Abstract
Polaprezinc, a chelate compound consisting of zinc and l-carnosine, is clinically used as a medicine for gastric ulcers. It has been shown that induction of heat shock protein (HSP) is involved in protective effects of polaprezinc against gastric mucosal injury. In the present study, we investigated whether polaprezinc and its components could induce HSP70 and prevent acetaminophen (APAP) toxicity in mouse primary cultured hepatocytes. Hepatocytes were treated with polaprezinc, zinc sulfate or l-carnosine at the concentration of 100 µM for 9 h, and then exposed to 10 mM APAP. Polaprezinc or zinc sulfate increased cellular HSP70 expression. However, l-carnosine had no influence on it. Pretreatment of the cells with polaprezinc or zinc sulfate significantly suppressed cell death as well as cellular lipid peroxidation after APAP treatment. In contrast, pretreatment with polaprezinc did not affect decrease in intracellular glutathione after APAP. Furthermore, treatment with KNK437, an HSP inhibitor, attenuated increase in HSP70 expression induced by polaprezinc, and abolished protective effect of polaprezinc on cell death after APAP. These results suggested that polaprezinc, in particular its zinc component, induces HSP70 expression in mouse primary cultured hepatocytes, and inhibits lipid peroxidation after APAP treatment, resulting in protection against APAP toxicity.
Keywords: acetaminophen, hepatotoxicity, zinc, polaprezinc, heat shock protein
Introduction
Acetaminophen (APAP) is a harmless analgesic and antipyretic drug at therapeutic doses. Generally, APAP at therapeutic doses is metabolized and eliminated as non-toxic glucuronate and sulfate conjugates [1, 2]. A small portion of APAP is bioactivated by the cytochrome P450 (CYP) system to the reactive intermediate N-acetyl-p-benzoquinoneimine (NAPQI) [3], which is normally detoxified by conjugation with reduced glutathione (GSH). APAP overdose leads to severe liver injury in experimental animals and humans [4, 5]. Excess NAPQI formation causes GSH depletion, resulting in covalent binding of NAPQI to cellular macromolecules [6, 7]. Formation of NAPQI adducts leads to mitochondria damage, oxidative/nitrosative stresses and lipid peroxidation, and ultimately to cell death such as apoptosis and necrosis [8–11]. Therefore, it has been considered that increase in NAPQI adducts followed by GSH depletion is involved in mechanism underlying APAP-induced hepatotoxicity. In this context, N-acetylcysteine, a cysteine prodrug, is clinically used as an antidote for APAP overdose.
We have focused on the repair of damaged hepatic proteins by heat shock protein (HSP) as an additional therapy for APAP toxicity in the previous studies [12, 13]. HSP is known to be highly conserved between prokaryotic and eukaryotic cells, ubiquitously expressed, and induced in response to various stresses [14]. It plays an important role in prevention of the incorrect aggregation of proteins and regulation of protein folding process. HSPs induced protect organs and cells against a variety of damages and stresses. In particular, overexpression of HSP70 has been reported to have potent cytoprotective effects [15–17]. Our previous study demonstrated that geranylgeranylacetone, an anti-ulcer drug, protected against APAP-induced liver injury [13] and LPS-mediated endotoxin shock [18] via induction of HSP70.
Polaprezinc [N-(3-aminopropionyl)-l-histidinato zinc], a chelating compound of zinc and l-carnosine, is commonly used in the treatment of gastric ulcers in Japan [19]. Previous studies have revealed that polaprezinc prevents gastric mucosal injury by its antioxidant activity [20, 21], membrane-stabilizing action [22] and stimulation of mucus production [23]. In addition, we have shown that polaprezinc suppresses indomethacin-induced apoptosis via inhibition of caspase-3 activation in rat gastric mucosal cell line, RGM1 [24]. Recently, it has been reported that polaprezinc induces HSP in gastric and colonic mucosa, and protects against mucosal damages induced by various stimuli [25–27].
In the present study, we investigated whether polaprezinc could induce HSP70 in mouse primary cultured hepatocytes and protect against APAP-induced toxicity.
Materials and Methods
Chemicals
APAP was purchased from Wako Pure Chemical Industries (Osaka, Japan). Polaprezinc was kindly provided by Zeria Pharmatheutical Co., Ltd. (Tokyo, Japan). Polaprezinc was dissolved in 0.4 M (400 mM) HCl. Zinc sulfate and l-carnosine were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in distilled water. KNK437, an inhibitor of heat shock factor-1, was purchased from Calbiochem (San Diego, CA) and dissolved in dimethyl sulfoxide. Rabbit anti-HSP70 polyclonal antibody was purchased from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). Dulbecco’s modified eagle medium (DMEM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Williams E Medium (WEM) was purchased from Gibco BRL (Grand Island, NY). All other chemicals used were of analytical grade.
Animals
Male C57BL/6 mice (8–12 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed 5–6 per cage in plastic cages. The animals were maintained on a 12-h light/dark cycle under controlled temperature (23 ± 3°C) and humidity (55 ± 5%) for a week before use in experiments. They were allowed free access to standard laboratory food and water.
