Abstract
Epigallocatechin gallate (EGCG), a green tea polyphenol, promotes vasodilation by phosphatidylinositol 3-kinase-dependent activation of Akt and endothelial nitric oxide synthase to stimulate production of nitric oxide. Reduction in endothelin-1 (ET-1) synthesis may also increase bioavailability of nitric oxide. We hypothesized that the phosphatidylinositol 3-kinase-dependent transcription factor FOXO1 may mediate effects of EGCG to regulate expression of ET-1 in endothelial cells. EGCG treatment (10 μm, 8 h) of human aortic endothelial cells reduced expression of ET-1 mRNA, protein, and ET-1 secretion. We identified a putative FOXO binding domain in the human ET-1 promoter 51 bp upstream from the transcription start site. Trans-activation of a human ET-1 (hET-1) promoter luciferase reporter was enhanced by coexpression of a constitutively nuclear FOXO1 mutant, whereas expression of a mutant FOXO1 with disrupted DNA binding domain did not trans-activate the hET-1 promoter. Disrupting the hET-1 putative FOXO binding domain by site-directed mutagenesis ablated promoter activity in response to overexpression of wild-type FOXO1. EGCG stimulated time-dependent phosphorylation of Akt (S473), FOXO1 (at Akt phosphorylation site T24), and AMP-activated protein kinase α (AMPKα) (T172). EGCG-induced nuclear exclusion of FOXO1, FOXO1 binding to the hET-1 promoter, and reduction of ET-1 expression was partially inhibited by the AMPK inhibitor Compound C. Basal ET-1 protein expression was enhanced by short interfering RNA knock-down of Akt and reduced by short interfering RNA knock-down of FOXO1 or adenovirus-mediated expression of dominant-negative Foxo1. We conclude that EGCG decreases ET-1 expression and secretion from endothelial cells, in part, via Akt- and AMPK-stimulated FOXO1 regulation of the ET-1 promoter. These findings may be relevant to beneficial cardiovascular actions of green tea.
Epigallocatechin gallate decreases ET-1 expression and secretion from endothelial cells, in part, via Akt- and AMPK-stimulated FOXO1 regulation of the ET-1 promoter that may be relevant to beneficial cardiovascular actions of green tea.
Green tea is a functional food whose consumption is linked to reduced cardiovascular morbidity and mortality in epidemiological studies (1). The bioactive polyphenol epigallocatechin gallate (EGCG) is the most abundant catechin in green tea constituting roughly 30% of green tea solids (2). EGCG has a number of biochemical, cellular, and physiological actions relevant to diseases characterized by reciprocal relationships between endothelial dysfunction and insulin resistance including diabetes, metabolic syndrome, and their vascular complications (3,4). For example, in hepatocytes, EGCG inhibits gluconeogenesis through activation of phosphatidylinositol 3-kinase- (PI3K) and/or AMP-activated protein kinase (AMPK)-dependent pathways (5,6). In vascular endothelial cells, we recently reported that EGCG acutely stimulates production of nitric oxide (NO) using signaling pathways that require activation of Fyn/PI3K/Akt/endothelial nitric oxide synthase (eNOS) resulting in vasodilation (7). Moreover, NO-dependent vasodilator actions of EGCG are implicated in effects of chronic EGCG treatment (3 wk) to simultaneously improve endothelial dysfunction, lower blood pressure, reduce insulin resistance, and protect against myocardial ischemia/reperfusion injury in the spontaneously hypertensive rat (SHR) (8). Beneficial effects of ACE-inhibitors and/or insulin sensitizers on cardiovascular and metabolic phenotypes of SHR may be mediated by both enhanced production of NO as well as reduction in elevated levels of the vasoconstrictor endothelin-1 (ET-1) (9). Endothelial dysfunction is linked to elevated levels of ET-1 (10,11) that oppose vasodilator actions of NO. ET-1 may also play a role in decreasing NO bioavailability (12,13). When ET-1 is overexpressed in transgenic mice, increases in blood pressure are seen only when endothelial nitric oxide synthase (eNOS) is knocked out (14). It is unknown whether beneficial actions of EGCG in endothelium involve reducing expression of vasoconstrictors, including ET-1, that contribute to endothelial dysfunction.
The forkhead box O family of transcription factors plays an important role in vascular homeostasis. Mice lacking Foxo1 die in utero from improper development of the vasculature (15), and FOXO1 integrates various cell signals at the transcriptional level that are relevant to endothelial function (16,17,18,19,20). Akt and AMPK both phosphorylate FOXO1 to help regulate its transcriptional function (21,22,23). Expression of ET-1 is regulated primarily at the level of transcription, and a host of responsive elements have been described within the ET-1 promoter. In combination with constant degradation of the ET-1 mRNA, this provides exquisite and acute regulation of ET-1 synthesis (reviewed in Ref. 24). Although we (25) and others (26) have described insulin-stimulated ET-1 secretion, we have also demonstrated that PI3K signaling may act to inhibit ET-1 expression (27). Therefore, we hypothesized that EGCG treatment may reduce ET-1 expression via FOXO1. This may represent an additional mechanism for EGCG to improve endothelial function and a generalizable mechanism for regulation of ET-1 synthesis and secretion that is relevant to other hormones with vasoactive actions in vascular endothelium.
Materials and Methods
Cell culture models
Primary bovine and human aortic endothelial cells (BAEC and HAEC, Cell Applications, San Diego, CA) were cultured as previously described (7,27). NIH-3T3 fibroblasts stably overexpressing human insulin receptor (NIH 3T3IR) were cultured as described (28). All cells were cultured at 37 C in a humidified atmosphere with 5% CO2.
Differential mRNA analysis and quantitative real-time PCR
HAECs were serum-deprived 2 h before EGCG treatment (10 μm, 8 h). RNA was isolated using Totally RNA and Turbo-Dnase kits from Ambion, Inc. (Austin, TX). RNA from cells treated without or with EGCG was subjected to differential gene analysis using the GeneFishing kit from Seegene (Seoul, South Korea). For quantitative real-time PCR analysis, RNA from BAEC and HAEC was isolated as described above. One microgram of total RNA was reverse transcribed into cDNA using the High-Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA). Quantitative real-time PCRs were performed using QuantiTect SYBR Green PCR Kit from QIAGEN (Valencia, CA) and analyzed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The data and calculation of ΔΔCt (cycle threshold) were analyzed using SDS 2.2 software from Applied Biosystems. Primers (all obtained from IDT, Coralville, IA) used for human ET-1 mRNA analysis were 5′-AAA CAG CAG TCT TAG GCG CTG A-3′ (forward) and 5′-GAC ACA CTC TTT ATC CAT CAG GGA C-3′ (reverse); for bovine ET-1 mRNA, primers used were 5′-AAG AAG TGT GTC TAC TTC TGC CAT CTG-3′ (forward) and 5′- AAA GAA GTC CTT TAA GGA GCG CT-3′ (reverse) (29); for human and bovine β-actin mRNA analysis, primers used were 5′-CTG GCA CCC AGC ACA ATG AAG-3′ (forward) and 5′-TAG AAG CAT TTG CGG TGG ACG-3′ (reverse). ET-1 mRNA expression was normalized to β-actin mRNA expression.
Measurement of ET-1 protein
The ET-1 ELISA kit from Assay Designs (Ann Arbor, MI) was used according to manufacturer’s instructions to measure ET-1 protein concentrations in conditioned cell culture media or whole cell lysates.
