Abstract
Recent work in sheep has identified a neuronal subpopulation in the arcuate nucleus that coexpresses kisspeptin, neurokinin B, and dynorphin (referred to here as KNDy cells) and that mediate the negative feedback influence of progesterone on GnRH secretion. We hypothesized that sex differences in progesterone negative feedback are due to sexual dimorphism of KNDy cells and compared neuropeptide and progesterone receptor immunoreactivity in this subpopulation between male and female sheep. In addition, because sex differences in progesterone negative feedback and neurokinin B are due to the influence of testosterone (T) during fetal life, we determined whether prenatal T exposure would mimic sex differences in KNDy cells. Adult rams had nearly half the number of kisspeptin, neurokinin B, dynorphin, and progesterone receptor-positive cells in the arcuate nucleus as did females, but the percentage of KNDy cells colocalizing progesterone receptors remained high in both sexes. Prenatal T treatment also reduced the number of dynorphin, neurokinin B, and progesterone receptor-positive cells in the female arcuate nucleus; however, the number of kisspeptin cells remained high and at levels comparable to control females. Thus, sex differences in kisspeptin in the arcuate nucleus, unlike that of dynorphin and neurokinin B, are not due solely to exposure to prenatal T, suggesting the existence of different critical periods for multiple peptides coexpressed within the same neuron. In addition, the imbalance between inhibitory (dynorphin) and stimulatory (kisspeptin) neuropeptides in this subpopulation provides a potential explanation for the decreased ability of progesterone to inhibit GnRH neurons in prenatal T-treated ewes.
Prenatal hormone exposure leads to an imbalance of neuropeptides in the adult brain that may be responsible for reproductive disease.
A central question in reproductive neuroendocrinology remains the precise identity of the specific sets of neurons and afferent pathways responsible for gonadal hormone feedback control of GnRH secretion. The temporal patterning of GnRH secretion into hypophyseal portal blood, and its subsequent control of LH secretion from the pituitary, is under the control of positive and negative feedback actions of gonadal steroid hormones progesterone (P4), estradiol (E2), and testosterone (T) (1,2). Of particular importance is P4 because it acts as a major inhibitory brake by suppressing pulsatile GnRH secretion during the luteal phase of the estrous cycle as well as blocking the ability of E2 to produce a preovulatory GnRH surge (1,2). Furthermore, the lack of nuclear P4 receptors (PR) in GnRH neurons (3) suggests that the influence of P4 is likely carried out by the afferent inputs to GnRH neurons.
Among the candidate afferent systems to GnRH neurons, a body of evidence, primarily using the sheep as a model, has accumulated suggesting that a subset of arcuate nucleus (ARC) neurons expressing the endogenous opioid peptide dynorphin (DYN) are critical mediators for P4 negative feedback (4,5,6). This evidence includes findings that 1) a very high percentage (>95%) of DYN cells in the ARC colocalize PR (4), 2) DYN cells provide direct synaptic input to GnRH neurons in the mediobasal hypothalamus (MBH) (4), 3) ovariectomy decreases pro-DYN mRNA levels in the ARC and DYN peptide levels in third ventricle cerebrospinal fluid (6), and 4) local administration in the MBH of antagonists to the κ-opioid receptor, that with the highest affinity to DYN, blocks the ability of P4 to inhibit LH pulse frequency during the luteal phase of the estrous cycle (5). Thus, DYN appears to act as an inhibitory brake on pulsatile GnRH secretion and mediates P4 negative feedback during the luteal phase of the estrous cycle (5).
In addition to the colocalization of PR with DYN, we have recently found that virtually all DYN cells in the ARC, but not elsewhere, coexpress two other peptides: the neurokinin B (NKB) (7) and kisspeptin (8). This colocalization is reciprocal, with an equally high percentage of NKB and kisspeptin cells that coexpress DYN (7,8). NKB is a tachykinin that has been implicated in steroid feedback control of GnRH secretion (9), although it is unclear whether it has stimulatory or inhibitory actions on GnRH neurons (10,11). Kisspeptin is one of a family of RFamide peptides and is now recognized as an extraordinarily potent stimulator of the GnRH neuroendocrine system in sheep and other mammals (12,13,14). Thus, this single ARC cell group coexpresses both stimulatory (kisspeptin) and inhibitory (DYN) neuropeptides, making alterations in the levels or release of each a potential mechanism for feedback control of GnRH neurons. In addition, the very high degree (>95%) of colocalization of this cell population with both E2 receptor-α (15) and PR (4) suggests that they are major targets for the actions of gonadal steroid hormones in the hypothalamus. Recent work has shown DYN and NKB to be colocalized in the rat (16), and complementary studies suggest that kisspeptin, NKB, and DYN may be also colocalized to a high degree in the ARC of the human (17), supporting the view that this neuropeptide subpopulation is conserved among mammals. For convenience, we have termed this cell group the KNDy subpopulation (coexpressing kisspeptin, NKB, and DYN).