All studies were approved by the Institutional Animal Care and Use Committee at Tottori University Faculty of Medicine, and were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Isolation and culture of mouse hepatocytes
Hepatocytes were isolated using a modification of the two-step collagenase perfusion method of Seglen [28]. In brief, the mice liver was perfused with calcium- and magnesium-free Hank’s balanced salt solution (HBSS) containing 0.5 mM EGTA and 10 mM HEPES (pH 7.2) for 5 min and then with HBBS containing 0.05% collagenase D, 0.005% trypsin inhibitor, 10 mM HEPES (pH 7.5) for 20 min. The digested liver was disrupted, filtered through a 150-µm filter and gently shaken in perfusion buffer for 5 min. After isolation, cells were centrifuged (25 × g, 2 min, three times) in DMEM containing 10% fetal bovine serum (FBS), 4 mM l-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin, the medium was replaced to WEM containing 5% FBS, 4 mM l-glutamine, 10−6 M dexamethason, 10−8 M insulin, 100 units/ml penicillin and 100 µg/ml streptomycin. The viability of hepatocytes was over 90% based on trypan blue exclusion test. Hepatocytes in WEM were plated in 90-mm collagen-coated dishes (1 × 106 cells/dish) or 96-well plate (1 × 104 cells/well) and incubated at 37°C in a humidified incubator with 5% CO2.
Exposure to reagents
Primary cultured mouse hepatocytes were treated with polaprezinc, zinc sulfate or l-carnosine at a concentration of 100 µM at 9 h before APAP treatment. APAP (10 mM) was dissolved in serum free WEM containing 4 mM l-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin and added to the culture medium. In some experiments, KNK437 (50 µM) was added at 6 h before polaprezinc treatment.
Analysis of HSP70 contents
HSP70 contents were determined by Western blotting as described previously [13, 29]. The cells were treated with polaprezinc, harvested and washed three times with ice-cold phosphate buffered saline (PBS). Cells were resuspended in lysis buffer containing 10 mM HEPES, 2 mM EDTA, 0.1% CHAPS, 5 mM dithiothreitol and 1 mM PMSF, left on ice 20 min and immediately centrifuged at 8,500 × g for 15 min at 4°C. The protein (30 µg) from each sample was separated by SDS polyacrylamide gel electrophoresis with 12.5% polyacrylamide gel. The gels were electroblotted onto polyvinylidene difluoride membranes. The membranes were incubated with rabbit anti-HSP70 polyclonal antibody (1:5,000), washed and then incubated with horseradish-peroxidase-conjugated goat anti-rabbit antibody (Sigma Chemical Co., St Louis, MO) (1:2,000). The immunoblot was revealed with an ECLTM Western blotting analysis system (GE Healthcare Bio-Sciences KK, Tokyo, Japan). Western blots were quantified with NIH Image.
Zinc concentrations
Hepatic zinc concentrations were determined by atomic absorption spectrophotometry (Fukuyama Medical Laboratory Co., Ltd., Hiroshima, Japan). After treatment of hepatocytes with polaprezinc, cells were collected, washed and lysed for 20 min on ice in lysis buffer (10 mM HEPES and 0.1% CHAPS). The lysates were centrifuged at 8,500 × g for 15 min at 4°C and the supernatants were used for zinc measurement.
Cell viability
The viability of hepatocytes was determined using a commercially available WST-8 assay kit (Seikagaku Biobusiness Co., Tokyo. Japan) according to the manufacture’s instruction. After attachment of cells to 96-well plate for 6 h, each well was treated with polaprezinc, zinc sulfate or l-carnosine and further incubated for 9 h. Thereafter cells in each well were washed twice with PBS and treated with APAP. Cell viability was assessed by measurement of the absorbance at 492 nm in a microplate reader after incubation of cells in WST-8 solution for 1 h at 37°C.
Lipid peroxidation and glutathione assay
Cells were collected and washed with PBS twice. The cells were resuspended in PBS and lysed by freezing and thawing. Then, the cell lysates were homogenated in 0.05 M phosphate buffer (0.2 M NaH2PO4, 0.2 M Na2HPO4, 0.2 M Na-EDTA, pH 7.4) under N2 stream. Lipid peroxidation in mouse primary cultured hepatocytes was measured by a fuluorometric reaction with thiobarbituric acid as previously described [13]. Lipid peroxide content was expressed as the amount of malondialdehyde (MDA) equivalents.
GSH contents in mouse primary cultured hepatocytes were determined fluorometrically using Thio-Glo1TM as previous described [30]. Briefly, after attachment of cells to dishes for 6 h, cells were incubated in the presence or absence of polaprezinc for 9 h, further incubated in the presence of APAP in serum free WEM for 3 h. Thereafter cells were lysed by the same procedure as that in lipid peroxidation assay. Immediately after addition of 10 mM ThioGlo-1 to the cell lysates, fluorescence was measured in a CytoFluor II (Applied Biosystems, Foster city, CA) fluorescence microplate reader using excitation at 360 ± 40 nm and emission at 530 ± 25 nm.
Protein assay
Protein contents were determined by the method of Bradford [31], with FBS as a standard.
Statistical evaluations
Data are expressed as means ± standard error (S.E.). Changes in variables for different assays were analyzed by Student’s t test or one-way ANOVA. Differences were considered to be significant at p<0.05.