Plasmid constructs
The human ET-1 promoter luciferase reporter constructs as described in figure legends were a kind gift from Cam Patterson (University of North Carolina, Chapel Hill, NC) (30). Site-directed mutagenesis of the human ET-1 (hET-1) promoter luciferase reporter was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The primers used for mutagenesis were 5′-GGG GCT GGA ATA AAG TCG GAG CTG TcT ACC CCC ACT CTA-3′ and 5′-GAG TGG GGG TAg ACA GCT CCG ACT TTA TTC CAG CCC CAG-3′. This introduced a point-mutation (T→C) 55 bps upstream of the hET-1 start-site and within the putative FOXO-binding domain. The FOXO1 expression vectors for wild-type (FOXO1-WT), constitutively nuclear mutant (FOXO1-AAA), and mutant with disrupted DNA binding domain (FOXO1-DBD) have been described elsewhere (31,32).
Cell treatments and transfections of DNA vectors and short interfering RNA (siRNA)
EGCG [stored as a 50 mm stock in 50% dimethylsulfoxide (DMSO)] and Compound C (stored as a 5 mm stock in 100% DMSO) were obtained from Sigma (St. Louis, MO). BAECs and HAECs were serum- and supplement-deprived before treatment with EGCG as described in figure legends. NIH-3T3IR cells were transfected with Polyfect (QIAGEN) according to the manufacturer’s protocol in 24-well plates using 200 ng of luciferase reporter and FOXO1 expression vector DNA and 10 ng of renilla expression vector DNA per well. Measurement of luciferase and renilla activity in cells 48 h after transfection was performed using the Dual-Glo reporter assay system kits from Promega (Madison, WI). Nontargeting (NT), Akt1, and FOXO1 siRNA ON-TARGETplus SMARTpool oligos were obtained from Dharmacon (Lafayette, CO) and prepared in 20 μm stocks according to the manufacturer’s suggestions. HAECs were transfected with Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol in 60-mm plates at 120 nm siRNA per plate. After 48 h, HAECs were then serum-starved 2 h before vehicle (DMSO) or EGCG (10 μm) treatment for 6 h.
Chromatin immunoprecipitation (ChIP) assay
HAECs were serum-deprived 2 h before DMSO vehicle or Compound C treatment (5 μm, 30 min). Cells were then treated with or without EGCG (10 μm, 30 min). The EZ-Magna ChIP A ChIP Kit from Millipore (Billerica, MA) was used according to their protocol. The immunoprecipitating antibody was anti-FOXO1 (FKHR, H128) from Santa Cruz Biotechnology (Santa Cruz, CA). For negative controls, nonimmune rabbit IgG from Millipore was used. The primer sequences for PCR of the FOXO binding domain region of the hET-1 promoter were 5′-GCC TGT TGG TGA CTA ATA ACA C-3′ (forward) and 5′-AGC TCT CTG CCG GCT TTT TAT A-3′ (reverse) which produced a 108-bp PCR product. PCR products were visualized by separation on agarose gels (3.5%) stained with ethidium bromide and quantified by NIH Image J software (Bethesda, MD).
Fractionation of membrane/cytosolic and nuclear proteins
HAECs were serum-starved 2 h before treatment with Compound C (5 μm, 30 min). Cells were then treated with EGCG (10 μm, 30 min) before fractionation as described elsewhere (33). A protein assay (BCA Protein Assay, Thermo Scientific, Rockford, IL) was performed on the lysates to ensure equivalent loading for immunoblot analysis.
Immunoblotting
Cells were harvested in Laemmli buffer for analysis by SDS-PAGE. The following antibodies were from Cell Signaling Technology (Danvers, MA): phosphoserine 473-Akt, Akt, phosphothreonine 24-FOXO1 (FKHR), phosphothreonine 172-AMPKα, AMPKα, phosphoserine 79-ACC, ACC, histone H4, caspase 3, and HA. FOXO1 (FKHR) and green fluorescent protein (GFP) antibodies were from Santa Cruz Biotechnology. The β-actin antibody was from Sigma. Incubations with HRP-linked antirabbit secondary antibodies (Amersham Biosciences, Piscataway, NJ) were performed for 1 h at room temperature. Immunoblots were visualized using Lumiglo (Cell Signaling Technology) and exposure to film (Amersham Hyperfilm MP, Buckinghamshire, UK).
Adenovirus infection
GFP adenovirus was obtained from Vector Biolabs (Philadelphia, PA). The HA-tagged WT- and Δ256-Foxo1 adenoviral constructs (34) were a kind gift from Domenico Accili (Columbia University, New York, NY). Quantification of virus particles was calculated using the QuickTiter Adenovirus Quantitation Kit (Cell Biolabs, Inc., San Diego, CA). HAECs were infected with 5 × 107 virus particles per 60-mm plate (for ET-1 ELISA) and 3 × 109 virus particles per chamber slide for immunocytochemistry (higher titers were required for detection with the anti-HA antibody). Experiments were performed 24 h after infection.
Immunocytochemistry
Following adenovirus infection to express HA-tagged WT-Foxo1, HAECs (cultured in chamber slides) were serum-starved 2 h, then treated with or without Compound C (5 μm) for 30 min, and finally treated with or without EGCG (10 μm) for an additional 30 min. The cells were then fixed with 2% paraformaldehyde for 10 min at room temperature. Cells were then washed in PBS containing 0.1% (vol/vol) Triton X-100 (PBST) and blocked in 10% mouse serum (Jackson ImmunoResearch, West Grove, PA) in PBST. HAECs were incubated overnight at 4 C in PBST containing anti-HA antibody conjugated to Alexa Fluor 488 (Cell Signaling Technology) at a dilution of 1:25. Immunofluorescence in cells was visualized using an Olympus IX81 inverted microscope with an attached charge-coupled device camera (Retiga Exi, Burnaby, British Columbia, Canada) using appropriate filters. Images were captured using IP Labs Software (Scanalytics, Inc., Fairfax, VA). When scoring Foxo1-infected HAECs, random images of the cells (four images per chamber slide) were scored by individuals who were unaware of treatment conditions. Only HA-positive cells were scored as either positive for cytoplasmic immunoreactivity, nuclear, or perinuclear (or no nuclear) immunoreactivity.
Statistics
Data are reported as mean ± sem. Statistical analyses were performed using the Student’s t test or ANOVA as described in the legends to the figures. Differences were considered significant at P < 0.05.
