotene [29]. If accumulation due to long-term administration of retinol exceeds the slight deficiency in hepatic β-carotene, adverse reactions due to retinol may occur. Retinol was therefore administered short-term in the induction stage of IFN α-2b plus ribavirin combination therapy.
Retinol reaches the liver and is converted to retinoic acid by hepatocytes [30]. Retinoic acid induces cellular gene expression via nuclear receptors, which acts as transcriptional factors [31]. Retinoic acid receptor (RAR) and retinoid X receptor (RXR) are the specific receptors for retinoic acids. These receptors bind to the cis-acting response element composed of direct repeats of the target gene. IFN receptor AR1 and AR2 gene sequence contains putative retinoic acid binding sequence in the promotor region. It is thought that these sequences may act as retinoic acid binding sites to promote transcription of type I IFN receptor genes [32, 33].
The dose of retinoic acid was 60 mg/m2 and 40 mg per day in patients with acute premyelocytic leukemia and psoriasis, respectively [34–36]. On the other hand, the dose of retinol administered in our study was equivalent to 3 mg/day retinoic acid. This dose is less than the conventional clinical dose. However, the putative concentration of retinoic acid in bodily fluids is 2 × 10−6 M if a dose of retinol equivalent to 3 mg/day retinoic acid is administered [13]. This concentration is more than the concentration of retinoic acid which increased the anti-HCV replication effect of IFN-α through up-regulation of type I IFN receptors in a hepatoma cell-line.
Muto et al. [37] reported that one-year administration of the acyclic retinoid polyretinoic acid, the molecular target of which is RXR, prevented second primary tumors in patients with hepatocellular carcinoma. In addition, some reports recently suggested that 5-fluorouracil (5-FU) and interferon combination therapy is effective in patients with advanced hepatocellular carcinoma complicated with portal thrombi [38–40]. The addition of retinol to 5-FU plus IFN combination treatment may improve the prognosis of advanced hepatocellular carcinoma.
We had concern that retinol may increase not only anti-HCV replication effect but the adverse reactions of IFN α-2b. No significant difference was observed, however, between the retinol and control groups in the frequency of dose reduction or treatment interruption during IFN α-2b plus ribavirin combination therapy. However, further study is necessary in the future to determine whether retinol has an effect on the adverse reactions of IFN.
In chronic hepatitis C patients, the prevalence of steatosis ranges from 40% to 86% (mean, 55%) [41, 42]. The majority of patients with steatosis (78%) have mild steatosis affecting less than 30% of hepatocytes. Thus, steatosis occurs more frequently in patients with chronic hepatitis C (55%) than in the general population (20%–30%) of adults in the Western world [43]. Hepatic steatosis in the patients with chronic hepatitis C relates to host factors (alcohol consumption, overweight, hyperlipidemia, diabetes, insulin resistance) as well as viral factor (HCV genotype 3) [44]. Hepatic steatosis can exacerbate T cell mediated liver injury, partly by polarizing T helper cells towards a TH-1 response with excess production of interferon-gamma and interleulin-2 [45, 46]. Furthermore, insulin resistance accompanied with hepatic steatosis interferes with IFN signaling cascade [71] through upregulation of SOCS-3, phosphoenolpyruvate carboxy kinase and activation of phosphatidylinositol-3-kinase (PI3K) that inhibit phosphorylation of STAT1 [47]. Hepatic steatosis are associated with enhanced lymphocyte responsiveness to hepatic chemokines, resulting in increased hepatic inflammatory cells [48]. Unfortunately, in recent study, we had not examined presence of fatty liver in the patients. In baseline characteristics, ALT in control group tended to be higher than that in retinol group despite of almost same viral load between both groups. It is possible that the patients with fatty liver in control group were more than that in retinol group. Further study involved host factors is necessary in the future.
In conclusion, we suggest that retinol supplement increases the antiviral effect of interferon α-2b plus ribavirin only during the administration of IFN α-2b, ribavirin and retinol in patients with chronic hepatitis C, and this mechanism may mediate increase of IFN receptors. However this early virological effect did not influence sustained virological response. Further trial of continuous retinol administration during the interferon plus ribavirin combination therapy is necessary.
Fig. 3.
Serum 2,5'AS protein concentration one week after IFN α-2b plus ribavirin combination therapy. Data are expressed as mean ± standard error (SE).
