Abstract
Clinical and experimental studies showed that the reflux of bile into the stomach contributes to the induction of intestinal metaplasia of the stomach and gastric carcinogenesis. Caudal-type homeobox 2 (Cdx2) plays a key role in the exhibition of intestinal phenotypes by regulating the expression of intestine-specific genes such as goblet-specific gene mucin 2 (MUC2). We investigated the involvement of the farnesoid X receptor (FXR), a nuclear receptor for bile acids, in the chenodeoxycholic acid (CDCA)-induced expression of Cdx2 and MUC2 in normal rat gastric epithelial cells (RGM-1 cells). RGM-1 cells were treated with CDCA or GW4064, an FXR agonist, in the presence or absence of guggulsterone, an FXR antagonist. CDCA induced dose-dependent expression of Cdx2 and MUC2 at both the mRNA and protein levels. The maximum stimulation of Cdx2 and MUC2 mRNA induced by CDCA was observed at 3 h and by 6 h, respectively. GW4064 also induced expression of these molecules. The effects of CDCA and GW4064 on expression of Cdx2 and MUC2 were abolished by guggulsterone. These findings suggest that bile acids may induce gastric intestinal metaplasia and carcinogenesis through the FXR.
Keywords: bile acid, intestinal metaplasia, FXR, Cdx2
Introduction
A high incidence of gastric cancer is known to occur in Asia and is one of the leading causes of cancer-related deaths worldwide. Although the precise mechanism that underlies gastric carcinogenesis is not yet fully understood, gastric carcino genesis, particularly the intestinal-type, is thought to be a multistep process triggered by chronic Helicobacter pylori infections. Atrophic gastritis, intestinal metaplasia and dysplasia represent different stages of the gastric carcinogenesis cascade and intestinal type gastric cancers are thought to develop within the gastric mucosa along with intestinal metaplasia [1–3].
Caudal-type homeobox 2 (Cdx2), a mammalian member of the caudal-related homeobox gene family, plays an important role in the differentiation of intestinal cells and in maintaining the intestinal phenotype by binding to the promoters of several intestine-specific genes including goblet-specific gene mucin 2 (MUC2) [4, 5]. Mizoshita et al. [6] reported that Cdx2 mRNA was widely present in human intestinal and colonic mucosa, but not in normal gastric mucosa. However, the nuclear expression of Cdx2 has been reported in gastric mucosa associated with intestinal metaplasia, as well as in relation to intestinal-type gastric carcinomas [7, 8]. Furthermore, Cdx2 transgenic mice developed intestinal metaplasia in the stomach with the induction of MUC2 [9, 10], suggesting that ectopically expressed Cdx2 may play a key role in gastric carcinogenesis via induction of intestinal metaplasia.
Clinical and experimental studies showed that the reflux of bile into the stomach and esophagus contributes to the induction of intestinal metaplasia of the stomach and gastric carcinogenesis [11, 12], as well as to esophageal injury, Barrett’s esophagus and esophageal adenocarcinoma [13, 14]. Furthermore, Hu et al. [15] demonstrated that deoxycholic acid caused the upregulation of Cdx2 and MUC2 mRNA using four human esophageal cell lines, including those derived from normal squamous, adenocarcinoma and squamous carcinoma tissues. The mechanisms associated with the bile acid induced expression of these molecules, however, remains unclear.
Bile acids are physiological ligands and activators of the farnesoid X receptor (FXR), a transcription factor belonging to the nuclear receptor superfamily [16–18]. Bile acids have been shown to exert signaling activities associated with the self-modulation of genes involved in their own synthesis and transport through the activation of the FXR [19].
In this study, we investigated whether two primary bile acids, chenodeoxycholic acid (CDCA) and secondary bile acid, deoxycholic acid (DCA), could induce the expression of Cdx2 and MUC2 in normal rat gastric epithelial cells. We also evaluated the involvement of the FXR in the CDCA-induced ectopic expression of these molecules.