Results
Treatment of endothelial cells with EGCG reduces expression of ET-1 mRNA and protein, and impairs secretion of ET-1
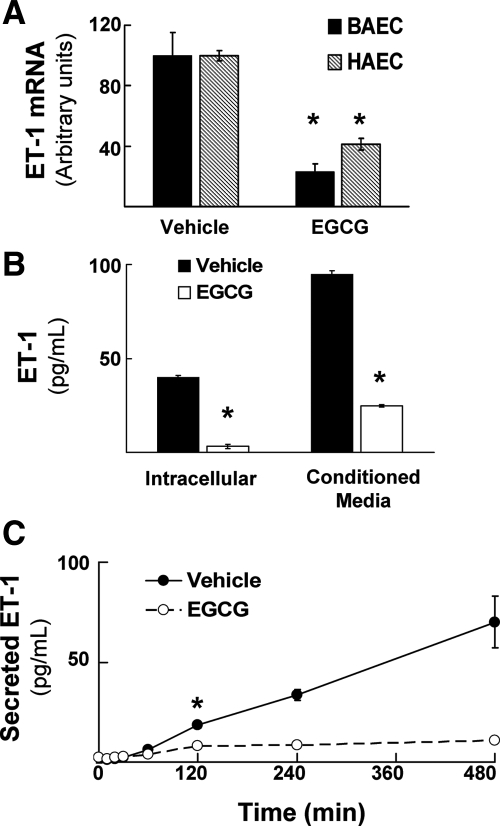
To identify novel biological actions of green tea polyphenols in vascular endothelium, we treated primary endothelial cells without or with EGCG (10 μm, 8 h) and performed differential mRNA analyses to discover genes that may be regulated by EGCG (GeneFishing kit from Seegene; see Materials and Methods). This concentration of EGCG used was chosen based on preliminary experiments showing that higher doses of EGCG reduced HAEC survival after 8 h (data not shown) and our previous observations that 10 μm EGCG is a minimal dose that still stimulates eNOS phosphorylation (7). This initial screening method suggested that EGCG treatment reduced ET-1 mRNA (data not shown). We confirmed these exploratory results using quantitative RT-PCR analysis of BAEC and HAEC treated without or with EGCG (10 μm, 8 h). EGCG treatment significantly reduced expression of ET-1 mRNA by more than approximately 60% in both BAEC and HAEC when compared with vehicle-treated control cells (Fig. 1A). Moreover, EGCG treatment of HAEC (Fig. 1B) and BAEC (data not shown) markedly reduced both intracellular ET-1 protein and ET-1 secreted from cells into conditioned media. In conditioned media from vehicle-treated control cells, there was a linear increase in ET-1 concentration of approximately 10-fold over 8 h. By contrast, in endothelial cells treated with EGCG, we observed nearly complete suppression of ET-1 secretion (Fig. 1C). Together, these results suggest that ET-1 mRNA, synthesis, and secretion in vascular endothelial cells are all potently inhibited by treatment with the green tea polyphenol EGCG.
Figure 1.
Treatment of vascular endothelial cells with EGCG reduces expression of ET-1 mRNA and protein. A, BAECs and HAECs were serum-starved overnight and then treated with vehicle (DMSO) or EGCG (10 μm, 8 h). Quantitative RT-PCR was then performed as described in Materials and Methods to evaluate expression of ET-1 mRNA in endothelial cells. Results shown are mean ± sem of six independent experiments normalized to β-actin mRNA expression. EGCG treatment significantly decreased expression of ET-1 mRNA when compared with vehicle-treated cells (*, P < 0.001 for BAEC and HAEC, by Student’s t test). B, HAECs were treated as in panel A, and then ET-1 protein concentration was measured by ELISA in cell lysates or in conditioned media as described in Materials and Methods. Results shown are the mean ± sem of three independent experiments. EGCG treatment caused a significant reduction in the concentration of ET-1 protein both intracellularly as well as in conditioned media when compared with vehicle-treated cells (*, P < 0.0005 for cell lysates and conditioned media measurements, by Student’s t test). C, BAECs were treated as in panel A and ET-1 protein concentration in conditioned media was determined as in panel B at the indicated times. Results are expressed as the mean ± sem of determinations performed in triplicate (*, P ≤ 0.003 at 120 min and beyond when compared with vehicle-treated cells; P = 0.06 at 60 min by Student’s t test).
Identification and characterization of a FOXO1 regulatory site in the hET-1 promoter and inhibition of promoter activity by EGCG
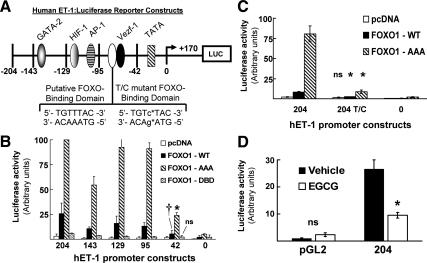
We next investigated potential mechanisms whereby EGCG may regulate expression of ET-1 mRNA in vascular endothelial cells. A number of transcription factors including GATA-2, HIF-1, AP-1, and Vezf-1 have previously been implicated in the regulation of ET-1 promoter activity (Fig. 2A) (24,30). Upon inspection of the sequence of the hET-1 promoter, we identified a region located 51 bps upstream from the transcription start site with a sequence 5′-TGTTTAC-3′ that matches a consensus binding site for FOXO1 (35). FOXO1 is a known downstream target of Akt, a kinase that we previously demonstrated is activated by EGCG treatment of primary endothelial cells (7). Therefore, we evaluated whether the hET-1 promoter is a novel target for direct regulation by FOXO1. In NIH-3T3IR cells transfected with the hET-1 promoter luciferase reporter (or by a series of deletion mutants of the hET-1 promoter), basal ET-1 promoter activity in cells cotransfected with the control empty expression vector was minimal (Fig. 2B, open bars). Overexpression of WT FOXO1 resulted in significant trans-activation of the 204-bp hET-1 promoter reporter. Moreover, this trans-activation was increased another 4-fold when a constitutively nuclear FOXO1 mutant (FOXO1-AAA missing three Akt phosphorylation sites; Ser24, Ser256, and Ser319 mutated to Ala) was cotransfected along with the hET-1 promoter reporter (Fig. 2B). Conversely, expression of a mutant FOXO1 with a disrupted DNA binding domain (FOXO1-DBD; His215 mutated to Arg) was unable to significantly trans-activate the hET-1 promoter reporter. We observed similar results using serially truncated deletion mutants of the 204-bp hET-1 promoter reporter that resulted in loss of GATA-2, HIF-1, and AP-1 binding sites (down to 95-bp promoter sequence). Importantly, with the deletion mutant of the hET-1 promoter containing only 42 bp (missing putative FOXO1 binding region), greatly reduced trans-activation of the reporter was observed with overexpression of WT FOXO1 (when compared with full-length promoter construct). Moreover, trans-activation of this 42-bp reporter in response to expression of FOXO1-AAA was substantially and significantly diminished when compared with results using reporters containing 204, 143, 129, or 95 bp of the hET-1 promoter sequence (Fig. 2B). A point mutant of the 204-bp hET-1 promoter reporter disrupting the putative FOXO1 binding motif (204 T/C; Fig. 2A) was unresponsive to coexpression of WT FOXO1 (Fig. 2C). Finally, activation of the 204 T/C mutant hET-1 promoter by coexpression of FOXO1-AAA was severely impaired when compared with the WT 204-bp reporter (Fig. 2C). Thus, we have identified a novel FOXO1 binding sequence on the hET-1 promoter that is responsive to FOXO1 regulation.
Figure 2.
FOXO1 drives activity of the hET-1 promoter through a specific FOXO1 DNA binding domain. A, Schematic representation of a 204-bp segment of the hET-1 promoter luciferase reporter construct with serial 5′ deletions at the indicated sites. Known binding sites of various transcription factors are indicated (GATA-2, HIF-1, AP-1, and Vezf-1). A putative FOXO-binding domain (pFBD) is located 51 bps upstream of the transcription start site. A point mutant of the 204-bp ET-1 reporter was created as indicated to disrupt the FOXO-binding domain. B, NIH-3T3IR cells were cotransfected with various hET-1-luciferase reporter constructs and expression vectors for either WT or mutant FOXO1 (pcDNA is an empty expression vector used as a negative control). Luciferase activity was measured as described in Materials and Methods. FOXO1-AAA is a constitutively nuclear mutant (*, P < 0.001 by ANOVA and Student-Newman-Keuls post-test when compared with −204, −143, −129, and −95 vectors). FOXO1-DBD is a mutant with a disrupted DNA binding domain (ns, not significantly different from other luciferase vectors). The ET-1 promoter reporter that is truncated to 42 bps is missing the pFBD (†, P < 0.05 by ANOVA and Student-Newman-Keuls post-test when compared with −204, −143, −129, and −95 vectors for WT FOXO1 cotransfections). C, NIH-3T3IR cells were cotransfected with the 204-bp hET-1-luciferase reporter containing either WT sequence or a mutation in the pFBD TGTcTAC (204 T/C) and WT or constitutively nuclear FOXO1 (pcDNA is an empty expression vector used as a negative control). FOXO1-AAA- and FOXO1-WT-induced activity of the WT hET-1 promoter was significantly greater than activity induced in the hET-1 promoter with the disrupted putative FOXO1 binding motif (*, P < 0.0005 by Student’s t test for both FOXO1-AAA and FOXO1-WT in 204 T/C vs. 204 transfected cells). D, HAECs were transiently transfected with the 204-bp hET-1 promoter reporter (as shown in panel A) or the promoterless pGL2 vector (Promega). Cells were treated with vehicle (DMSO) or EGCG (10 μm) 24 h posttransfection. Luciferase activity (normalized to renilla lucifierase) was measured 18 h later (*, P = 0.008 by Student’s t test comparing vehicle-treated vs. EGCG-treated HAECs with the 204-bp reporter; n = 3 per condition).