References
- 1.Berg T., von Wagner M., Nasser S., Sarrazin C., Heintges T., Gerlach T., Buggisch P., Goeser T., Rasenack J., Pape G.R., Schmidt W.E., Kallinowski B., Klinker H., Spengler U., Martus P., Alshuth U., Zeuzem S. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Taliani G., Gemignani G., Ferrari C., Aceti A., Bartolozzi D., Blanc P.L., Capanni M., Esperti F., Forte P., Guadaqnino V., Mari T., Marino N., Milani S., Pasquazzi C., Rosina F., Tacconi D., Toti M., Ziqnego A.L., Messerini L., Stroffolini T. Pegylated interferon alfa-2b plus ribavirin in the retreatment of interferon-ribavirin nonresponder patients. Gastroenterology. 2006;130:1098–1106. doi: 10.1053/j.gastro.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson I.M., Gonzalez S.A., Ahmed F., Lebovics E., Min A.D., Bodenheimer H.C. Jr., Esposito S.P., Brown R.S. Jr., Brau N., Klion F.M., Tobias H., Bini E.J., Brodsky N., Cerulli M.A., Aytaman A., Gardner P.W., Geders J.M., Spivack J.E., Rahmin M.G., Berman D.H., Ehrlich J., Russo M.W., Chait M., Rovner D., Edlin B.R. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am. J. Gastroenterol. 2005;100:2453–2462. doi: 10.1111/j.1572-0241.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 4.Huber M., Weber R., Oppliger R., Vernazza P., Schmid P., Schonbucher P., Bertisch B., Meili D., Renner E.L. Interferon alpha-2a Plus Ribavirin 1,000/1,200 mg versus Interferon alpha-2a Plus Ribavirin 600 mg for Chronic Hepatitis C Infection in Patients on Opiate Maintenance Treat. Infection. 2005;33:25–29. doi: 10.1007/s15010-005-4043-2. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl K., Stahle L., Bruchfeld A., Schvarcz R. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology. 2005;41:234–236. doi: 10.1002/hep.20563. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman M.L., Di Bisceglie A.M., Lindsay K.L., Morishima C., Wright E.C., Everson G.T., Lok A.S., Morgan T.R., Bonkovsky H.L., Lee W.M., Dienstag J.L., Ghany M.G., Goodman Z.D., Everhart J.E. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;125:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Diago M., Castellano G., Garcia-Samaniego J., Perez C., Fernandez I., Romero M., Iacono O.L., Garcia-Monzon C. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akuta N., Suzuki F., Tsubota A., Suzuki Y., Someya T., Kobayashi M., Saitoh S., Arase Y., Ikeda K., Kumada H. Efficacy of interferon monotherapy to 394 consecutive naive cases infected with hepatitis C virus genotype 2a in Japan: therapy efficacy as consequence of tripartite interaction of viral, host and interferon treatment-related factors. J. Hepatol. 2002;37:831–836. doi: 10.1016/s0168-8278(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 9.Tabaru A., Narita R., Hiura M., Abe S., Otsuki M. Efficacy of short-term interferon therapy for patients infected with hepatitis C virus genotype 2a. Am. J. Gastroenterol. 2005;100:862–867. doi: 10.1111/j.1572-0241.2005.40826.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda R., Ishimura N., Kushiyama Y., Moriyama N., Ishihara S., Nagasawa S., Miyake T., Niigaki M., Satoh S., Sakai S., Akagi S., Watanabe M., Fukumoto S. Effectiveness of interferon-alpha therapy in chronic hepatitis C is associated with the amount of interferon-alpha receptor mRNA in the liver. J. Hepatol. 1997;26:455–461. doi: 10.1016/s0168-8278(97)80407-2. [DOI] [PubMed] [Google Scholar]
- 11.Madsen B., Georg B., Vissing H., Fahrenkrug J. Retinoic acid down-regulates the expression of vasoactive intestinal polypeptide receptor type-I human breast carcinoma cell lines. Cancer Res. 1998;58:4845–4850. [PubMed] [Google Scholar]
- 12.Buck J., Myc A., Grabe A., Cathomas G. Differences in the action and metabolismbetween retinol and retinoic acid in B lymphocytes. J. Cell Biol. 1991;115:851–859. doi: 10.1083/jcb.115.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamamoto S., Fukuda R., Ishimura N., Rumi M.A., Kazumori H., Uchida Y., Kadowaki Y., Ishihara S., Kinoshita Y. 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor. J. Lab. Clin. Med. 2003;141:58–66. doi: 10.1067/mlc.2003.8. [DOI] [PubMed] [Google Scholar]
