Abstract
We investigated energy expenditure in hospitalized patients with Crohn’s disease (CD), and determined optimal energy requirements for nutritional therapy. Sixteen patients (5 women and 11 men, mean age 36 year old, mean BMI 18.7 kg/m2) and 8 healthy volunteers were enrolled in this study. Measured resting energy expenditure (mREE) levels were determined by indirect calorimetry. The mREEs in CD patients were significantly higher than those of healthy controls (24.4 ± 2.4 kcal/kg/day vs 21.3 ± 1.7 kcal/kg/day). However, mREEs in CD patients were significantly lower than predicted REEs (pREEs) calculated by the Harris-Benedict equation (26.4 ± 2.5 kcal/kg/day). Furthermore, mREE/pREE values were lower in undernourished patients than in well-nourished patients. CD patients had hyper-metabolic statuses evaluated by mREE/body weight, but increased energy expenditure did not contribute to weight loss in these patients. In conclusion, nutritional therapy with 25–30 kcal/ideal body weight/day (calculated by mREE × active factor) may be optimal for active CD patients, while higher energy intake values pose the risk of overfeeding.
Keywords: Crohn’s disease, resting energy expenditure, indirect calorimetry
Introduction
Crohn’s disease (CD) is a chronic inflammatory disease of the digestive tract of unknown etiology. Patients with CD are accompanied by various nutrition deficiencies. Body weight loss and malnutrition are common features, and nutritional deficiencies occur in CD patients in both active and remission phases. Emaciation is found in 20–75% of patients with CD [1, 2], and approximately 75% of hospitalized CD patients are undernourished [3, 4]. Malnutrition in CD patients is associated with decreased dietary intake, malabsorption, and protein-losing enteropathy. Furthermore, micronutrient deficiencies including vitamin D, vitamin B12, folic acid, zinc and selenium are frequent in these patients [5–8].
Nutritional support is essential for CD patients, and nutritional therapy such as enteral nutrition (EN) or total parenteral nutrition (TPN) has been established as primary therapy for CD as well as infliximab therapy and steroid therapy. Especially, elemental diet therapies are effective in both induction and maintenance therapies [9–11], although their therapeutic mechanisms are still unknown.
Energy metabolism changes to a hyper-metabolic status in CD patients [12–16]. However, Melchior et al. [17] reported that measured REE (mREE) was approximately 33 kcal/kg/day in patients with CD, which was lower than the REE observed in other diseases. Furthermore, Schneeweiss et al. [18] reported that mREE was not significantly different between CD patients and healthy subjects. In Japan, nutritional therapies such as EN or TPN therapies are widely accepted, but energy metabolism in Japanese patients with CD remains unclear. Total energy of EN or TPN is usually determined by using predicted resting energy expenditure (pREE) calculated by the Harris-Benedict equation [19], and total energy requirements are calculated by pREE × active factor × stress factor [20]. On the other hand, mREE can be determined by indirect calorimetry. Theoretically, pREE is equal to mREE in healthy humans, and mREE/pBEE ratios are markers of hyper-metabolic status.
In this study, we evaluated energy metabolism in Japanese patients with CD and suggested optimal energy requirements for nutritional therapies.
Subjects and Methods
Patients
Sixteen patients with CD (5 women and 11 men, median age 36 years old) and 8 healthy volunteers were enrolled in this study. Patients were admitted to the Gastroenterology Unit of Shiga University of Medical Science Hospital. The ethics committee of the Shiga University of Medical Science approved this study. All patients had the diagnosis of CD established by radiological, histological, and clinical criteria. Among these patients, two patients had colitic disease, six patients had ileal disease and eight patients had ileocolitic disease. Twelve patients were receiving TPN, and three patients were receiving elemental diet therapy. Infliximab therapy has just been started in four patients, and (oral) prednisone (10–30 mg/day) was taken by five patients.