Next, in HAECs transfected with the luciferase reporter construct driven by 204 bp of the hET-1 promoter, EGCG treatment (10 μm, 18 h) significantly reduced ET-1 promoter activity when compared with DMSO-treated control cells (Fig. 2D). Together, these data suggest that EGCG reduces activity of the hET-1 promoter, and this contributes to a reduction of ET-1 mRNA and protein expression (Fig. 1).
EGCG stimulates phosphorylation of Akt, AMPK, ACC, and FOXO1 in endothelial cells
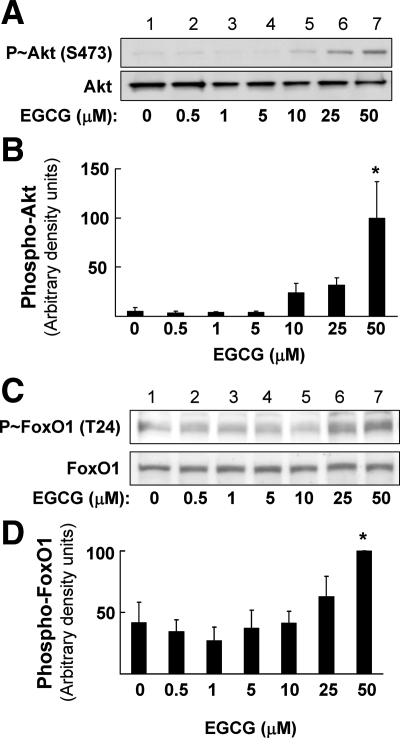
We previously demonstrated that EGCG has endothelium-dependent vasodilator actions mediated by activation of a signaling pathway involving PI 3-kinase/Akt/eNOS that leads to increased production of NO (7,8). Because FOXO1 may directly bind to and regulate the ET-1 promoter (Fig. 2), we examined the ability of EGCG to stimulate phosphorylation of Akt and AMPK, two upstream kinases that regulate FOXO1 action through phosphorylation (21,22,23). Using BAECs, we performed experiments in which cells were serum-starved overnight followed by treatment with varying concentrations of EGCG (ranging from 0.5 to 50 μm) for 10 min (Fig. 3). Phosphorylation of Akt (Ser473) (Fig. 3, A and B) and FoxO1 (Thr24) (Fig. 3, C and D) increased in response to EGCG treatment in a concentration-dependent manner (determined by immunoblotting with phospho-specific antibodies).
Figure 3.
EGCG increases Akt (Ser473) and FoxO1 (Thr24) phosphorylation in a dose-dependent manner. BAECs were serum-starved overnight and then treated for 10 min with the indicated doses of EGCG. Cell lysates were then analyzed my immunobloting with phospho- and nonphospho-specific antibodies. A, Representative immunoblot of Akt phosphorylation stimulated with the indicated doses of EGCG. B, Quantification (mean ± sem) of multiple independent experiments shown in panel A (*, P < 0.01 by ANOVA and Dunnett’s posttest). C, Representative immunoblot of FoxO1 phosphorylation stimulated with the indicated doses of EGCG. D, Quantification (mean ± sem) of multiple independent experiments shown in panel C (*, P < 0.05 by ANOVA and Dunnett’s posttest).
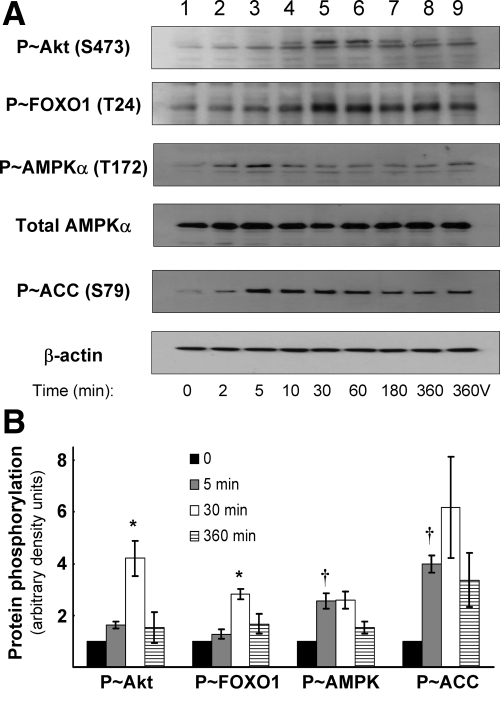
To examine the time course of phosphorylation, HAECs were serum starved for 2 h, followed by EGCG treatment (10 μm) for various times. Concentrations of EGCG greater than 10 μm were toxic to the cells over a 6-h time course (data not shown). As we previously reported (7), EGCG stimulated phosphorylation of Akt at Ser473 in a time-dependent manner (maximal stimulation at 30 min; Fig. 4 A, panel 1, B, open bars). FOXO1 phosphorylation at Thr24 (an Akt phosphorylation site) was also stimulated by EGCG treatment in a time-dependent manner that mirrored the time course of Akt phosphorylation (Fig. 4, A, panel 2, and B, open bars). EGCG also acutely stimulated phosphorylation of AMPKα at Thr172 (maximal stimulation by 5 min; Fig. 4, A, panel 3, and B, gray bars). Phosphorylation of ACC at Ser79 (an AMPK target) in response to EGCG stimulation was also maximal after 5 min (Fig. 4, A, panel 5, and B, gray bars). Together, our data suggest that EGCG acutely stimulates phosphoyrylation (and presumably activation) of Akt and AMPK. These upstream kinases may then phosphorylate their substrate FOXO1 in endothelial cells.
Figure 4.
EGCG stimulates phosphorylation of Akt, AMPK, and FOXO1 in vascular endothelial cells. HAECs were serum-starved for 2 h followed by treatment with EGCG (10 μm) for the indicated times. As a control, some cells were treated with vehicle for 360 min (360V). A, Cells lysates were immunoblotted with antibodies against phosphorylated and nonphosphorylated proteins. Total AMPK and β-actin expression remained constant throughout the experiment and served as loading controls. Images shown are representative of experiments that were repeated independently three times. B, Quantification of the immunoblot analysis in part A. Basal (time 0) phosphorylation is arbitrarily set to 1 for each protein. Phospho-Akt (S473) and phospho-FOXO1 (T24; Akt phosphorylation site) were maximally phosphorylated after 30 min (lane 5 in panel A; *, P < 0.05 by ANOVA and Dunnett’s posttest). Phospho-AMPKα (T172) and phospho-ACC (S79; AMPK phosphorylation site) were maximally stimulated after 5 min (lane 3 in panel A; †, P < 0.05 by ANOVA and Dunnett’s posttest).