Results
Expression of HSP70 after polaprezinc, zinc sulfate or l-carnosine treatment
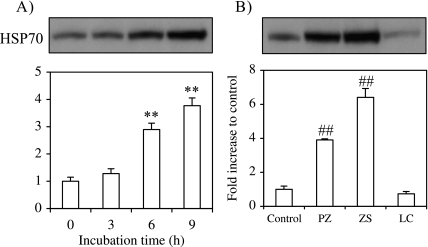
Induction of HSP70 expression in mouse primary cultured hepatocytes after polaprezinc treatment was assessed by Western Blotting analysis. Cells were treated with polaprezinc and harvested after 3, 6 or 9 h. The expression of HSP70 increased in a time-dependent manner and HSP70 content was increased 3.9-fold of control level at 9 h after polaprezinc (Fig. 1A). We also examined whether zinc sulfate or l-carnosine, polaprezinc components, induces HSP70. Treatment with zinc sulfate increased HSP70 to 6.4-fold of control level, while l-carnosine treatment did not enhance HSP70 expression (Fig. 1B).
Fig. 1.
Effect of polaprezinc, zinc sulfate or l-carnosine on HSP70 expression. HSP70 expression was analyzed by Western blot as described in materials and methods. (A) Time course of the expression of HSP70 after polaprezinc treatment. Cells were incubated in the presence of 100 µM polaprezinc for 0, 3, 6 or 9 h. (B) HSP70 expression 9 h after treatment with polaprezinc, zinc sulfate or l-carnosine at a concentration of 100 µM. Western blots were quantified with NIH Image. The data are expressed as means ± SE of 3 separate experiments. PZ; polaprezinc, ZS; zinc sulfate, LC; l-carnosine. **p<0.01 vs initial (0 h) time point (in A); ##p<0.01 vs control (in B).
Intracellular zinc concentrations after polaprezinc or zinc sulfate treatment
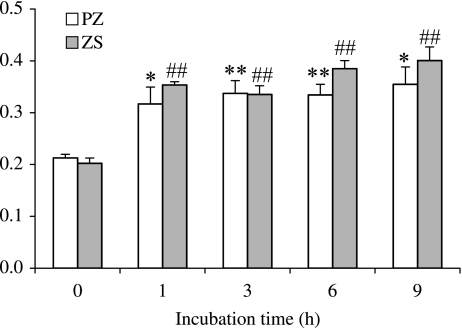
Since zinc reportedly induced HSPs [32, 33], we evaluated zinc concentrations in mouse hepatocytes after polaprezinc or zinc sulfate treatment. The intracellular zinc concentrations increased after treatment with each reagent, reached a peak level after 1 h (1.5- and 1.7-fold of control level, respectively) and remained unchanged from 1 h onwards (Fig. 2).
Fig. 2.
Changes in zinc concentrations after polaprezinc or zinc sulfate treatment. Cells were incubated in the presence of polaprezinc or zinc sulfate at a concentration of 100 µM for 0, 1, 3, 6 or 9 h. Zinc concentration was measured by atomic absorption spectrophotometry. The data are expressed as means ± S.E. of 5 separate experiments. PZ; polaprezinc, ZS; zinc sulfate. **p<0.01, *p<0.05 vs initial (0 h) time point of PZ treatment, ##p<0.01 vs initial (0 h) time point of ZS treatment.
Effect of polaprezinc, zinc sulfate or l-carnosine pretreatment on APAP toxicity
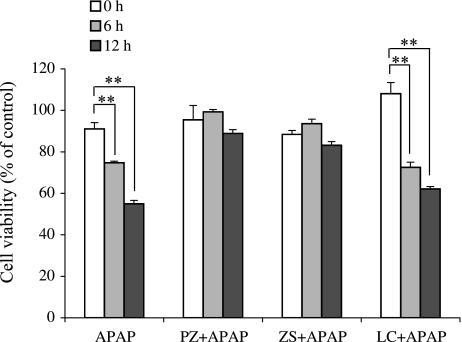
While APAP overdoses cause liver damage in vivo and in vitro, it has been reported that overexpression of HSP70 protects APAP toxicity [13, 34, 35]. We next investigated whether polaprezinc, zinc sulfate or l-carnosine improves cell viability in mouse hepatocytes after APAP treatment (Fig. 3). The cell viability after APAP decreased in a time-dependent manner and was 55% of normal level after 12 h. Treatments of mouse hepatocytes with polaprezinc and zinc sulfate 9 h before APAP improved cell viability to 89% and 83% of normal level at 12 h, respectively, while l-carnosine treatment failed to improve the cell viability (62%).
Fig. 3.
Effect of polaprezinc, zinc sulfate or l-carnosine on cell viability after APAP exposure. The cell viability was analyzed using WST-8 assay. Cells were treated with polaprezinc, zinc sulfate or l-carnosine at a concentration of 100 µM at 9 h before APAP (10 mM) treatment. The cell viability was measured 0, 6 or 12 h after APAP. The data are expressed as means ± SE of 7 separate experiments. PZ; polaprezinc, ZS; zinc sulfate, LC; l-carnosine. **p<0.01 vs APAP alone.