Materials and Methods
Cell culture and reagents
Normal rat gastric epithelial cells, RGM-1 cells, established by Hirofumi Matsui (Riken Cell Bank, Tsukuba, Japan), were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 Ham (Sigma-Aldrich, St. Louis, MO) supplemented with 20% fetal bovine serum (FBS) at 37°C in an humidified 95% air, 5% CO2 atmosphere. The cells were seeded in 100 mm dishes and were routinely grown in DMEM/F12 supplemented with 20% FBS. Once the cells reached 70% confluency, they were serum-deprived for 24 h. The cells were then treated with CDCA (0–50 µM), 100 µM DCA, or 5 nM GW4064 (an FXR agonist) in the presence or absence of 50 µM guggulsterone (an FXR antagonist). CDCA, DCA, and guggulsterone were purchased from Sigma-Aldrich and GW4064 was purchased from Tocris Bioscience (Ellisville, MO). They were dissolved in DMSO.
Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR)
Real-time quantitative RT-PCR analyses were performed as previously described [20]. In brief, an ISOGEN kit (Nippon Gene, Tokyo, Japan) was used to isolate total RNA according to the manufacturer’s protocol. Quantitative RT-PCR analyses were performed using an ABI PRISM 7700 Sequence Detection System and accompanying software (PE Applied Biosystems, Foster City, CA). The reaction mixture was prepared according to the manufacturer’s protocol using the Platinum qRT-PCR ThermoScript One-Step System from Invitrogen (Carlsbad, CA). The thermal cycling conditions were 50°C for 30 min and 95°C for 5 min, followed by 45 cycles of amplification using 95°C for 15 s and 60°C for 1 min. Total RNA was subjected to quantitative RT-PCR to assess the levels of the targeted gene sequences. GAPDH served as an internal standard using TaqMan GAPDH control reagents purchased from PE Applied Biosystems. The expression of Cdx2 and MUC2 mRNA was standardized against GAPDH mRNA, and the mRNA levels were expressed as ratios compared to the mean value obtained for vehicle (DMSO)-treated cells. Specific probe sets for Cdx2, MUC2 and GAPDH were also obtained from PE Applied Biosystems.
Western blotting
RGM-1 cells were routinely cultured and lysed on ice after harvesting in a lysis buffer containing 0.5% NP-40, 40 mM Tris HCl (pH 8.0), 120 mM NaCl and a protease inhibitor cocktail (Complete Mini, Pierce, Rockford, IL). The protein concentration of the lysate was measured using a modified bicinchoninic acid method (Pierce). The proteins were denatured with SDS sample buffer and subjected to SDS-PAGE. The separated proteins were then transferred onto an Immun-Blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked in TBS-T buffer (10 mM Tris HCl, pH 7.5, 100 mM NaCl, 0.1% Tween-20) containing 5% skim-milk and these were then incubated in a 1:250 dilution of a mouse anti-Cdx2 or a rabbit anti-MUC2 antibody (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. Subsequently, the membranes were washed in TBS and incubated with the appropriate HRP-conjugated secondary antibody before washing again in TBS. The reactive bands were visualized using enhanced chemiluminescence (Amersham, Arlington Heights, IL) in accordance with the manufacturer’s instructions.
Immunofluorescence
RGM-1 cells were fixed in 4% paraformaldehyde for 20 min and fixed with cold acetone for 5 min. The cells were immunostained using a primary rabbit polyclonal anti-FXR antibody (1:100 dilution; Santa Cruz Biotechnology, Inc.) followed by a secondary donkey anti-rabbit IgG antibody conjugated to Alexa Fluor-488 (1:200 dilution; Invitrogen). Then slides were then examined using a Nikon epifluorescence microscope (Tokyo, Japan).
Statistical analysis
Values are the means ± standard deviation. A one-way analysis of variance was used to test for significant differences among the treatment group means, and results were examined using a Fisher’s protected least-significant-difference test. Differences with p values less than 0.05 were considered significant.