NKB expression in the ARC has been shown to be sexually dimorphic in the sheep, with greater numbers of cells present in the female compared with the male (9). Similarly, the ability of P4 to inhibit GnRH pulses is also decreased in males compared with females (18). Given the role of DYN in mediating P4 negative feedback, we hypothesized that fewer DYN cells would be present in the ARC of males compared with females. In addition, because PR and kisspeptin are also present in KNDy neurons, and their expression has been shown to be sexually dimorphic in the hypothalami of rodents (19,20,21,22), we explored potential sex differences in their expression as well, predicting that fewer PR cells would be present in the male than female ARC. Finally, sex differences in NKB and responsiveness to P4 negative feedback in the sheep have been shown to be due to the organizational influence of prenatal T during fetal life (9,18). Therefore, we also tested the hypothesis that prenatal T exposure decreases the number of DYN, PR, and other neuropeptide-positive cells in prenatal T-treated female sheep compared with control animals. Surprisingly, we found that prenatal T treatment did not completely mimic the sex differences we observed in normal sheep, resulting in an imbalance of neuropeptide expression within the KNDy subpopulation.
Materials and Methods
Animals
Experiment 1: sex differences in normal adult female and male sheep
Adult, gonadally intact rams (n = 4) and ewes (n = 4, perfused during the midluteal phase of the estrous cycle) of Suffolk breed were used in this study. Animals were maintained in an open barn with a daily maintenance feeding and free access to water. They were moved indoors 2 d before the experiment. Once indoors, animals were housed in a light-dark cycle that mimicked the hours of daylight outdoors. Experimental designs and protocol were approved for this study by the Animal Use and Care Committee at the West Virginia University (Morgantown, WV). Animals were perfused during breeding season (October through January). Ewes had demonstrated at least two normal estrous cycles (16–17 d) before ovariectomy, as determined by monitoring either blood P4 level or estrous behavior with a vasectomized ram.
Experiment 2: sex differences in control and prenatal T-treated ewes
To examine the effects of prenatal T on the number of DYN, NKB, and kisspeptin cells, and their colocalization with PR, 2-yr-old control and prenatal T-treated ewes of Suffolk breed were used. Experimental procedures were approved by the University Animal Care and Use Committee at the University of Michigan (Ann Arbor, MI).
Pregnant ewes were administered twice weekly im injections of T propionate (100 mg/injection, catalog item T1875; Sigma-Aldrich, St. Louis, MO) suspended in cottonseed oil (catalog item C7767; Sigma-Aldrich) in the hind leg from 30–90 d of pregnancy (term = 147 d). The dose of T propionate administered results in levels of T in the female fetus comparable to those in fetal males (23). Control ewes received an equal volume of vehicle (2 ml cottonseed oil) in the same regimen as T.
Lambs were born in March and April. After weaning, they were maintained outdoors under natural photoperiods with a daily maintenance feeding and free access to water at the Sheep Research Facility of the University of Michigan (Ann Arbor, MI) until the age of 2 yr. Four prenatal T-treated ewes and five control, vehicle-treated ewes were used in this experiment. As part of studies to characterize the estrous cycles of these animals (24), ovarian cycles of all ewes were synchronized by two im injections of 10 mg prostaglandin F2 (Luteolyse; Pharmacia & Upjohn, Kalamazoo, MI) given 11 d apart. On d 11 from the second injection (midluteal phase of the estrous cycle), blood samples were collected every 20 min for 8 h by jugular venipuncture. These samples were used for P4 and LH RIAs (see below) to confirm that ewes were in the luteal phase and to examine expected differences in responsiveness to P4 negative feedback between control and prenatal T animals (18).