Effect of polaprezinc, zinc sulfate or l-carnosine pretreatment on lipid peroxidation following APAP exposure
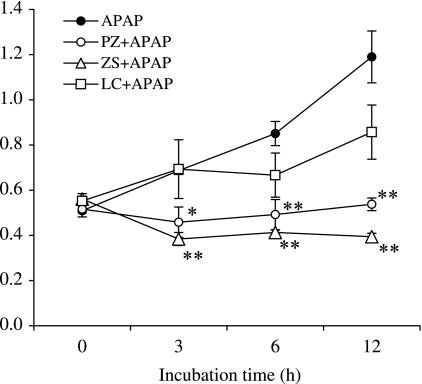
We examined the effect of polaprezinc, zinc sulfate or l-carnosine on lipid peroxidation in mouse hepatocytes after APAP treatment (Fig. 4). The lipid peroxide content increased to 2.3-fold of normal level at 12 h after APAP. Treatment with polaprezinc or zinc sulfate, but not l-carnosine, at 9 h before APAP significantly reduced the increased lipid peroxide content to normal level.
Fig. 4.
Time course of lipid peroxide content after APAP exposure. Cells were incubated in the presence of polaprezinc, zinc sulfate or l-carnosine at a concentration of 100 µM for 9 h before APAP (10 mM) treatment. The lipid peroxide content was measured at 0, 3, 6 or 12 h after APAP. The data are expressed as means ± SE of 3–7 separate experiments. PZ; polaprezinc, ZS; zinc sulfate, LC; l-carnosine. **p<0.01, *p<0.05 vs APAP alone at each time point.
Effects of polaprezinc on reduced glutathione contents following APAP exposure
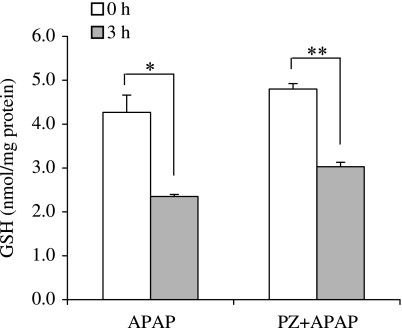
To determine whether polaprezinc affects GSH contents, we measured intracellular GSH contents in mouse hepatocytes after APAP treatment (Fig. 5). The GSH contents after APAP decreased to 55% of normal level at 3 h. Treatment with polaprezinc did not suppressed the decrease in GSH contents.
Fig. 5.
Effect of polaprezinc on intracellular GSH contents after APAP treatment. Cells were treated with 100 µM polaprezinc (PZ) 9 h before APAP (10 mM) exposure. GSH contents were measured at 3 h after APAP. The data are expressed as means ± SE of 3 separate experiments. **p<0.01, *p<0.05 vs initial (0 h) time point, respectively.
KNK437 inhibits HSP70 induction by polaprezinc and abolishes a protective effect of polaprezinc against APAP toxiciy
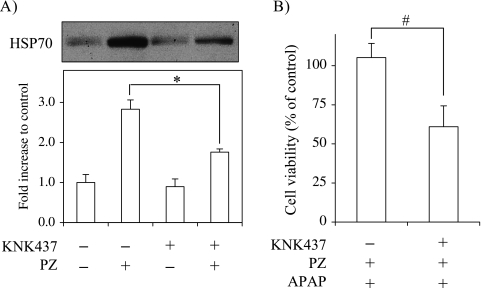
To confirm that increase of HSP70 expression by polaprezinc exerts a protective effect against APAP toxicity, we investigated whether KNK437 inhibits HSP70 expression in mouse hepatocytes following polaprezinc treatment and abrogates the protective effect of polaprezinc on APAP toxicity. Treatment with KNK437 at 6 h before polaprezinc suppressed the polaprezinc-induced HSP70 expression by 58% (Fig. 6A). Furthermore, the protective effect of polaprezinc on the cell viability at 12 h after APAP treatment was completely suppressed by KNK437 pretreatment (Fig. 6B).
Fig. 6.
Inhibition by KNK437 against HSP70 induction and prevention of APAP toxicity by polaprezinc. Cells were treated with KNK437 of 50 µM at 6 h before polaprezinc (PZ) treatment. (A) After 6 h, cells were incubated in the presence of 100 µM polaprezinc for 9 h. HSP70 expression was analyzed by Western blot. (B) After incubation with KNK437 for 6 h followed by polaprezinc for 9 h, cells were treated with 10 mM APAP. The cell viability was measured at 12 h after APAP. The data are expressed as means ± SE of 3–7 separate experiments. *p<0.05 vs PZ alone (in A), #p<0.05 vs PZ + APAP (in B).
Discussion
The present study demonstrated for the first time that polaprezinc, in particular its zinc component, induced HSP70 in mouse primary cultured hepatocytes and protected against APAP toxicity.
APAP is converted to a highly reactive intermediate NAPQI by multiple CYPs including CYP2E1, CYP1A2, CYP3A, CYP2A6 and CYP2D6 [36–39] when it is taken in a large dose. A growing body of evidence suggests that CYP2E1 is a primary contributor to APAP biotransformation among CYPs [40–42]. In our previous study, we found that S-allylmercaptocysteine inhibits CYP2E1 activities, resulting in reduction of NAPQI formation and suppression of GSH depletion [43]. As mentioned above, NAPQI is detoxified by GSH conjugation, resulting in a rapid decrease in hepatocellular GSH to the bottom level about 3 h after APAP treatment [12, 44]. Zinc, a component of polaprezinc, is known to increase transcription of γ-glutamylcysteine synthetase (rate-limiting enzyme of glutathione synthesis) heavy chain gene via activation of metal-responsive transcription factor 1 [45]. Furthermore, it has been reported in the study using primary cultured rat hepatocytes that intracellular zinc content increased to 5-fold of normal level at 24 h after treatment with 100 µM zinc, and cellular GSH and metallothionein (MT) levels increased to 2-fold of normal levels [46]. Therefore, we investigated whether polaprezinc treatment increases the amount of intracellular GSH and inhibited APAP-induced GSH consumption. As shown in Fig. 5, polaprezinc neither affected cellular GSH content nor inhibited GSH depletion after APAP treatment. A possible explanation of the discrepancy with the results for GSH is that treatment of mouse hepatocytes with 100 µM polaprezinc increased cellular zinc content twice after 9 h in our study, but much more intracellular zinc may be required to increase GSH. These results indicated that polaprezinc exhibited its protective effects downstream of APAP biotransformation by CYP2E1 and detoxication by GSH conjugation.