Indirect calorimetry
mREEs and respiratory quotients (RQ) were measured by computed open-circuit indirect calorimetery (AE-300S; Minato Medical Science Co., Osaka, Japan). Indirect calorimetry (IC) was performed in the hospital room the morning after a 10-h overnight fasting, but infusion of total parenteral nutrition solution were kept on going. Period flow and gas calibration were performed prior to measurements. After resting for a minimum of 30 min, patients were assessed in a supine position with a facemask. A pump drew ambient air through a facemask at a constant rate. After equilibrium was reached for 10 min, respiratory exchange was performed continuously over 30 min. mREE and RQ data were obtained every minute.
mREE was calculated from oxygen consumption (VO2) and carbon dioxide production (VCO2) by the Weir equation [21].
mREE = (3.94 × VO2 + 1.11 × VCO2) × 1.44
Measurement of the RQ was calculated as RQ = VCO2/VO2. The measured mREE was compared with pREE calculated by the Harris and Benedict equation.
Man; pREE = 66.47 + 13.75 × W + 5.0 × H – 6.75 × A
Woman; pREE = 665.09 + 9.56 × W + 1.84 × H – 4.67 × A
Where pREE stands for resting energy expenditure (kcal/day), W for weight (kg), H for height (cm) and A for age (years).
Statistical analyses
Differences between groups were analyzed with Kruskal-Wallis tests. A p value <0.05 was considered to be statistically significant. Correlations were investigated with Spearman rank correlation tests.
Results
Patients were not significantly different from control subjects in age and height, but had significantly lower body weights, and thus lower BMIs (Table 1). Crohn’s disease activity index from these patients was 231.3 ± 84.8, and C-reactive protein was 3.24 ± 4.6 mg/dl. Average Hemoglobin, red blood cell count, white blood cell count and platelet count were 10.64 ± 2.1 g/dl, 400.8 ± 62.6 × 104/mm3, 7540 ± 3490/mm2, 35.4 ± 12.2 × 104/mm3 respectively. Serum albumin and total-cholesterol is 3.46 ± 0.44 g/dl and 127.9 ± 47.6 mg/dl.
Table 1.
Characteristics, energy expenditure and respiratory quotient (RQ) in CD patients (n = 16) and control subjects (n = 8).
| CD patients | controls | p | |
|---|---|---|---|
| Patients number | 16 | 8 | |
| Female/male | 5/11 | 2/6 | |
| Age (y) | 36.0 ± 9.8 | 44.8 ± 19.0 | 0.131 |
| Height (cm) | 167.8 ± 10.3 | 165.6 ± 8.1 | 0.602 |
| Body weight (kg) | 52.7 ± 9.4 | 64.9 ± 12.4 | 0.013 |
| BMI (kg/mm2) | 18.7 ± 2.6 | 23.5 ± 2.6 | <0.001 |
| pREE (kcal/day) | 1372.7 ± 143.7 | 1467.9 ± 238.5 | 0.233 |
| mREE (kcal/day) | 1271.0 ± 181.7 | 1378.0 ± 253.3 | 0.245 |
| mREE/pREE (%) | 92.5 ± 8.5 | 94.2 ± 11.4 | 0.685 |
| pREE/body weight(kcal/kg/day) | 26.4 ± 2.5 | 22.9 ± 2.6 | 0.003 |
| mREE/body weight(kcal/kg/day) | 24.4 ± 2.4 | 21.3 ± 1.7 | 0.003 |
| RQ | 0.94 ± 0.14 | 0.85 ± 0.04 | 0.107 |
BMI; body mass index, pREE; predicted resting energy expenditure calculated by the Harris-Benedict equation. mREE; measured resting energy expenditure by using indirect calorimetry, RQ; respiratory quotient.
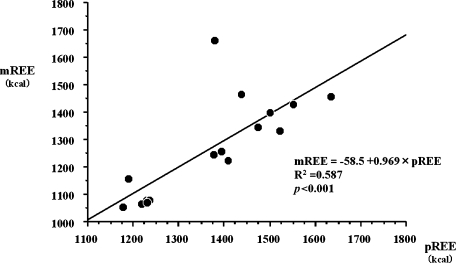
In CD patients, mREE determined by indirect calorimetry was 1271 ± 181.7 kcal/day and pREE calculated by the Harris-Benedict equation was 1372.7 ± 143.7 kcal/day, respectively. As shown in Fig. 1, there were significant correlations between mREE and pREE in CD patients (p<0.001) as well as in healthy controls (p<0.05). Both mREE and pREE in CD patients were slightly lower than those of healthy controls, but there was no significant difference.