- 14.Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988;333, 16:669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- 15.Samuel C.E. Antiviral action of interferon. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams B.R.G. The role of the dsRNA-activated protein kinase, PKR, in signal transduction. Semin. Virol. 1995;6:191–202. [Google Scholar]
- 17.Garcia M.A., Gil J., Ventoso I., Guerra S., Domingo E., Rivas C., Esteban M. The impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2007;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Der S.D., Yang Y.I., Weissmann C., Williams B.R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamanian-Daryoush M., Der S.D., Williams B.R. Cell cycle regulation of the double stranded RNA activated protein kinase, PKR. Oncogene. 1999;18:315–326. doi: 10.1038/sj.onc.1202293. [DOI] [PubMed] [Google Scholar]
- 20.Muto Y., Moriwaki H., Ninomiya M., Adachi S., Saito A., Tanaka T., Tsurumi K., Okuno M., Tomita E., Nakamura T., Kojima T., Takasaki K.T. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N. Engl. J. Med. 1996;334:1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Levin M.S. Suppression of FGF signaling: a putative mechanism for the chemopreventive effects of acyclic retinoid in hepatocellular carcinoma. Gastroenterology. 2005;128:228–231. doi: 10.1053/j.gastro.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 22.van Der Vliet H.J., Roberson A.E., Hogan M.C., Morales C.E., Crader S.C., Letendre L., Pruthi R.K. All-trans-retinoic acid-induced myositis: a description of two patients. Am. J. Hematol. 2000;63:94–98. doi: 10.1002/(sici)1096-8652(200002)63:2<94::aid-ajh7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Okuno M., Moriwaki H., Imai S., Muto Y., Kawada N., Suzuki Y., Kojima S. Retinoids exacerbate rat liver fibrosis by inducing the activation of latent TGF-beta in liver stellate cells. Hepatology. 1997;26:913–921. doi: 10.1053/jhep.1997.v26.pm0009328313. [DOI] [PubMed] [Google Scholar]
- 24.Alles A.J., Sulik K.K. Pathogenesis of retinoid-induced hindbrain malformations in an experimental model. Clin. Dysmorphol. 1992;1:187–200. [PubMed] [Google Scholar]
- 25.Perea G., Salar A., Altes A., Brunet S., Sierra J. Acute hepatomegaly with severe liver toxicity due to all-trans-retinoic acid. Haematologica. 2000;85:551–552. [PubMed] [Google Scholar]
- 26.Okuno M., Moriwaki H., Imai S., Muto Y., Kawada N., Suzuki Y., Kojima S. Retinoids exacerbate rat liver fibrosis by inducing the activation of latent TGF-beta in liver stellate cells. Hepatology. 1997;26:913–921. doi: 10.1053/jhep.1997.v26.pm0009328313. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan S.K., Thomas S., Ramachandran A., Pulimood A.B., Balasubramanian K.A. Retinoid metabolism during development of liver cirrhosis. Arch. Biochem. Biophys. 2005;443:93–100. doi: 10.1016/j.abb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Clemente C., Elba S., Buongiorno G., Berloco P., Guerra V., Di Leo A. Serum retinol and risk of hepatocellular carcinoma in patients with child-Pugh class A cirrhosis. Cancer Lett. 2002;178:123–129. doi: 10.1016/s0304-3835(01)00843-6. [DOI] [PubMed] [Google Scholar]
- 29.Newsome P.N., Beldon I., Moussa Y., Delahooke T.E., Poulopoulos G., Hayes P.C., Plevris J.N. Low serum retinol levels are associated with hepatocellular carcinoma in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2000;14:1295–1301. doi: 10.1046/j.1365-2036.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 30.Lippel K., Olson J.A. Biosynthesis of beta-glucuronides of retinol and of retinoic acid in vivo and in vitro. J. Lipid Res. 1968;9:168–175. [PubMed] [Google Scholar]