In our previous studies, we revealed that HSPs play a critical role in protection against APAP hepatotoxicity [12, 13]. Moreover the importance of HSPs is strongly supported by the finding that HSP70 knockout mice are sensitive to APAP-induced hepatotoxicity [47]. It is considered that HSP might function downstream of NAPQI formation and repair the damaged hepatic proteins as so-called “molecular chaperone” in APAP toxicity. Recently, polaprezinc is reported to induce HSP70 (HSP72) in rat gastric mucosa, mouse colonic mucosa, human colon cells, and protect against injuries induced by a variety of stimuli [25–27, 48]. Therefore, we examined whether polaprezinc could also prevent APAP toxicity by inducing HSP70. Treatment with polaprezinc increased HSP70 expression in mouse hepatocytes in a time-dependent manner (Fig. 1A), and treatment with zinc sulfate significantly increased HSP70 in the hepatocytes after 9 h (Fig. 1B). Contrarily, l-carnosine, the other component of polaprezinc, did not affect HSP70 levels. Zinc sulfate at low concentrations is shown to induce HSP70 in HeLa cells and rat hepatocytes [32, 33]. Zinc and other heavy metals could activate metal responsive element that is present in the HSP70 promoter region [49]. Therefore, it is considered that zinc and other heavy metals might induce HSP synthesis by activation of HSP gene transcription. Recent reports have shown that HSP70 exhibits anti-apoptotic function by interacting with components of apoptotic pathway [50, 51]. As shown in Fig. 6, KNK437, an inhibitor of HSP synthesis, decreased the induction of HSP70 in mouse hepatocytes by polaprezinc and abolished the protective effect of polaprezinc on APAP toxicity. Given that APAP-induced liver injury is caused by both necrosis and apoptosis [52], these findings suggest that the protective effect of polaprezinc might be at least in part due to inhibition against APAP-induced apoptosis via HSP70 induction. In the present study, treatments with polaprezinc and zinc sulfate increased HSP70 expression in mouse hepatocytes about 3.9-fold and 6.4-fold of control level, respectively. These data indicate that zinc sulfate may be more effective in inducing HSP70 and in protecting against APAP hepatotoxicity than polaprezinc. However, Seiki et al. [53] have demonstrated that zinc concentration after intragastric administration of polaprezinc is higher than that of zinc sulfate and that l-carnosine enhances the adhesive and/or permeable action of zinc on gastric mucosa. Zinc concentration in mouse hepatocytes after treatment with polaprezinc or zinc sulfate was almost the same (Fig. 2). However, it is known that l-carnosine exerts a remarkable enhancing effect on zinc uptake [54]. Therefore, polaprezinc could be a more promising HSP inducer than only zinc treatment in vivo. We examined using mouse model whether polaprezinc could be a promising drug for the HSP-based therapy of APAP insult. Oral administration of polaprezinc (100 mg/kg) to mice significantly increased plasma zinc concentration after 1 h and enhanced hepatic HSP70 induction after APAP (500 mg/kg, p.o.) administration. Furthermore, treatment with polaprezinc at 4 h before APAP administration suppressed the increase in plasma ALT activity (Nishida, unpublished data).
In addition to HSP-inducing activity, polaprezinc is known to have an antioxidant activity to inhibit lipid peroxidation via scavenging superoxide and hydroxyl radicals [20]. It is well known that oxidative stress plays an important role in the development of APAP toxicity [11, 55–57]. Recently, it has been reported that the APAP-induced reactive oxygen species (ROS) generation followed by lipid peroxidation in mouse primary hepatocytes induced cell death, and pretreatment with melatonin known as a potent antioxidant suppressed the increase in ROS and lipid peroxides, resulting in prevention of cell death [58]. In the present study, polaprezinc or zinc sulfate pretreatment significantly inhibited lipid peroxidation induced by APAP (Fig. 5), and improved the decrease in cell viability (Fig. 3). It is likely that the antioxidant activity of polaprezinc contributes to its protective effect on APAP toxicity in hepatocytes. However, it remains to be elucidated whether HSP70 induction by polaprezinc could be linked to its antioxidant function.