Fig. 1.
Correlation between measured resting energy expenditure (mREE) by indirect calorimetry and predicted resting energy expenditure (pREE) calculated by the Harris-Benedict equation in CD patients (n = 16). There is a positive correlation between mREE and pREE in CD patients.
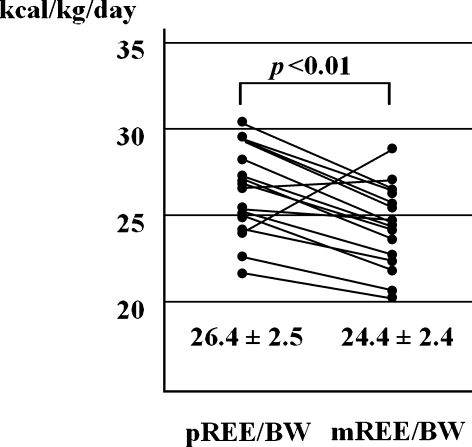
On the other hand, mREE/body weight of CD patients (24.4 ± 2.4 kcal/kg/day) was significantly higher than that of healthy control subjects (21.3 ± 1.7 kcal/kg/day) (Table 1). Similarly, pREE/body weight of CD patients (26.4 ± 2.6 kcal/kg/day) was also significantly higher than that of healthy control subjects (22.9 ± 2.6 kcal/kg/day) (Table 1).
In CD patients, mREE/body weight was significantly lower than pREE/body weight (26.4 ± 2.5 kcal/kg/day) (Fig. 2). Percentage of mREE/pREE was 93.6 ± 13.7% in CD patients. In only two patients, mREE/body weight was higher than pBEE/body weight, and one of them developed complications including an abdominal abscess with fever.
Fig. 2.
Comparison of resting energy expenditure measured by using indirect calorimetry (mREE) and predicted resting energy expenditure (pREE) calculated by Harris-Benedict equation in CD patients (n = 16). mREE is significantly lower than pREE in CD patients.
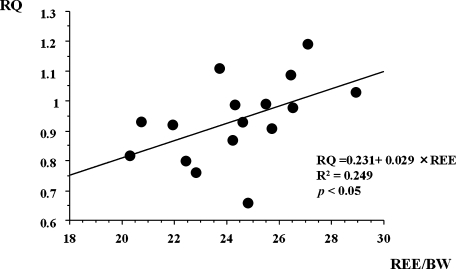
RQ in these patients was 0.94 ± 0.14, and there were significant positive correlations between mREE and RQ (Fig. 3), whereas there were no significant correlations between mREE and RQ in healthy controls (p = 0.075). However, there were no significant correlations between mREE and BMI, or between RQ and BMI in CD patients.
Fig. 3.
Correlation between resting mREE and respiratory quotient (RQ) in CD patients (n = 16). RQ in CD patients exhibited a positive correlation with mREE.
Subjects were also categorized into two groups based on body mass index (group A; <18.5 vs group B; >18.5). Group A included seven patients and group B included nine patients. There were no significant differences in height, body weight, pREE, mREE and between groups, but there were significant differences in pREE/body weight (p<0.021). The mREE/body weight in group A (24.7 ± 1.2 kcal/kg/day) was almost same as that of group B (24.1 ± 3.0 kcal/kg/day).