- 31.Yu V.C., Delsert C., Andersen B., Holloway J.M., Devary O.V., Naar A.M., Kim S.Y., Boutin J.M., Glass C.K., Rosenfeld M.G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991;67:1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- 32.Umesono K., Evans R.M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 33.Kato S., Sasaki H., Suzawa M., Masushige S., Tora L., Chambon P., Gronemeyer H. Widely spaced, directly repeated PuGGTCA elements act as promiscuous enhancers for different classes of nuclear receptors. Mol. Cell. Biol. 1995;15:5858–5867. doi: 10.1128/mcb.15.11.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel S.R., Eardley A., Heller G., Berman E., Miller W.H. Jr., Dmitrovsky E., Warrell R.P. Jr. All-trans retinoic acid for acute promyelocytic leukemia. Results of the New York Study. Ann. Intern. Med. 1994;120:278–286. doi: 10.7326/0003-4819-120-4-199402150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Usuki K., Endo M., Osawa M., Kitazume K., Iki S., Urabe A. Pharmacokinetics of all-trans-retinoic acid in Japanese patients with acute promyelocytic leukemia. Int. J. Hematol. 1996;63:19–23. doi: 10.1016/0925-5710(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 36.Kragballe K., Jansen C.T., Geiger J.M., Bjerke J.R., Falk E.S., Gip L., Hjorth N., Lauharanta J., Mork N.J., Reunala T. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta. Derm. Venereol. 1989;69:35–40. [PubMed] [Google Scholar]
- 37.Muto Y., Moriwaki H., Shiratori Y. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Digestion. 1998;59:89–91. doi: 10.1159/000051435. [DOI] [PubMed] [Google Scholar]
- 38.Kurokawa Y., Matoba R., Nagano H., Sakon M., Takemasa I., Nakamori S., Umeshita K., Sakon M., Ueno N., Oba S., Ishii S., Kato K., Monden M. Molecular prediction of response to 5-fluorouracil and interferon-alpha combination chemotherapy in advanced hepatocellular carcinoma. Clin. Cancer Res. 2004;10:6029–6038. doi: 10.1158/1078-0432.CCR-04-0243. [DOI] [PubMed] [Google Scholar]
- 39.Leung T.W., Tang A.M., Zee B., Yu S.C., Lai P.B., Lau W.Y., Johnson P.J. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421–427. doi: 10.1002/cncr.10236. [DOI] [PubMed] [Google Scholar]
- 40.Sakon M., Nagano H., Dono K., Nakamori S., Umeshita K., Yamada A., Kawata S., Imai Y., Iijima S., Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435–442. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- 41.Mihm S., Fayyazi A., Hartmann H., Ramadori G. Analysis of histopathological manifestations of chronic hepatitis C virus infection with respect to virus genotype. Hepatology. 1997;25:735–739. doi: 10.1002/hep.510250340. [DOI] [PubMed] [Google Scholar]
- 42.Rubbia-Brandt L., Quadri R., Abid K., Giostra E., Male PJ., Mentha G., Spahr L., Zarski JP., Borisch B., Hadengue A., Negro F. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J. Hepatol. 2000;33:106–115. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 43.Clark J.M., Brancati F.L., Diehl A.M. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 44.Asselah T., Rubbia-Brandt L., Marcellin P., Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–130. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kremer M., Hines I.N., Milton R.J., Wheeler M.D. Favred T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology. 2006;44:216–227. doi: 10.1002/hep.21221. [DOI] [PubMed] [Google Scholar]
- 46.Li Z., Soloski M.J., Diehl A.M. Dietary factors alterhepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 47.Bloomgarden Z.T. The 1st World Congress on the Insulin Resistance Syndrome. Diabetes Care. 2004;27:602–609. doi: 10.2337/diacare.27.2.602. [DOI] [PubMed] [Google Scholar]
- 48.Bigorgne A.E., Bouchet-Delbos L., Naveau S., Dagher I., Prévot S., Durand-Gasselin I., Couderc J., Valet P., Emilie D., Perlemuter G. Obesity-induced lymphocyte hyperresponsiveness to cytokines: a new mechanism of fatty liver inflammation in obese mice. Gastroenterology. 2008;134:1459–1469. doi: 10.1053/j.gastro.2008.02.055. [DOI] [PubMed] [Google Scholar]