Endogenous FOXO1 directly binds to the hET-1 promoter to regulate its activity
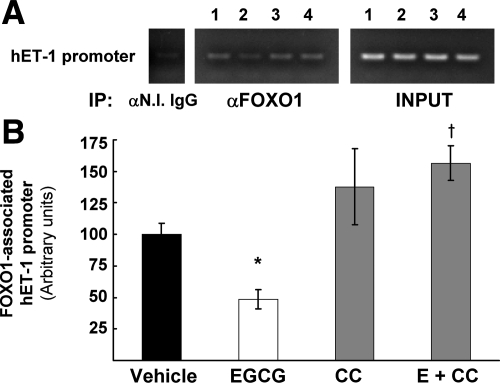
Because FOXO1 is phosphorylated in response to EGCG treatment of primary endothelial cells, we next examined interactions between endogenous FOXO1 and the endogenous ET-1 promoter in primary human endothelial cells using a ChIP assay. EGCG treatment of HAEC (10 μm, 30 min) decreased the amount of FOXO1 bound to the ET-1 promoter by approximately 54% (Fig. 5, A and B). We also assessed the role of AMPK by using a specific inhibitor. Pretreatment with Compound C (5 μm, 30 min) slightly increased FOXO1 association with the hET-1 promoter, but importantly, Compound C blocked the effect of EGCG to reduce FOXO1 binding to the hET-1 promoter compared with EGCG treatment alone (Fig. 5, A, lane 4, and B). Therefore, our data suggest that EGCG activates an intracellular signaling cascade in which FOXO1 is phosphorylated and dissociated from the hET-1 promoter in conjunction with reduced ET-1 expression.
Figure 5.
EGCG reduces endogenous FOXO1 association with the endogenous hET-1 promoter and decreases overall promoter activity. A, ChIP assay was performed on HAECs as described in Materials and Methods after treatment with vehicle alone (lane 1), EGCG (lane 2), Compound C (lane 3), or EGCG with pretreatment with Compound C (lane 4). In immunoprecipitated (IP) samples with FOXO1 antibodies, the amount of PCR product encompassing the putative FOXO1 binding domain on the hET-1 promoter was reduced following EGCG treatment. Pretreatment with Compound C blocked the effects of EGCG. The images are from the same gel and are representative of at least three independent experiments. B, Quantification of the PCR reactions was performed by normalizing the intensity of the FOXO1 immunopricipitated band of the hET-1 promoter with its respective input band using NIH Image J software. The vehicle controls were arbitrarily set to 100% for each experiment (mean ± sem). EGCG (E) treatment reduced the amount of hET-1 promoter associated with FOXO1 by 51%, and the effect of EGCG was blocked by pretreatment with the AMPK inhibitor Compound C (CC) (*, P = 0.028 and †, P = 0.021 by two-way ANOVA).
AMPK plays a role in nuclear exclusion of FOXO1 and the regulation of ET-1 mRNA and protein expression by EGCG
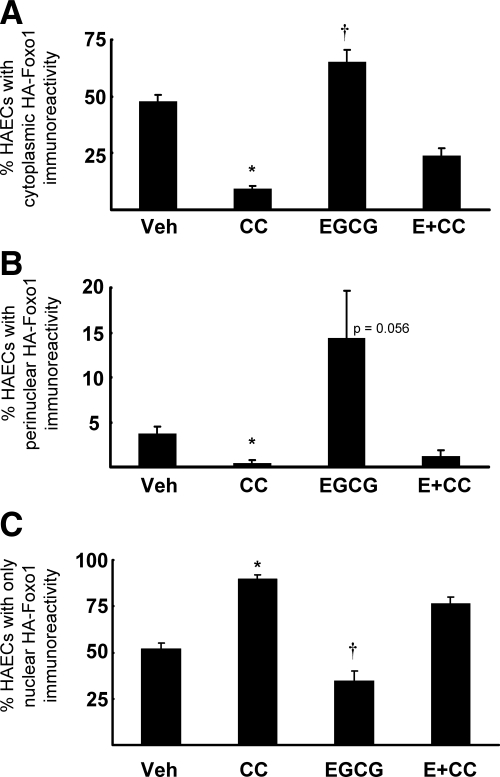
We used a specific inhibitor of AMPK (Compound C) to evaluate the role of AMPK in modulating translocation of FOXO1 from the nucleus into the cytoplasm in vascular endothelial cells in response to EGCG treatment. HAECs infected with adenoviral constructs to express HA-tagged Foxo1 were pretreated with vehicle control or Compound C for 30 min followed by treatment without or with EGCG (10 μm, 30 min; Fig. 6 and supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Cells were then fixed and incubated with a fluorescently labeled anti-HA antibody to detect Foxo1 localization (supplemental Fig. S1). Images from each of the control and experimental conditions were scored in a blinded fashion (as described in Materials and Methods and supplemental Fig. S1) to quantify the subcellular localization of HA-WT-Foxo1. The quantification of these results from multiple indepdendent experiments is shown in Fig. 6, A–C. When compared with vehicle pretreated control cells in the absence of EGCG treatment, pretreatment with Compound C resulted in a significantly higher fraction of cells with nuclear Foxo1 localization (Fig. 6C; supplemental Fig. S1, panel 4) and less Foxo1 in the cytoplasm (Fig. 6A) or perinuclear region (Fig. 6B). EGCG treatment in vehicle pretreated cells increased both cytoplasmic and perinuclear Foxo1 localization (Fig. 6, A and B; supplemental Fig. S1, panel 6) consistent with increased FOXO1 phosphorylation shown in Figs. 3 and 4. When cells were pretreated with Compound C, the ability of EGCG to promote cytoplasmic localization of Foxo1 was significantly impaired (Fig. 6, A and B; supplemental Fig. S1, panel 8). Together with data in Figs. 4 and 5, these results strongly suggest AMPK plays an important role in the ability of EGCG to inhibit expression of ET-1 through phosphorylation, and subsequent translocation, of FOXO1 in vascular endothelial cells.
Figure 6.
Inhibition of AMPK increases nuclear localization of FOXO1 and impairs effects of EGCG (E) to cause nuclear translocation of FOXO1. Bar graphs represent the mean ± sem of four independent experiments. A, Cells were scored in a blinded fashion based on the appearance of cytoplasmic HA immunoreactivity (see supplemental Fig. S1). Compound C (CC) pretreatment reduced basal and EGCG-stimulated HA-Foxo1 in the cytoplasm (*, P < 0.0001 by two-way ANOVA), whereas EGCG treatment increased cytoplasmic HA immunoreactivity (†, P = 0.0009 by two-way ANOVA). B, Cells were scored based on the appearance of perinuclear HA-WT-Foxo1. EGCG treatment tended to increase perinuclear Foxo1 localization (P = 0.056 by two-way ANOVA), whereas pretreatment of cells with Compound C almost completely blocked perinuclear localization of Foxo1 and substantially inhibited the effect of EGCG to increase perinuclear localization of Foxo1 (*, P = 0.01 by two-way ANOVA). C, Cells were scored based on the appearance of nuclear HA-WT-Foxo1 localization. Compound C pretreatment significantly increased nuclear Foxo1 localization in the absence or presence of EGCG treatment, whereas EGCG treatment alone significantly reduced nuclear Foxo1 localization when compared with vehicle-treated (Veh) cells (*, P < 0.0001 and †, P = 0.0012 by two-way ANOVA for Compound C and EGCG treatments, respectively).