Discussion
This is the first report of resting energy expenditure in Japanese patients with CD. This study showed that measured REE by IC in hospitalized CD patients was 24.4 ± 2.4 kcal/kg/day, and this was significantly higher than that in healthy controls. This result suggests that CD patients apparently have a hyper-metabolic status, and supports observations from previous studies [12–16]. However, mREE was significantly lower than pREE calculated by the Harris-Benedict equation in CD patients. Usually, energy requirements in hospitalized patients are calculated by pREE × active factor × stress factor. Stress factor in patients with inflammatory bowel disease is recommended as 1.1–1.3 [22] as well as in patients with cancer and chronic obstructive pulmonary disease. This adjustment may result in the actual energy expenditure as approximately 45 kcal/kg/day. However, Barot et al. reported that the pBEE was equivalent to mREE in non-septic CD patients. They decided that energy requirement were often not greater than the predicted requirement by pREE × active factor × stress factor [23]. The inflammatory bowel disease study group of the Japanese Ministry of Health, Labor and Welfare also recommends that total energy of TPN or EN should be 40–45 kcal/ideal body weight/day in active CD patients [24]. However, our study suggests that 30–35 kcal/kg/day (25–30 kcal/ideal body weight) may be ideal as total energy for EN or TPN therapies for CD patients by calculating REE × 1.2–1.3 as the active factor. CD patients certainly have hyper-metabolic statuses, and but high-energy intakes during EN or TPN therapies may have a risk of overfeeding. European guidelines [25] recommend that 25–30 kcal/ideal body weight/day is optimal for active Crohn’s disease.
A previous study showed that RQ in active CD is significant lower than in health controls (CD patients; 0.78 ± 0.05 versus healthy controls; 0.86 ± 0.05) and energy metabolism in active CD resembled starvation patterns [18]. In this study, there is a positive correlation between mREE/body weight and RQ, and this finding shows that fat is mainly oxidated in low metabolic patients. However, the present results revealed that (the mean) RQ in hospitalized CD patients was 0.93 ± 0.14, and there was no significant difference from that found in controls. Furthermore, there was no significant difference between RQ and BMI, and RQ was lower than 0.8 in only a few subjects (12.5%). The aforementioned result may be explained by the fact that our patients had already received nutritional therapy. Based on these observations, our results did not support a previous report that energy metabolism of active CD patients mimic starvation patterns.
It has long been hypothesized that increased energy expenditure contributes to weight loss in patients with CD. However, Vaisman et al. [26] reported that mREE/pREE was higher in a well-nourished CD group than in an undernourished CD group. In this study, mREE/body weight in the undernourished group was found to be equivalent to that in well-nourished group, and mREE/pREE was slightly higher in the well-nourished group than the undernourished group. These observations support that increased energy expenditure is not a contributor to the underweight status of CD patients. Mingrone et al. reported that prednisone therapy did not change energy expenditure, but stimulated food intake [27]. This means that steroid treatment promotes an overall positive energy balance. However, patients receiving this oral steroid were few in this study (31.2%).
Recently, Wise et al. [28] reported that REE did not change after Infliximab therapy, whereas RQ increased after Infliximab therapy. Their result revealed that lipolysis decreased and starvation improved in CD patients receiving Infliximab therapy. Infliximab therapy is now going to be a main therapeutic tool in active CD patients, but nutritional management is necessary in these patients. REE and RQ determinations by indirect calorimetry are useful for CD patients receiving Infliximab therapy as well as nutritional therapies.
In conclusion, we determined resting energy expenditure in Japanese patients with CD. Nutritional therapies consisting of 25–30 kcal/ideal body weight/day are recommended as optimal for active CD patients.
Table 2.
Energy expenditure and respiratory quotient (RQ) in CD patients (n = 16).