After estrous cycle characterization studies, animals were ovariectomized, and for 2 months before use, they were treated sequentially for 11–12 d with two controlled internal drug release P4 implants (CIDR) (InterAG, Hamilton, Waikato, New Zealand) and then for 2 d with four 3-cm-long E2 implants to simulate ovarian steroid levels during the normal cycle (Fig. 1). After the last E2 treatment, one 1-cm-long E2 implant and two CIDRs were inserted. The CIDRs were removed on d 6, one CIDR was inserted on d 10 and a second CIDR on d 13, and tissue was collected on d 16. Thus, the hormonal milieu of animals at the time of perfusion mimicked the midluteal phase of the estrous cycle. Animals were moved indoors 2 d before the experiment. Once indoors, animals were housed in an environment under a 12-h light,12-h dark cycle.
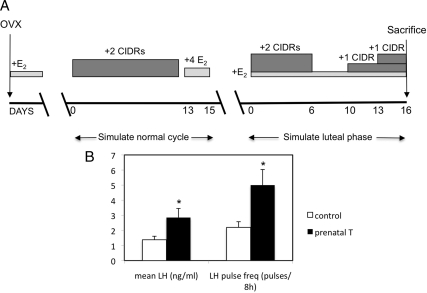
Figure 1.
A, Schema illustrating the design for experiment 2 of this study. Two-year-old control and prenatal T-treated ewes were ovariectomized (OVX) and over the following 2 months received an artificial hormonal regimen that simulated the normal estrous cycle and, just before killing, the midluteal phase of the estrous cycle. E2, One 1-cm-long E2 implant; 4 E2, four 3-cm-long E2 implants. See text for additional details. B, Mean LH (ng/ml) and LH pulse frequency (pulses/8 h) in control and prenatal T females taken from samples collected every 20 min during the midluteal phase of the estrous cycle before ovariectomy in control (n = 5) and prenatal T (n = 4) animals. Mean LH levels and pulse frequency were significantly higher in prenatal T animals than in controls. *, P < 0.05.
Tissue collection and preparation
On the day of killing, animals were received two iv injections of heparin (25,000 U given 10 min apart; catalog item 402588B; Abraxiz Pharmaceutical Products, Schumberry, IL) and then deeply anesthetized with sodium pentobarbital (2–3 g, iv, catalog item P3761; Sigma-Aldrich) and rapidly decapitated. The heads were perfused via both internal carotids with 6 liters 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.3) containing 0.1% sodium nitrate and 10 U/ml heparin. The brains were removed after perfusion, and a block of tissue containing the preoptic area and hypothalamus was dissected out. Tissues were placed in 4% paraformaldehyde in 0.1 m PB overnight for postfixation at 4 C and then transferred into 30% sucrose in 0.1 m PB for cryoprotection until the filtration was completed. Frozen coronal sections (50 μm) were cut using a freezing microtome (Microm HM400R, Walldorf, Germany) and stored at −20 C in a cryopreservative solution (25) until being processed for DYN, NKB, kisspeptin, and PR immunohistochemistry or immunofluorescent staining. Within each experiment, tissue sections from all experimental groups were processed simultaneously as described below.
RIA
LH concentrations were measured in duplicate 200-μl aliquots of serum by RIA as previously described (26) and expressed in terms of NIH-LH-S12. The sensitivity of the LH assay averages 0.44 ng/ml (mean ± sem; n = 2 assays). Mean intraassay coefficients of variation (CV) based on four quality control pools measuring 2.9, 7.7, 13.5, and 23.0 ng/ml averaged 11.5, 2.3, 9.7, and 5.3%, respectively. The corresponding interassay CV were 5.7, 10.2, 14.5, and 9.9%, respectively. Plasma P4 concentrations were measured in duplicate 100-μl aliquots using a solid-phase RIA kit (Coat-A-Count P; Diagnostic Products Corp., Los Angeles, CA) as previously described (27). The assay sensitivity of this assay averaged 0.23 ± 0.01 ng/ml (mean ± sem, n = 4 assays). Intraassay CV, based on three quality control pools measuring 0.15, 1.68, and 15.07 ng/ml average 6.02 ± 2.70, 3.53 ± 1.10, and 3.41 ± 0.81%, respectively. Corresponding interassay CV were 16.1, 4.4, and 3.3%, respectively.