Results and Discussion
Effect of CDCA on the expression of Cdx2 and MUC2 in RGM-1 cells
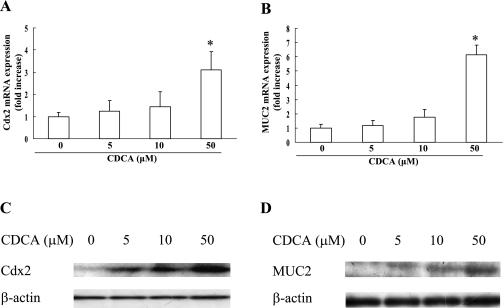
CDCA (0 to 50 µM) increased the expression level of Cdx2 and MUC2 mRNA in a dose-dependent fashion (Fig. 1A and B). Therefore, we used CDCA at a dose of 50 µM in this study. A dose-dependent upregulation of Cdx2 and MUC2 protein expression by CDCA was also observed as assessed by Western blotting (Fig. 1C and D).
Fig. 1.
Effect of CDCA on the expression of Cdx2 and MUC2 in RGM-1 cells. RGM-1 cells treated with CDCA at doses ranging from 0 to 50 µM for 3 h or 6 h were subjected to measurement of expression level of mRNA for Cdx2 (A) or MUC2 (B) by real-time RT-PCR, respectively. n = 3, * p<0.01 compared with controls (vehicle-treated group). (C and D) Western blot analysis of Cdx2 (C) and MUC2 (D) in RGM-1 cells treated CDCA at doses ranging from 0 to 50 µM.
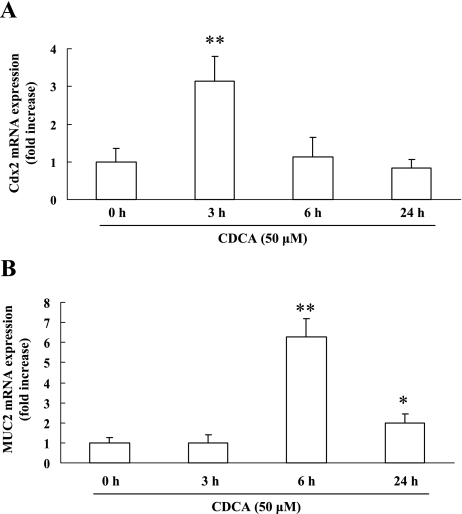
The maximum effect of CDCA on the mRNA expression levels observed for Cdx2 and MUC2 occurred at 3 h and 6 h, respectively (Fig. 2A and B). Because CDCA did not affect MUC2 mRNA expression by 3 h, we suspect that the increase in Cdx2 expression induced by CDCA may be related to the upregulation of MUC2 expression in RGM-1 cells.
Fig. 2.
Time-course of Cdx2 and MUC2 mRNA expression in RGM-1 cells stimulated with CDCA. RGM-1 cells were treated with 50 µM CDCA for 24 h, and the expression levels of Cdx2 (A) and MUC2 (B) mRNA were evaluated by real-time RT-PCR. n = 3. * p<0.05, ** p<0.01 compared with controls (vehicle-treated group).
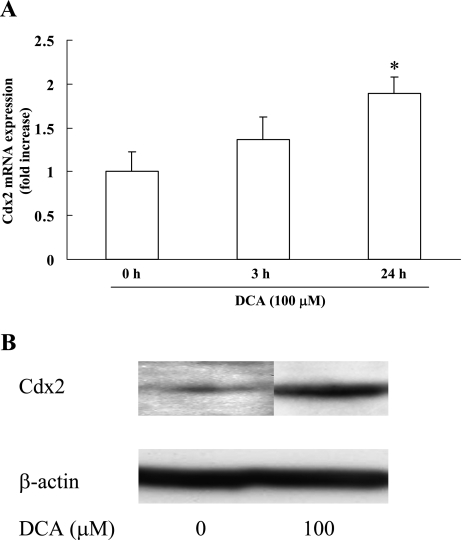
We also investigated the effect of DCA on Cdx2 expression in RGM-1 cells. Similar to CDCA, DCA induced Cdx2 expression at both mRNA and protein levels (Fig. 3A and B).