| BMI<18.5 | BMI>18.5 | p | |
|---|---|---|---|
| Patients number | 7 | 9 | |
| Female/male | 3/4 | 2/7 | |
| Age (y) | 30.3 ± 2.5 | 40.0 ± 11.2 | 0.037 |
| Height (cm) | 170.6 ± 11.2 | 165.6 ± 9.7 | 0.36 |
| Body weight (kg) | 47.7 ± 7.4 | 56.6 ± 9.3 | 0.056 |
| BMI (kg/mm2) | 16.3 ± 1.2 | 20.6 ± 1.5 | <0.001 |
| pREE (kcal/day) | 1327.9 ± 160.1 | 1407.6 ± 128.1 | 0.287 |
| mREE (kcal/day) | 1173.6 ± 147.9 | 1347.2 ± 175.2 | 0.054 |
| mREE/pREE (%) | 88.2 ± 1.3 | 95.8 ± 10.3 | 0.073 |
| pREE/body weight(kcal/kg/day) | 28.0 ± 1.5 | 25.2 ± 2.5 | 0.021 |
| mREE/body weight(kcal/kg/day) | 24.7 ± 1.2 | 24.1 ± 3.0 | 0.615 |
| RQ | 0.97 ± 0.12 | 0.91 ± 0.15 | 0.422 |
These patients were categorized into two groups based on body mass index 18.5. There are significant differences in BMI, pREE/body weight between these groups. The mREE/body weight in undernourished CD patients (n = 7) was almost same as that of well-nourished CD patients (n = 9), and there are no significant differences in pREE, mREE and mREE/pREE value. BMI; body mass index, pREE; predicted resting energy expenditure calculated by the Harris-Benedict equation. mREE; measured resting energy expenditure by using indirect calorimetry, RQ; respiratory quotient.
References
- 1.Farmer R.G., Hawk W.A., Turnbull R.B. Jr. Clinical patterns in Crohn’s disease: a statistical study of 615 cases. Gastroenterology. 1975;68:627–635. [PubMed] [Google Scholar]
- 2.Mekhjian H.S., Switz D.M., Melnyk C.S., Rankin G.B., Brooks R.K. Clinical features and natural history of Crohn’s disease. Gastroenterology. 1979;77:898–906. [PubMed] [Google Scholar]
- 3.Afonso J.J., Rombeau J.L. Nutritional care for patients with Crohn’s disease. Hepatogastroenterology. 1990;37:32–41. [PubMed] [Google Scholar]
- 4.Driscoll R.H., Resenberg J.L. Total parenteral nutrition in inflammatory bowel disease. Med. Clin. North Am. 1978;62:185–201. doi: 10.1016/s0025-7125(16)31831-4. [DOI] [PubMed] [Google Scholar]
- 5.Fillipi J., Al-Jaouni R., Wiroth J.B., Hebuterne X., Schneider S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel. Dis. 2006;12:185–191. doi: 10.1097/01.MIB.0000206541.15963.c3. [DOI] [PubMed] [Google Scholar]
- 6.Hodges P., Gee M., Grace M., Sherbaniuk R.W., Wensel R.H., Thomson A.B. Protein-energy intake and malnutrition in Crohn’s disease. J. Am. Diet Assoc. 1984;84:1460–1464. [PubMed] [Google Scholar]
- 7.Geerling B.J., Badart-Smook A., Stockbrugger R.W., Brummer R.J. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am. J. Clin. Nutr. 1998;67:919–926. doi: 10.1093/ajcn/67.5.919. [DOI] [PubMed] [Google Scholar]
- 8.Johtatsu T., Andoh A., Kurihara M., Iwakawa H., Tsujikawa T., Kashiwagi A., Fujiyama Y., Sasaki M. Serum concentration of trace elements in patients with Crohn’s disease receiving enteral nutrition. J. Clin. Biochem. Nutr. 2007;41:197–201. doi: 10.3164/jcbn.2007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Morain C., Segal A.W., Levi A.J. Elemental diet therapy as primary therapy of acute Crohn’s disease. Br. Med. J. 1984;288:1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter J.O. Nutrition factors in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 1988;10:235–237. doi: 10.1097/00042737-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Takagi S., Utsunomiya K., Kuriyama S., Yokoyama H., Takahashi S., Iwabuchi M., Takahashi H., Takahashi S., Kinouchi Y., Hiwatashi N., Funayama Y., Sasaki I., Tsuji I., Shimosegawa T. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006;24:1333–1340. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan A.T., Fleming C.R., O’Fallon W.M., Huizenga K.A. Estimated versus measured basal energy requirement in patients with Crohn’s disease. Gastroenterology. 1986;91:75–78. doi: 10.1016/0016-5085(86)90441-5. [DOI] [PubMed] [Google Scholar]