Polaprezinc comprises zinc and l-carnosine as mentioned above. Zinc has several important biological activities, including prevention of ROS generation, induction of MT and inhibition of apoptosis. Zinc treatment has been reported to protect against hepatic injuries by a variety of hepatotoxicants such as carbon tetrachloride, ethanol, thioacetamide and APAP [59–62]. MT, an important protein induced by metals, is shown to play a protective role in a lot of experimental models [63]. The study using MT-null mice has demonstrated that zinc pretreatment protects against APAP-induced hepatotoxicity in wild type mice, but not MT-null mice [64], indicating that the protective effect of zinc treatment on APAP toxicity may be related to its MT induction. Although we examined the expression of MT after treatment with polaprezinc, unexpectedly it did not affect cellular MT expression (data not shown). This discrepancy may also be ascribed to the difference in intracellular zinc contents as mentioned above. These findings suggest that MT induction is not involved in the protective effect of polaprezinc or zinc sulfate on APAP toxicity in this model. l-Carnosine is also known as an antioxidant [65]. A recent study showed that treatment with carnosine inhibited an increase in lipid peroxidation and prevented hepatotoxicity in thioacetamide-treated rats [66]. In our experiments, l-carnosine alone neither suppressed APAP-induced lipid peroxidation nor improved cell viability in mouse hepatocytes (Figs. 3 and 5).
In conclusion, the present results indicate that polaprezinc, an anti-ulcer drug, protects mouse primary cultured hepatocytes against APAP insult due to HSP70 induction and inhibition of lipid peroxidation, and suggest that zinc subcomponent in polaprezinc may play a pivotal role in cytoprotective actions of the drug.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Black M. Acetaminophen hepatotoxicity. Gastroenterology. 1980;78:382–392. [PubMed] [Google Scholar]
- 2.Pacifici G.M., Back D.J., Orme M.L. Sulphation and glucuronidation of paracetamol in human liver: Assay conditions. Biochem. Pharmacol. 1988;37:4405–4407. doi: 10.1016/0006-2952(88)90624-7. [DOI] [PubMed] [Google Scholar]
- 3.Dahlin D.C., Miwa G.T., Lu A.Y., Nelson S.D. N-Acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson D.G., Eastham W.N. Acute liver necrosis following overdose of paracetamol. Br. Med. J. 1966;5512:497–499. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell J.R. Acetaminophen toxicity. N. Engl. J. Med. 1988;319:1601–1602. doi: 10.1056/NEJM198812153192409. [DOI] [PubMed] [Google Scholar]
- 6.Jollow D.J., Mitchell J.R., Potter W.Z., Davis D.C., Gillrtte J.R., Brodie B.B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 7.Potter D.W., Hinson J.A. Reactions of N-acetyl-p-benzoquinoneimine with reduced glutathione, acetaminophen, and NADPH. Mol. Pharmacol. 1986;30:33–41. [PubMed] [Google Scholar]
- 8.Meyers L.L., Beierchmitt W.P., Khairallah E.A., Cohen S.D. Acetaminophen-induced inhibition of mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay R.R., Rashed M.S., Nelson S.D. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch. Biochem. Biophys. 1989;273:663–673. doi: 10.1016/0003-9861(89)90504-3. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H., Knight T.R., Bajt M.L. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 11.Amimoto T., Matsura T., Koyama S., Nakanishi T., Yamada K., Kajiyama J. Acetaminopheninduced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and a-tocopherol. Free Radic. Biol. Med. 1995;19:169–176. doi: 10.1016/0891-5849(94)00233-a. [DOI] [PubMed] [Google Scholar]
- 12.Sumioka I., Matsura T., Kai M., Yamada K. Potential roles of hepatic heat shock protein 25 and 70i in protection of mice against acetaminophen-induced liver injury. Life Sci. 2004;74:2551–2561. doi: 10.1016/j.lfs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Nishida T., Matsura T., Nakada J., Togawa A., Kai M., Sumioka I., Minami Y., Inagaki Y., Ishibe Y., Ito H., Ohta Y., Yamada K. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology. 2006;219:187–196. doi: 10.1016/j.tox.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Hendrick J.P., Hartl F. Molecular chaperone functions of heat-shock protein. Ann. Rev. Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 15.Kabakov A.E., Budagova K.R., Bryantsev A.L., Latchman D.S. Heat shock protein or heat shock protein 27 overexpressed in juman endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperon. 2003;8:335–347. doi: 10.1379/1466-1268(2003)008<0335:hspohs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.P., Wang X., Zhang J., Suh G.Y., Benjamin I.J., Ryter S.W., Choi A.M. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: Involvement of p38 beta MAPK and heat shock factor-1. J. Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara E.H., Martin J.L., Griffin T.M., Moriscot A.S., Mestril R. Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am. J. Phisiol. Cell Physiol. 2006;290:1128–1138. doi: 10.1152/ajpcell.00399.2005. [DOI] [PubMed] [Google Scholar]
- 18.Nakada J., Matsura T., Okazaki N., Nishida T., Togawa A., Minami Y., Inagaki Y., Ito H., Yamada K., Ishibe Y. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and HSP70 induction. Shock. 2005;24:482–487. doi: 10.1097/01.shk.0000180980.63247.a9. [DOI] [PubMed] [Google Scholar]