Fig. 3.
Effect of DCA on the expression of Cdx2 in RGM-1 cells. RGM-1 cells were treated with 100 µM DCA and were subjected to measurement of expression levels of Cdx2 by real-time RT-PCR (A) and Western blot analysis (B). n = 3. * p<0.05 compared with controls (vehicle-treated group).
To investigate the mechanism by which bile acids might induce intestinal metaplasia in the stomach, we performed in vitro studies using normal gastric cells (RGM-1 cells), instead of gastric cancer cells. We observed that CDCA markedly upregulated the expression of both Cdx2 and MUC2. Together with the finding that DCA also induced Cdx2 expression in RGM-1 cells, these results suggest that bile acids likely cause gastric intestinal metaplasia by inducing the ectopic expression of Cdx2 in normal epithelial cells.
Expression of the FXR in RGM-1 cells
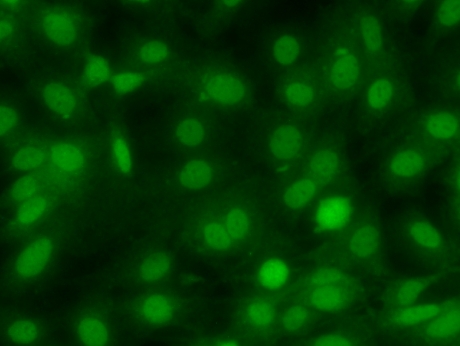
During normal physiological conditions, the expression of the FXR is generally thought to be limited to the liver, intestine, kidney and adrenal glands [21, 22]. Zhang et al., [23] however, reported that the FXR is also expressed in the stomach. But because there are few reports documenting the expression of the FXR in the stomach, we investigated whether RGM-1 cells expressed the FXR. As shown in Fig. 4, immunofluorescence staining clearly demonstrated the nuclear localization of the FXR in these cells.
Fig. 4.
Expression of the FXR in RGM-1 cells. The expression of the FXR was assessed by immunofluorescence.
Involvement of the FXR in the CDCA-induced upregulation of Cdx2 and MUC2
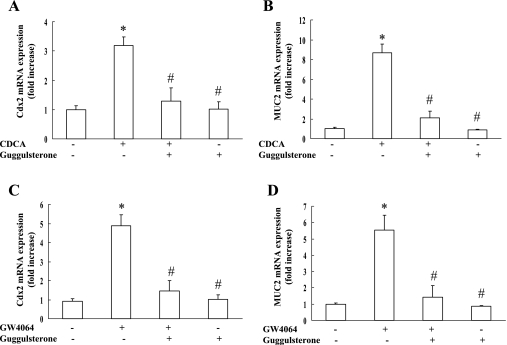
Next, we investigated the effect of guggulsterone, an FXR antagonist, on the expression of Cdx2 and MUC2 in RGM-1 cells stimulated by CDCA. As shown in Fig. 5A and B, the effect of CDCA stimulation on the expression of Cdx2 and MUC2 mRNA was completely abolished by guggulsterone. Guggulsterone alone did not affect the expression levels of these molecules.
Fig. 5.
Effect of FXR agonists on the expression of Cdx2 and MUC2 in the presence or absence of guggulsterone, an FXR antagonist. RGM-1 cells were treated with FXR agonists (50 µM CDCA and GW4064) in the presence or absence of guggulsterone, an FXR antagonist, for 3 h or 6 h and the levels of Cdx2 (A and C) or MUC2 (B and D) were examined. n = 3. * p<0.05 compared with controls (vehicle-treated group), # p<0.01 compared with CDCA group (A and B) or GW4064 group (C and D).