Dual-label immunohistochemistry
An alternative series of every fourth section from each animal was processed for PR and either DYN, NKB, or kisspeptin using a modified dual-immunoperoxidase protocol (4) in which nuclear PR was first detected with nickel sulfate-enhanced diaminobenzidine as chromogen (blue-black reaction product), followed by detection of either cytoplasmic DYN, NKB, or kisspeptin using unenhanced diaminobenzidine (brown reaction product). All steps in this dual-immunoperoxidase procedure were performed at room temperature. Blocking solutions used consisted of 0.1 m PBS, 0.4% Triton X-100 (catalog item BP151-500; Sigma-Aldrich) containing 4% normal goat serum (catalog item 005-000-121; Jackson ImmunoResearch Laboratories, West Grove, PA). Unless otherwise specified, tissue sections were washed with 0.1 m PBS (pH 7.35) between steps.
Free-floating sections were washed thoroughly in PBS for several hours to remove the cryopreservative solution. Sections were then incubated with 1% hydrogen peroxide (H2O2; catalog item H325; Fisher Scientific, Pittsburgh, PA) for 10 min to eliminate the endogenous peroxidase activity. After several washes, sections were incubated in blocking solution for 1 h and then incubated with mouse monoclonal anti-PR antibody (catalog item 1408; Immunotech, Marseille, France) at a 1:50 dilution in blocking solutions for 40 h.
After incubation in primary antiserum, sections were washed and incubated with biotinylated goat antimouse IgG (1:500, catalog item BA-9200; Vector Laboratories, Burlingame, CA) for 1 h, followed by ABC reagent (1:500, avidin and biotinylated horseradish peroxidase macromolecular complex, catalog item PK-6100; Vector Laboratories) diluted in 0.1 m PBS for 1 h. Nuclear PR was visualized by incubating tissue sections with 3,3′-diaminobenzidine tetrahydrochloride (catalog item D5905; Sigma-Aldrich) with 0.02% nickel sulfate (catalog item N73; Fisher Scientific) and 0.003% H2O2 as a substrate for 10 min.
After washing with PB, sections were incubated with 1% H2O2 for 10 min to remove any unreacted peroxidase. Tissue sections were then incubated overnight with the second primary antibody against DYN, NKB, or kisspeptin diluted in blocking solutions for 17 h. The dilution and source for the second primary antibodies were rabbit polyclonal anti-DYN (1:10,000, catalog item H-021-03; Phoenix Pharmaceuticals, Belmont, CA), rabbit polyclonal anti-NKB (1:15,000, catalog item T-4450; Peninsula Laboratories, San Carlos, CA), and rabbit polyclonal anti-kisspeptin serum (1:100,000; kp10, lot 564; gift from Dr. Alain Caraty, Nouzilly, France).
After incubation in the second primary antibody, sections were washed and then incubated with biotinylated goat antirabbit IgG (1:500, catalog item BA-1000; Vector) for 1 h. Sections were again washed and then incubated with ABC reagent (1:500) diluted in 0.1 m PBS for 1 h. DYN, NKB, and Kisspeptin staining were visualized using unenhanced 3,3′-diaminobenzidine with 0.003% H2O2 as substrate for 10 min. After washing, sections were mounted on glass slides, air dried, cleared, dehydrated, and coverslipped with mounting medium.
Immunohistochemical controls included omission of one or both primary antibodies in the dual staining procedures; the absence of primary antibody resulted in completed elimination of the staining for the corresponding antigen. In addition, preabsorption controls have previously been performed for each of the antibodies as part of previous studies (4,8); in each case, preincubation of the diluted antiserum with nanomolar concentrations of purified antigen was sufficient to eliminate all specific staining in sheep hypothalamic sections. In addition, the kisspeptin antibody used has been shown to be specific for kisspeptin cells in the ovine brain and not cross-react with other RFamide peptides (8).