- 19.Ueki S., Seiki M., Yoneta T., Omata T., Hori Y., Ishikawa M., Tagashira E. Effect of Z-103 on compound 48/80-induced gastric lesions in rats. Scand. J. Gastroenterol. Suppl. 1989;162:202–205. doi: 10.3109/00365528909091161. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim. Biophys. Acta. 1991;1115:15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Yasuda M., Ueda S., Oyamada H., Kondo M. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free. Radic. Res. Commun. 1991;14:289–296. doi: 10.3109/10715769109088958. [DOI] [PubMed] [Google Scholar]
- 22.Cho C.H., Luk C.T., Ogle C.W. The membrane-stabilizing action of zinc carnosine (Z-103) in stress-induced gastric ulceration in rats. Life Sci. 1991;49:189–194. doi: 10.1016/0024-3205(91)90321-2. [DOI] [PubMed] [Google Scholar]
- 23.Arakawa T., Satoh H., Nakamura A., Nebiki H., Fukuda T., Sakuma H., Nakamura H., Ishikawa M., Seiki M., Kobayashi K. Effects of zinc L-carnosine on gastric mucosal and cell damage caused by ethanol in rats. Correlation with endogenous prostaglandin E2. Dig. Dis. Sci. 1990;35:559–566. doi: 10.1007/BF01540402. [DOI] [PubMed] [Google Scholar]
- 24.Fujii Y., Matsura T., Kai M., Kawasaki H., Yamada K. Protection by Polaprezinc, an Anti-ulcer Drug, Against Indomethacin-Induced Apoptosis in Rat Gastric Mucosal Cells. Jpn. J. Pharmacol. 2000;84:63–70. doi: 10.1254/jjp.84.63. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawara T., Takeda H., Kato K., Miyashita K., Kato M., Iwanaga T., Asaka M. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand. J. Gastroenterol. 2005;40:1321–1327. doi: 10.1080/00365520510023530. [DOI] [PubMed] [Google Scholar]
- 26.Mikami K., Otaka M., Watanabe D., Goto T., Endoh A., Miura K., Ohshima S., Yoneyama K., Sato M., Shibuya T., Segawa D., Kataoka E., Yoshino R., Takeuchi S., Sato W., Odashima M., Watanabe S. Zinc L-carnosine protects against mucosal injury in portal hypertensive gastrophathy through induction of heat shock protein 72. J. Gastroenterol. Hepatol. 2006;21:1669–1674. doi: 10.1111/j.1440-1746.2006.04328.x. [DOI] [PubMed] [Google Scholar]
- 27.Otaka M., Konishi N., Odashima M., Jin M., Wada I., Matsuhashi T., Horikawa Y., Ohba R., Watanabe S. Is Mongolian gerbil really adequate host animal for study of Helicobacter pylori infection-induced gastritis and cancer? Biochem. Biophys. Res. Commun. 2006;347:297–300. doi: 10.1016/j.bbrc.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 28.Seglen P.O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp. Cell Res. 1973;82:391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 29.Mochida S., Matsura T., Yamashita A., Horie S., Ohata S., Kusumoto C., Nishida T., Minami Y., Inagaki Y., Ishibe Y., Nakada J., Ohta Y., Yamada K. Geranylgeranylacetone ameliorates inflammatory response to lipopolysaccharide (LPS) in murine macrophages: Inhibition of LPS binding to the cell surface. J. Clin. Biochem. Nutr. 2007;41:115–123. doi: 10.3164/jcbn.2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsura T., Serinkan B.F., Jiang J., Kagan V.E. Phosphatidylserine peroxidation/externalizaton during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002;524:25–30. doi: 10.1016/s0014-5793(02)02990-3. [DOI] [PubMed] [Google Scholar]
- 31.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Hatayama T., Asai Y., Wakatsuki T., Kitamura T., Imahara H. Regulation of Hsp70 synthesis induced by cupric sulfate and zinc sulfate in thermotolerant HeLa cells. J. Biochem. 1993;114:592–597. doi: 10.1093/oxfordjournals.jbchem.a124222. [DOI] [PubMed] [Google Scholar]
- 33.Bauman J.W., Liu J., Klaassen C.D. Production of metallothionein and heat shock proteins in response to metals. Fund. Appl. Toxicol. 1993;21:15–22. doi: 10.1006/faat.1993.1066. [DOI] [PubMed] [Google Scholar]
- 34.Masubuchi Y., Bourdi M., Reilly T., Graf M.L.M., George J.W., Pohl L.R. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem. Biophys. Res. Commun. 2003;304:207–212. doi: 10.1016/s0006-291x(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 35.Bao X.Q., Liu G.T. Bicyclol: a novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice. Cell Stress Chaperon. 2008;13:347–355. doi: 10.1007/s12192-008-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raucy J.L., Lasker J.M., Lieber C.S., Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch. Biochem. Biophys. 1989;271:270–283. doi: 10.1016/0003-9861(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 37.Guo G.L., Moffit J.S., Nicol C.J., Ward J.M., Aleksunes L.A., Slitt A.L., Kliewer S.A., Manautou J.E., Gonzalez F.J. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol. Sci. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 38.Chen W., Koenigs L.L., Thompson S.J., Peter R.M., Rettie A.E., Trager W.F., Nelson S.D. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem. Res. Toxicol. 1998;11:295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- 39.Dong H., Haining R.L., Thummel K.E., Rettie A.E., Nelson S.D. Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab. Dispos. 2000;28:1397–1400. [PubMed] [Google Scholar]