To further confirm that inhibition of AMPK by Compound C can block the nuclear-to-cytoplasmic translocation of FOXO1, we performed subcellular fractionation and immunoblot analysis of endogenous FOXO1 in HAECs. Cells were serum-starved and treated with Compound C and EGCG as in Fig. 6. With EGCG treatment alone, FOXO1 in the nuclear-enriched (Triton X-100-insoluble) fraction is reduced (supplemental Fig. S2A, lanes 5 and 6, supplemental Fig. S2B). Pretreatment with Compound C increases FOXO1 appearance in the nuclear-enriched (Triton X-100-insoluble) fraction (supplemental Fig. S2A, lane 7, and supplemental Fig. S2B). Together with Fig. 6, these data add further support to the notion that EGCG promotes FOXO1 phosphorylation and translocation from the nucleus to the cytoplasm in HAECs.
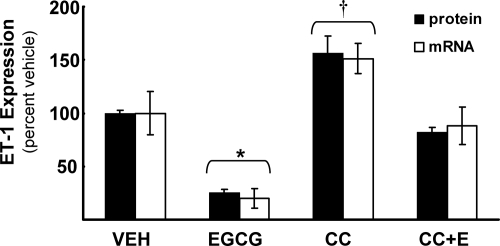
We next used Compound C to help evaluate the role of AMPK in EGCG-stimulated inhibition of ET-1 mRNA and protein (Fig. 7). In agreement with data presented in Fig. 1, EGCG significantly reduced ET-1 mRNA and protein expression in HAECs. Inhibition of AMPK activity with Compound C increased expression of both ET-1 mRNA and protein even in the absence of EGCG treatment (when compared with vehicle treated-control cells). Importantly, pretreatment of HAECs with Compound C significantly blunted the effects of EGCG to reduce expression of ET-1 mRNA and protein (not significantly different from vehicle-treated cells). Thus, AMPK plays a role in the actions of EGCG to inhibit ET-1 expression that is likely mediated (at least in part) through FOXO1.
Figure 7.
Compound C impairs the inhibitory actions of EGCG on ET-1 mRNA expression and ET-1 secretion. HAECs were serum-starved for 2 h, pretreated without or with the AMPK inhibitor Compound C (5 μm, 30 min), and then treated without or with EGCG (10 μm, 6 h). ET-1 mRNA expression and ET-1 secretion was significantly reduced following EGCG treatment when compared with all other treatment groups (*, P = 0.0019 and P < 0.0001 by two-way ANOVA for mRNA and protein, respectively). Compound C blocked the effect of EGCG while significantly increasing ET-1 mRNA and protein expression (†, P = 0.0055 and P < 0.0001 by two-way ANOVA for mRNA and protein, respectively). The bar graph represents the mean ± sem of at least three independent experiments.
Akt and FOXO1 regulate basal ET-1 secretion in vascular endothelial cells
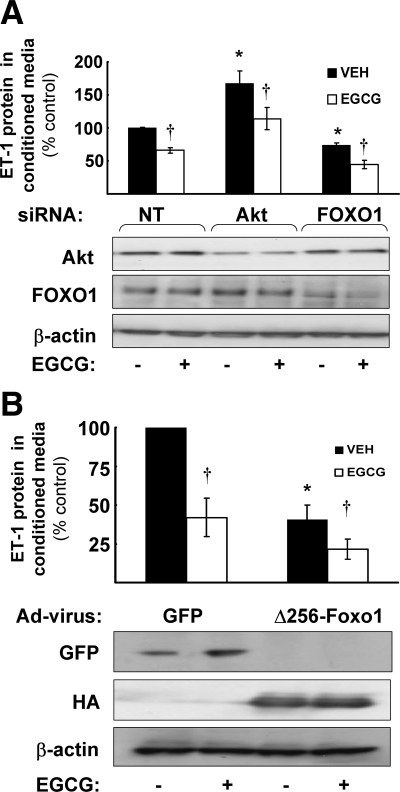
To further explore the role of Akt and FOXO1 in regulation of ET-1 secretion, we reduced Akt or FOXO1 expression by transfecting HAECs with specific siRNA oligos (Fig. 8A). A NT siRNA oligo that has four or more mismatches with all human RNA sequences was used as a negative control. After cells were transfected with siRNA to reduce expression of Akt1 or FOXO1, they were then serum-starved for 2 h before treatment without or with EGCG (10 μm, 6 h). EGCG treatment of control NT-transfected cells resulted in reduced ET-1 secretion into conditioned media consistent with results shown in Fig. 1. Knockdown of FOXO1 resulted in reduced secretion of ET-1 in the basal state (absence of EGCG stimulation) when compared with control cells in the basal state (Fig. 8A). Knockdown of Akt resulted in increased secretion of ET-1 in the basal state (absence of EGCG stimulation) when compared with control cells in the basal state. Reduction of Akt is likely to reduce FOXO1 phosphorylation resulting in a relative increase in nuclear localization of FOXO1. Together, these data suggest that FOXO1 plays a role to promote basal ET-1 secretion in vascular endothelium and that this function of FOXO1 is regulated by its upstream kinase Akt. Interestingly, EGCG treatment of cells with knockdown of FOXO1 or Akt resulted in further reduction of ET-1 secretion (when compared with transfected cells not treated with EGCG). This suggests that Akt/FOXO1 interactions do not explain all of the regulation of ET-1 secretion by EGCG. Indeed, we obtained similar results when we infected HAEC with an adenoviral construct expressing a dominant-negative mutant of FOXO1 (Δ256-Foxo1) to inhibit endogenous FOXO1 function (Fig. 8B). The Δ256-Foxo1 mutant retains the DNA-binding portion of the protein, but lacks trans-activating capabilities (34). In control cells infected with GFP, we observed a significant reduction in ET-1 secretion in response to EGCG treatment. When compared with control cells in the basal state, ET-1 secretion was reduced by expression of Δ256-Foxo1 (in the absence of EGCG treatment). Nevertheless, EGCG treatment of cells expressing Δ256-Foxo1 was still able to reduce ET-1 secretion even further (Fig. 8B).
Figure 8.
siRNA knockdown of Akt and FOXO1 or expression of a dominant negative Foxo1 mutant in endothelial cells alters ET-1 levels in conditioned media. Results shown in bar graphs are the mean ± sem of at least four independent experiments. A, HAECs were transfected with siRNA to reduce expression of Akt1 or FOXO1 as described in Materials and Methods. A NT siRNA was used as a negative control. In the absence of EGCG treatment, when compared with conditioned media from vehicle-treated control cells transfected with NT, conditioned media from vehicle-treated cells transfected with Akt1 siRNA or FOXO1 siRNA had significantly increased (67%) or decreased (27%) ET-1 levels, respectively (*, P < 0.0001 by two-way ANOVA). EGCG treatment reduced ET-1 expression in all three siRNA transfection groups (†, P < 0.0001 by two-way ANOVA). Representative immunoblots demonstrate appropriate knock-down of Akt1 and FOXO1 protein by respective siRNA constructs. B, HAECs were infected with adenoviral constructs to express GFP (control) or a dominant-negative Foxo1 mutant (HA-tagged Δ256-Foxo1) and then treated with vehicle or EGCG as described in Materials and Methods. In conditioned media from vehicle-treated (VEH) cells expressing Δ256-Foxo1, ET-1 levels were decreased by 60% when compared with vehicle-treated control cells (*, P = 0.025 by two-way ANOVA). EGCG treatment reduced ET-1 expression in both GFP and Δ256-Foxo1 infected cells (†, P = 0.017 by two-way ANOVA). Representative immunoblots demonstrate expression of appropriate adenoviral constructs.