To confirm involvement of the FXR in upregulating the expression of Cdx2 and MUC2, RGM-1 cells were stimulated with GW4064, an FXR agonist. As expected, GW4064 increased the expression of Cdx2 and MUC2 mRNA by 4.9-fold and 5.5-fold, respectively (Fig. 5C and D). The effect of GW4064 on the expression of these molecules was also abolished by guggulsterone. These results suggest that the activation of the FXR may cause the ectopic expression of Cdx2 and MUC2 in the gastric mucosa, and that CDCA may be a potent activator of the FXR in the stomach.
While the main function of the FXR is to prevent intracellular bile acid overload and toxicity by acting as a bile acid sensor that stimulates bile acid export and absorption [16–18], other studies suggested that the FXR performs a pro-inflammatory role in the liver [24] and esophagus [25]. We demonstrated that the FXR directly upregulates the expression of Cdx2 for the first time. Our findings suggest that the FXR may be a key molecule involved in the intestinal metaplasia of the stomach. Bile acid reflux into the stomach may therefore, cause gastric carcinogenesis by activating the FXR, a nuclear bile acid receptor.
The activation of epidermal growth factor receptors (EGFR) is also important for gastric intestinal carcinogenesis [26] and several reports suggest that bile acids activate EGFR in gastrointestinal cells [27, 28]. Yasuda et al. [29] reported that deoxycholate rapidly activated EGFR through a membrane-type bile acid receptor-dependent mechanism in AGS cells, a human gastric adenocarcinoma cell line. These results suggest that bile acids may promote gastric carcinogenesis through the activation of both nuclear and membrane receptors.
In conclusion, bile acids may induce an ectopic expression of Cdx2 and MUC2 by activating the FXR in gastric epithelial cells. This activation may play an important role in the induction of intestinal metaplasia and gastric carcinogenesis by bile acids.
Acknowledgments
This study was supported by the Japan China Medical Association and the Nippon Foundation, and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat. Rev. Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil R.A., Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J. Gastroenterol. Hepatol. 2009;24:193–201. doi: 10.1111/j.1440-1746.2008.05774.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki M., Suzuki H., Hibi T. Proton pump inhibitors and gastritis. J. Clin. Biochem. Nutr. 2008;42:71–75. doi: 10.3164/jcbn.2008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh E., Traber P.G. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol. Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesquita P., Jonckheere N., Almeida R., Ducourouble M.P., Serpa J., Silva E., Pigny P., Silva F.S., Reis C., Silberg D., Van Seuningen I., David L. Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J. Biol. Chem. 2003;278:51549–51556. doi: 10.1074/jbc.M309019200. [DOI] [PubMed] [Google Scholar]
- 6.Mizoshita T., Inada K., Tsukamoto T., Kodera Y., Yamamura Y., Hirai T., Kato T., Joh T., Itoh M., Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa—with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric. Cancer. 2001;4:185–191. doi: 10.1007/pl00011741. [DOI] [PubMed] [Google Scholar]
- 7.Satoh K., Mutoh H., Eda A., Yanaka I., Osawa H., Honda S., Kawata H., Kihira K., Sugano K. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter. 2002;7:192–198. doi: 10.1046/j.1523-5378.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 8.Seno H., Oshima M., Taniguchi M.A., Usami K., Ishikawa T.O., Chiba T., Taketo M.M. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int. J. Oncol. 2002;21:769–774. doi: 10.3892/ijo.21.4.769. [DOI] [PubMed] [Google Scholar]