- 40.Lee S.S., Buters J.T., Pineau T., Fernandez-Salguero P., Gonzalez F.J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 41.Tonge R.P., Kelly E.J., Bruschi S.A., Kalhorn T., Eaton D.L., Nebert D.W., Nelson S.D. Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice. Toxicol. Appl. Pharmacol. 1998;153:102–108. doi: 10.1006/taap.1998.8543. [DOI] [PubMed] [Google Scholar]
- 42.Zaher H., Buters J.T., Ward J.M., Bruno M.K., Lucas A.M., Stern S.T., Cohen S.D., Gonzalez F.J. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol. Appl. Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 43.Sumioka I., Matsura T., Kasuga S., Itakura Y., Yamada K. Mechanisms of protection by S-allylmercaptocystein against acetaminophen-induced liver injury in mice. Jpn. J. Pharmacol. 1998;78:199–207. doi: 10.1254/jjp.78.199. [DOI] [PubMed] [Google Scholar]
- 44.Bajt M.L., Knight T.R., Lemasters J.J., Jaesckhe H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 45.Andrews G.K. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 46.Steinebach O.M., Wolterbeek H.T. Effects of zinc on rat hepatoma HTC cells and primary cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 1993;118:245–254. doi: 10.1006/taap.1993.1030. [DOI] [PubMed] [Google Scholar]
- 47.Tolson J.K., Dix D.J., Voellmy R.W., Roberts S.M. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol. Appl. Pharmacol. 2006;210:157–162. doi: 10.1016/j.taap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Ohkawara T., Nishihira J., Nagashima R., Takeda H., Asaka M. Polaprezinc protects human colon cells from oxidative injury induced by hydrogen peroxide: relevant to cytoprotective heat shock proteins. World J. Gastroenterol. 2006;12:6178–6181. doi: 10.3748/wjg.v12.i38.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu B.J., Kingston R.E., Morimoto R.I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc. Natl. Acad. Sci. USA. 1986;83:629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arya R., Mallik M., Lakhotia SC. Heat shock genes—integrating cell survival and death. J. Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 51.Kalmar B., Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliver. Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Jaeschke H., Gujral J.S., Bajt M.L. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–89. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- 53.Seiki M., Aita H., Mera Y., Arai K., Toyama S., Furuta S., Morita H., Hori Y., Yoneta T., Tagashira E. The gastric mucosal adhesiveness of Z-103 in rats with chronic ulcer. Nippon Yakurigaku Zasshi. 1992;99:255–263. doi: 10.1254/fpj.99.255. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura Y., Matsukura T. Zinc uptake enhancing effect of L-carnosine. Biomed. Res. Trace Elements. 2000;11:347–348. [Google Scholar]
- 55.Nordblom G.D., Coon M.J. Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Arch. Biochem. Biophys. 1977;180:343–347. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- 56.Kuthan H., Tsuji H., Gral H., Ullrich V. Generation of superoxide anion as a source of hydrogen peroxide in a reconstituted monooxygenase system. FEBS Lett. 1978;91:343–345. doi: 10.1016/0014-5793(78)81206-x. [DOI] [PubMed] [Google Scholar]
- 57.Matsura T., Nishida T., Togawa A., Horie S., Kusumoto C., Ohata S., Nakada J., Ishibe Y., Yamada K., Ohta Y. Mechanisms of protection by melatonin against acetaminophen-induced liver injury in mice. J. Pineal Res. 2006;41:211–219. doi: 10.1111/j.1600-079X.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 58.Kanno S., Tomizawa A., Hiura T., Osanai Y., Kakuta M., Kitajima Y., Koiwai K., Ohtake T., Ujibe M., Ishikawa M. Melatonin protects on toxicity by acetaminophen but not on pharmacological effects in mice. Biol. Pharm. Bull. 2006;29:472–476. doi: 10.1248/bpb.29.472. [DOI] [PubMed] [Google Scholar]
- 59.Dhawan D., Goel A., Gautam C.S. Effects of zinc intake on liver enzymes in toxicity carbontetrachloride induced liver injury. Med. Sci. Res. 1992;20:55–56. [Google Scholar]
- 60.Zhou Z., Wang L., Song Z., Saari J.T., McClain C.J., Kang Y.J. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am. J. Pathol. 2005;166:1681–1690. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Y.M., Chen M.D. Zinc supplementation attenuates thioacetamide-induced liver injury and hyperglycemia in mice. Biol. Trace Elem. Res. 2003;92:173–180. doi: 10.1385/BTER:92:2:173. [DOI] [PubMed] [Google Scholar]
- 62.Woo P.C., Kaan S.K., Cho C.H. Evidence for potential application of zinc as an antidote to acetaminophen-induced hepatotoxicity. Eur. J. Pharmacol. 1995;293:217–224. doi: 10.1016/0926-6917(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 63.Formigari A., Irato P., Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 64.Liu L., Liu Y., Hartley D., Klaassen C.D., Shehin-Johnson S.E., Lucas A. Metallothionein-I/II knockout mice are sensitive to acetaminophen-induced hepatotoxicity. J. Pharmacol. Exp. Ther. 1999;289:580–586. [PubMed] [Google Scholar]
- 65.Babizhayev M.A., Seguin N.C., Gueyne J., Evstigneeva R.P., Ageyeva E.A., Zheltukhina G.A. L-carnosine (beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities. Biochem. J. 1994;304:509–516. doi: 10.1042/bj3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehmetçik G., Özdemirler G., Koçak-Toker N., Çevikbas U., Uysal M. Role of carnosine in preventing thioacetamide-induced liver injury in the rat. Peptides. 2008;29:425–429. doi: 10.1016/j.peptides.2007.11.008. [DOI] [PubMed] [Google Scholar]