Discussion
Putative beneficial effects of green tea consumption to improve cardiovascular health (1) may be mediated, in part, by the ability of EGCG to stimulate production of NO from vascular endothelium resulting in vasodilation (7). It is likely that acute PI3K/Akt-dependent vasodilator actions of EGCG contribute to chronic effects of EGCG treatment to simultaneously improve the cardiovascular and metabolic phenotype of SHR rats (8). However, additional mechanisms by which EGCG improves endothelial dysfunction may also exist. In the present study, we focused on the hypothesis that EGCG may inhibit production of ET-1 in endothelial cells.
EGCG reduces expression and secretion of ET-1 from endothelial cells
We demonstrated that treatment of primary endothelial cells with EGCG significantly decreased ET-1 mRNA, protein, and protein secretion. Thus, beneficial effects of EGCG treatment to improve endothelial dysfunction may be mediated by decreased ET-1 levels in addition to increased NO production. Our findings are relevant to understanding potential therapeutic actions of EGCG in diseases characterized by reciprocal relationships between insulin resistance and endothelial dysfunction. In endothelial cells, insulin stimulates both production of NO by PI3K/Akt-depedent pathways and secretion of ET-1 via MAPK-dependent pathways (36,37,38). Insulin resistance with pathway-selective impairment in PI3K-dependent pathways causes an imbalance with decreased NO production and increased ET-1 secretion in response to compensatory hyperinsulinemia that accompanies insulin resistance (4,39,40). EGCG treatment has the potential to restore balance between NO production and ET-1 secretion, because it both enhances production of NO and inhibits secretion of ET-1. Furthermore, reduced ET-1 expression may also directly increase NO bioavailability. Reports by Lteif et al. (12) and Mather et al. (13) suggest that endogenous ET-1 in obese humans contributes to reduced NO production and decreased glucose disposal. Long-term ET-1 exposure disrupts insulin-stimulated IRS-1/PI3K/Akt signaling resulting in lower glucose uptake in adipocytes (41). ET-1-stimulated PKC activity may also contribute to insulin resistance (42). Therefore, ET-1 signaling may disrupt the IRS-1/PI3K/Akt pathway in skeletal muscle and vascular endothelium that drives substrate metabolism and vasodilation, respectively.
The hET-1 promoter is a novel target for trans-activation by FOXO1
By inspection, we observed that the hET-1 promoter contains a consensus binding site for FOXO1 51 bps upstream from the transcription start site (35). In the present study, we validated this location as a bona fide binding site for FOXO1 in the hET-1 promoter. Overexpression of WT FOXO1 trans-activated the ET-1 promoter, and this was even more pronounced with expression of a constitutively-nuclear FOXO1 mutant. Disruption of the putative FOXO1 binding motif on the ET-1 promoter by deletion or point mutation abrogated the ability of the ET-1 promoter to be trans-activated by a constitutively nuclear FOXO1 mutant. A FOXO1 mutant with disrupted DNA binding domain was unable to trans-activate the ET-1 promoter. Moreover, siRNA knockdown of FOXO1 or expression of a dominant-negative Foxo1 reduced basal ET-1 expression in human vascular endothelial cells. Finally, EGCG treatment of endothelial cells resulted in decreased ET-1 promoter activity and decreased association between endogenous FOXO1 and endogenous ET-1 promoter. Together, these results establish a novel role for FOXO1 in vascular endothelium to regulate the ET-1 gene. Moreover, our results provide an additional mechanism for EGCG to improve endothelial function. That is, in addition to stimulating production of NO through PI3K/Akt/eNOS, EGCG may also inhibit ET-1 expression and secretion through FOXO1. Thus, in endothelial cells, EGCG treatment may simultaneously increase NO production and decrease ET-1 secretion leading to an improvement in endothelial function and, potentially, insulin action (4). Our results in endothelial cells are consistent with previous findings that EGCG induces FOXO1 phosphorylation in HEK 293 cells (43), and reduces ET-1 expression in ovarian cancer cells (44). It is important to note that primary endothelial cells are more sensitive to EGCG than HEK 293 cells, because they respond to lower doses of EGCG (10 μm) that increase FoxO1 phosphorylation more acutely (<30 min). Finally, the molecular mechanism for EGCG-induced ET-1 down-regulation in primary endothelial cells described here may also be generalizable to other compounds or conditions that promote phosphorylation of FOXO1 in endothelial cells (16,18,19,21,45).
EGCG activates AMPK and Akt to regulate FOXO1 and modulate ET-1 expression
One of the primary mechanisms for regulation of FOXO1 function is phosphorylation by upstream kinases including Akt and AMPK that subsequently cause translocation of FOXO1 from the nucleus to the cytoplasm (21,22,23). We previously demonstrated that EGCG treatment phosphorylates and activates Akt in endothelial cells (7) and AMPK in hepatocytes (5). In the present study, we showed that EGCG treatment of endothelial cells causes phosphorylation of both Akt and AMPK in a time and dose-dependent manner that is accompanied by phosphorylation of their substrates FOXO1 and ACC at their Akt and AMPK phosphorylation sites, respectively. This is associated with translocation of FOXO1 from the nucleus to the cytoplasm. Using compound C to inhibit AMPK activity resulted in increased nuclear localization of FOXO1 and impairment in the ability to EGCG to promote translocation of FOXO1 from the nucleus to the cytoplasm. Pretreatment with compound C significantly increased basal ET-1 mRNA and protein expression and blunted the effect of EGCG to reduce ET-1 mRNA, protein, and FOXO1-association with the hET-1 promoter. Thus, one mechanism for EGCG to reduce ET-1 synthesis and secretion is likely to involve phosphorylation of FOXO1 by AMPK. In other studies, AMPK alters FOXO function to regulate life-span (46) and response to fluid-shear stress (21). AMPK may also mediate some effects on ET-1 through the Krüppel-like factor 2 transcription factor (47). In skeletal muscle, AMPK stimulates glucose uptake (48), whereas in the endothelium, AMPK activates eNOS by phosphorylating serine 1177 (49). Metformin, may promote beneficial effects to improve endothelial dysfunction and insulin resistance, in part, by activating AMPK, and thus, eNOS (50). Results from our study suggest that down-regulation of ET-1 may also play a role in the beneficial metabolic and vascular actions of metformin.
Both Akt signaling (51) and FOXO1 gene regulation (15,20,52) have previously been implicated in maintaining vascular homeostasis, and both are activated or inhibited, respectively, by insulin. Insulin, however, creates a net increase in ET-1 expression (26), but EGCG has distinct signaling characteristics from insulin by activating Fyn upstream of PI3K/Akt and not the insulin receptor (7). Furthermore, we have demonstrated that stimulation of ET-1 secretion in response to DHEA and insulin is MAPK-dependent, whereas inhibition of ET-1 secretion in response to DHEA and insulin is PI3K-dependent (25,27). Therefore, PI3K/Akt and MEK/MAPK pathways maintain a physiological balance that act together to control net ET-1 expression and secretion. In the present study, we focused on effects of EGCG on the PI3K/Akt pathway and the downstream transcription factor, FOXO1. Using siRNA to knockdown Akt increased basal expression of ET-1 protein, whereas siRNA knockdown of FOXO1 or expression of a dominant inhibitory mutant of FOXO1 significantly reduced basal expression of ET-1 protein. These results are consistent with the concept that Akt is upstream of FOXO1 and that phosphorylation of FOXO1 by Akt may down-regulate ET-1 expression. However, even with Akt or FOXO1-knock-down or inhibition, there was still a residual effect of EGCG to further reduce ET-1 expression. This may be due to residual endogenous Akt or FOXO1, the presence of AMPK, or other unidentified regulatory factors that regulate ET-1 expression in response to EGCG.