- 13.Kushner R.F., Schoeller D.A. Resting and total energy expenditure in patients with inflammatory bowel disease. Am. J. Clin. Nutr. 1991;53:161–165. doi: 10.1093/ajcn/53.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Stokes M.A., Hill G.L. Total energy expenditure in patients with Crohn’s disease: measured by the combined body scan technique. JPEN. 1993;17:3–7. doi: 10.1177/014860719301700103. [DOI] [PubMed] [Google Scholar]
- 15.Rigaud D., Cerf M., Angel Alberto. L., Sobhani I., Mignon M. Increase of resting energy expenditure during flare-ups in Crohn’s disease. Gastroenterol. Clin. Biol. 1993;17:932–937. [PubMed] [Google Scholar]
- 16.AI-Janouni R., Hebuterne X., Pouget I., Rampal P. Energy metabolism and substrate oxidation in patients with Crohn’s disease. Nutrition. 2000;16:173–178. doi: 10.1016/s0899-9007(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 17.Melchior J.C., Salmon D., Rigaud D., Leport C., Bouvet E., Detruchis P., Vilde J.L., Vachon F., Coulaud J.P., Apfelbaum M. Resting energy expenditure is increased in stable, malnourished HIV-infected patients. Am. J. Clin. Nutr. 1991;53:437–441. doi: 10.1093/ajcn/53.2.437. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss B., Lochs H., Zauner C., Fischer M., Wyatt J., Maier-Dobersberger T., Schneider B. Energy and substrate metabolism in patients with active Crohn’s disease. J. Nutr. 1999;129:844–848. doi: 10.1093/jn/129.4.844. [DOI] [PubMed] [Google Scholar]
- 19.Harris J.A., Benedict F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. U.S.A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long C.L., Schaffel N., Geiger J.W., Schiller W.R., Blakemore W.S. Metabolic response to injury and illness: Estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J. Parenteral. Entr. Nutr. 1979;3:452–456. doi: 10.1177/014860717900300609. [DOI] [PubMed] [Google Scholar]
- 21.Weir J.B.V. New methods for calculating metabolic rate with special reference to protein matabolism. J. Physiol. 1949;109:254–259. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han P.D., Burke A., Baldassano R.N., Rombeau J.L., Lichtenstein G.R. Nutritional and inflammatory bowel disease. Gatroenterol. Clin. North Am. 1999;28:423–443. doi: 10.1016/s0889-8553(05)70063-7. [DOI] [PubMed] [Google Scholar]
- 23.Barot L.R., Rombeau J.L., Feurer I.D., Mullen J.L. Caloric requirement in patients with inflammatory bowel disease. Ann. Surg. 1982;195:214–218. doi: 10.1097/00000658-198202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ida M. Annual Report of the Research Committee of Inflammatory Bowel Disease, The Japanese Ministry of Health, Labor and Welfare. 2003. Pharmacological therapy in Crohn’s disease; pp. 21–22. (In Japanese) [Google Scholar]
- 25.Lochs H., Dejong C., Hammarqvist F. ESPEN guidelines on enteral nutrition: gastroenterology. Clin. Nutr. 2006;25:260–274. doi: 10.1016/j.clnu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Vaisman N., Dotan I., Halack A., Niv E. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition. 2006;22:855–859. doi: 10.1016/j.nut.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Mingrone G., Benedetti G., Capristo E., De Gaetano A., Greco A.V., Tataranmi P.A., Gasbarrini G. Twenty-four-hour energy balance in Crohn’s disease patients: metabolic implication of steroid treatment. Am. J. Clin. Nutr. 1998;67:118–123. doi: 10.1093/ajcn/67.1.118. [DOI] [PubMed] [Google Scholar]
- 28.Wiese D., Lashner B., Seidner D. Measurement of nutrition status in Crohn’s disease patients receiving Infliximab therapy. Nutr. Clin. Pract. 2008;23:551–556. doi: 10.1177/0884533608323421. [DOI] [PMC free article] [PubMed] [Google Scholar]