- 9.Mutoh H., Hakamata Y., Sato K., Eda A., Yanaka I., Honda S., Osawa H., Kaneko Y., Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem. Biophys. Res. Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 10.Silberg D.G., Sullivan J., Kang E., Swain G.P., Moffett J., Sund N.J., Sackett S.D., Kaestner K.H. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 11.Sobala G.M., O’Connor H.J., Dewar E.P., King R.F., Axon A.T., Dixon M.F. Bile reflux and intestinal metaplasia in gastric mucosa. J. Clin. Pathol. 1993;46:235–240. doi: 10.1136/jcp.46.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein H., Bernstein C., Payne C.M., Dvorakova K., Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Dixon M.F., Neville P.M., Mapstone N.P., Moayyedi P., Axon A.T. Bile reflux gastritis and Barrett’s oesophagus: further evidence of a role for duodenogastro-oesophageal reflux? Gut. 2001;49:359–363. doi: 10.1136/gut.49.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song S., Guha S., Liu K., Buttar N.S., Bresalier R.S. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett’s oesophagus and oesophageal adenocarcinoma. Gut. 2007;56:1512–1521. doi: 10.1136/gut.2007.121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y., Jones C., Gellersen O., Williams V.A., Watson T.J., Peters J.H. Pathogenesis of Barrett esophagus: deoxycholic acid up-regulates goblet-specific gene MUC2 in concert with CDX2 in human esophageal cells. Arch. Surg. 2007;142:540–544. doi: 10.1001/archsurg.142.6.540. discussion 544–545, [DOI] [PubMed] [Google Scholar]
- 16.Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 17.Parks D.J., Blanchard S.G., Bledsoe R.K., Chandra G., Consler T.G., Kliewer S.A., Stimmel J.B., Willson T.M., Zavacki A.M., Moore D.D., Lehmann J.M. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 18.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 19.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa T., Watanabe T., Higuchi K., Machida H., Okazaki H., Yamagami H., Watanabe K., Tominaga K., Fujiwara Y., Oshitani N., Arakawa T. Lansoprazole, a Proton Pump Inhibitor, Suppresses Production of Tumor Necrosis Factor-alpha and Interleukin-1beta Induced by Lipopolysaccharide and Helicobacter Pylori Bacterial Components in Human Monocytic Cells via Inhibition of Activation of Nuclear Factor-kappaB and Extracellular Signal-Regulated Kinase. J. Clin. Biochem. Nutr. 2009;45:86–92. doi: 10.3164/jcbn.08-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman B.M., Goode E., Chen J., Oro A.E., Bradley D.J., Perlmann T., Noonan D.J., Burka L.T., McMorris T., Lamph W.W., Evans R.M., Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 22.Lu T.T., Repa J.J., Mangelsdorf D.J. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J. Biol. Chem. 2001;276:37735–37738. doi: 10.1074/jbc.R100035200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Kast-Woelbern H.R., Edwards P.A. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J. Biol. Chem. 2003;278:104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 24.Qin P., Borges-Marcucci L.A., Evans M.J., Harnish D.C. Bile acid signaling through FXR induces intracellular adhesion molecule-1 expression in mouse liver and human hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G267–273. doi: 10.1152/ajpgi.00043.2005. [DOI] [PubMed] [Google Scholar]
- 25.Capello A., Moons L.M., Van de Winkel A., Siersema P.D., van Dekken H., Kuipers E.J., Kusters J.G. Bile acid-stimulated expression of the farnesoid X receptor enhances the immune response in Barrett esophagus. Am. J. Gastroenterol. 2008;103:1510–1516. doi: 10.1111/j.1572-0241.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts R.B., Min L., Washington M.K., Olsen S.J., Settle S.H., Coffey R.J., Threadgill D.W. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng K., Raufman J.P. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem. Pharmacol. 2005;70:1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Werneburg N.W., Yoon J.H., Higuchi H., Gores G.J. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G31–36. doi: 10.1152/ajpgi.00536.2002. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H., Hirata S., Inoue K., Mashima H., Ohnishi H., Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem. Biophys. Res. Commun. 2007;354:154–159. doi: 10.1016/j.bbrc.2006.12.168. [DOI] [PubMed] [Google Scholar]