In summary, we report multiple novel findings. First, EGCG has a robust effect to reduce ET-1 synthesis and secretion in vascular endothelial cells. Second, we have identified a novel bona fide FOXO1 binding site in the hET-1 promoter. Third, EGCG regulates ET-1 synthesis and secretion, in part, through AMPK- and Akt-mediated phosphorylation of their downstream target FOXO1 that results in dissociation of FOXO1 from the hET-1 promoter and nuclear exclusion of FOXO1. The reduction of ET-1 expression by EGCG in endothelial cells may contribute to the reduced cardiovascular morbidity and mortality associated with drinking green tea. Moreover, our results provide a generalizable mechanism for Akt, AMPK, and FOXO1 to contribute to the complex regulation of ET-1 and overall vascular homeostasis.
Supplementary Material
Acknowledgments
We thank Domenico Accili for the gift of Foxo1 adenoviral constructs and Cam Patterson for the gift of hET-1 promoter reporter constructs.
Footnotes
This work was supported by the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2009
Abbreviations: AMPK, AMP-activated protein kinase; BAEC, bovine aortic endothelial cell; ChIP, chromatin immunoprecipitation; DMSO, dimethylsulfoxide; EGCG, Epigallocatechin gallate; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; GFP, green fluorescent protein; HAEC, human aortic endothelial cell; hET-1, human ET-1; NO, nitric oxide; NT, nontargeting; PI3K, phosphatidylinositol 3-kinase; SHR, spontaneously hypertensive rat; siRNA, short interfering RNA; WT, wild type.
References
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I 2006 Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296:1255–1265 [DOI] [PubMed] [Google Scholar]
- Graham HN 1992 Green tea composition, consumption, and polyphenol chemistry. Prev Med 21:334–350 [DOI] [PubMed] [Google Scholar]
- Cheng TO 2006 All teas are not created equal: the Chinese green tea and cardiovascular health. Int J Cardiol 108:301–308 [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ 2006 Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904 [DOI] [PubMed] [Google Scholar]
- Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W 2007 Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282:30143–30149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK 2002 Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277:34933–34940 [DOI] [PubMed] [Google Scholar]
- Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ 2007 Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282:13736–13745 [DOI] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M 2007 EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292:E1378–E1387 [DOI] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M 2006 Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55:3594–3603 [DOI] [PubMed] [Google Scholar]
- Marasciulo FL, Montagnani M, Potenza MA 2006 Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13:1655–1665 [DOI] [PubMed] [Google Scholar]
- Schneider MP, Hilgers KF, Klingbeil AU, John S, Veelken R, Schmieder RE 2000 Plasma endothelin is increased in early essential hypertension. Am J Hypertens 13:579–585 [DOI] [PubMed] [Google Scholar]
- Lteif A, Vaishnava P, Baron AD, Mather KJ 2007 Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 56:728–734 [DOI] [PubMed] [Google Scholar]
- Mather KJ, Lteif A, Steinberg HO, Baron AD 2004 Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes 53:2060–2066 [DOI] [PubMed] [Google Scholar]
- Quaschning T, Voss F, Relle K, Kalk P, Vignon-Zellweger N, Pfab T, Bauer C, Theilig F, Bachmann S, Kraemer-Guth A, Wanner C, Theuring F, Galle J, Hocher B 2007 Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol 18:730–740 [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N 2004 Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem 279:34741–34749 [DOI] [PubMed] [Google Scholar]
- Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A 2007 Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett 581:673–680 [DOI] [PubMed] [Google Scholar]
- Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS 2004 Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 18:1060–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I, Keseru B, Walsh K, Busse R 2007 Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res 100:e12–e21 [DOI] [PubMed] [Google Scholar]
- Potente M, Fisslthaler B, Busse R, Fleming I 2003 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem 278:29619–29625 [DOI] [PubMed] [Google Scholar]
- Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S 2005 Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115:2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit M, Bess E, Fisslthaler B, Härtel FV, Noll T, Busse R, Fleming I 2008 Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 77:160–168 [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P 1999 Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274:17179–17183 [DOI] [PubMed] [Google Scholar]
- Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P 2002 Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J 21:2263–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawji IA, Marsden PA 2003 Perturbations in paracrine control of the circulation: role of the endothelial-derived vasomediators, endothelin-1 and nitric oxide. Microsc Res Tech 60:46–58 [DOI] [PubMed] [Google Scholar]
- Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ 2006 Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol 20:1153–1163 [DOI] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, Quertermous T, King GL 1991 Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem 266:23251–23256 [PubMed] [Google Scholar]
- Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ 2008 Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem 283:29228–29238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Yeh DC, Ver M, Li Y, Carranza A, Conrads TP, Veenstra TD, Harrington MA, Quon MJ 2005 Phosphorylation of ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by mouse pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J Biol Chem 280:23173–23183 [DOI] [PubMed] [Google Scholar]
- Sethi AS, Lees DM, Douthwaite JA, Dawnay AB, Corder R 2006 Homocysteine-induced endothelin-1 release is dependent on hyperglycaemia and reactive oxygen species production in bovine aortic endothelial cells. J Vasc Res 43:175–183 [DOI] [PubMed] [Google Scholar]
- Aitsebaomo J, Kingsley-Kallesen ML, Wu Y, Quertermous T, Patterson C 2001 Vezf1/DB1 is an endothelial cell-specific transcription factor that regulates expression of the endothelin-1 promoter. J Biol Chem 276:39197–39205 [DOI] [PubMed] [Google Scholar]
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E 2006 FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem 281:19881–19891 [DOI] [PubMed] [Google Scholar]
- Tang ED, Nuñez G, Barr FG, Guan KL 1999 Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274:16741–16746 [DOI] [PubMed] [Google Scholar]
- Li Y, Bor YC, Misawa Y, Xue Y, Rekosh D, Hammarskjöld ML 2006 An intron with a constitutive transport element is retained in a Tap messenger RNA. Nature 443:234–237 [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Silver DL, Accili D 2001 The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N 2000 Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ 2000 Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545 [DOI] [PubMed] [Google Scholar]
- Zeng G, Quon MJ 1996 Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98:894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M 2005 Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289:H813–H822 [DOI] [PubMed] [Google Scholar]
- Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B 2002 Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277:1794–1799 [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Iantorno M, Quon MJ 2008 An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am 37:685–711, ix–x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi KI, Imamura T, Sharma PM, Huang J, Ugi S, Olefsky JM 2001 Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J Clin Invest 107:1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Zhou QL, Chatterjee A, Feener EP, Myers Jr MG, White MF, King GL 1999 Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes 48:1120–1130 [DOI] [PubMed] [Google Scholar]
- Anton S, Melville L, Rena G 2007 Epigallocatechin gallate (EGCG) mimics insulin action on the transcription factor FOXO1a and elicits cellular responses in the presence and absence of insulin. Cell Signal 19:378–383 [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Decandia S, Albini A, Nicotra MR, Natali PG, Bagnato A 2006 Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol Cancer Ther 5:1483–1492 [DOI] [PubMed] [Google Scholar]
- Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, Fukamizu A, Lauro R, Federici M 2006 Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes 55:2231–2237 [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A 2007 An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17:1646–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G 2009 Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol 29:1902–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW 1997 AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273:E1107–E1112 [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE 1999 AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH 2006 Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505 [DOI] [PubMed] [Google Scholar]
- Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK 2006 Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest 116:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA 2007 FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128:309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.