Dual immunofluorescent labeling
To assess colocalization of neuropeptide expression in tissue sections from prenatal T-treated ewes, we performed dual-label immunofluorescent staining for DYN and either NKB or kisspeptin in sections through the middle division of the ARC. All immunofluorescent procedures were performed at room temperature. Free-floating sections were washed with PBS followed by incubation in 1% H2O2 and blocking solutions as described in the dual-immunoperoxidase procedure above. A series of every fourth section was incubated for 17 h with rabbit anti-NKB (1:6000, catalog item H-046-026; Phoenix Pharmaceuticals), whereas an alternate series of sections was incubated overnight with rabbit anti-kisspeptin serum (1:300,000, kp10, lot 564), both diluted in blocking solution. After incubation in primary antibody, sections were then washed and incubated with biotinylated goat antirabbit IgG (1:500) for 1 h, followed by washes and incubation with ABC reagent (1:500) for an additional 1 h. After this, sections were washed and incubated with tyramide signal amplification reagent (1:250, TSA biotin system, catalog item NEL700A; PerkinElmer Life Sciences, Boston, MA) diluted in 0.1 m PBS with 0.003% H2O2 as substrate for 10 min. Sections were then incubated with streptavidin-conjugated AlexaFluor 488 (catalog item S-32354; Invitrogen, Carlsbad, CA) 1:100 diluted in 0.1 m PBS for 30 min with light protection from this step forward. Sections were subsequently incubated for 17 h with the second primary antibody, rabbit anti-DYN (1:1000, catalog item H-021-03; Phoenix Pharmaceuticals) diluted in blocking solutions. After washes, sections were incubated with AlexaFluor 555-conjugated goat antirabbit IgG (catalog item A-21428; Invitrogen) 1:100 diluted in 0.1 m PBS for 30 min. Sections were mounted onto slides, air dried, and coverslipped with Gelvatol mounting medium. Slides were stored covered at 4 C until analyzed.
Data analysis and image capture
Sections through the preoptic area (POA) and hypothalamus in each animal were examined for DYN-, NKB-, and kisspeptin-positive cells and their coexpression with PR. For quantitative analysis, we analyzed three sections from the POA and three sections each from the rostral, middle, and caudal divisions of the ARC from each animal. Cell counts were performed by two independent observers, blinded to the animal number or group, to obtain the mean number of single- and dual-labeled PR-, DYN-, NKB-, and kisspeptin-expressing cells in each animal. The mean percentages of DYN-, NKB-, or kisspeptin-expressing cells that contained PR-positive nuclei were calculated for each animal and expressed for each group as mean ± sem.
Images of immunostained sections were captured at original magnifications of ×10 and ×40 using an Optronics digital microscope Camera (Goleta, CA) attached to a Leica DMRD microscope (Leica Microsystems GmbH, Wetzlar, Germany). Immunofluorescent staining images were captured at original magnifications of ×10 using a Leica DFC340FX digital camera (Leica Microsystems Ltd., Heerbrugg, Switzerland) attached to a Leica DM5000B Microscope (Leica Microsystems CMS GmbH, Mannheim, Germany).
Statistical analysis
The statistical software SigmaStat 3.0 for Windows (SPSS Inc., Chicago, IL) was used for all the statistical analysis in this study. LH pulses were identified using well-established statistical criteria (28). Mean LH concentrations and LH pulse frequencies were compared between control and prenatal T animals by t test and Mann-Whitney U test, respectively. Comparisons of cell number and percentage colocalization between adult female and male sheep and between control and prenatal T-treated ewes were analyzed by t test. P < 0.05 was considered statistically significant for all analyses.
Results
Mean LH levels and LH pulse frequency during the midluteal phase of the estrous cycle was significantly higher in prenatal T-treated ewes than in control animals (Fig. 1); by contrast, mean serum P4 levels did not differ between these animals (data not shown). Thus, these data confirm the ability of prenatal T treatment to decrease responsiveness of the GnRH system to the negative feedback influence of P4, consistent with previous findings (18).
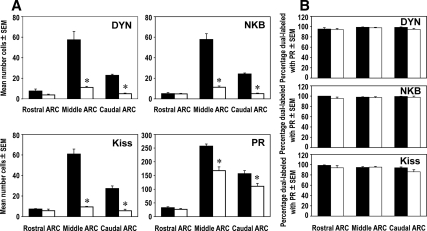
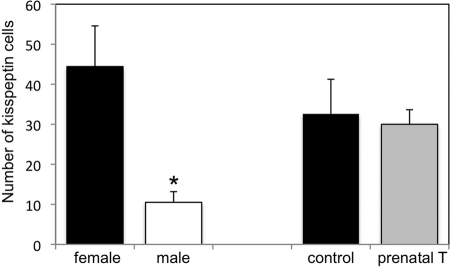
We found significantly fewer DYN-, NKB-, kisspeptin-, and PR-positive cells in the ARC of males compared with females (Figs. 2 and 3A). Regional analysis of rostral, middle, and caudal levels of the ARC (Fig. 3A) revealed that sex differences for each neuropeptide/receptor were limited to the middle and caudal subdivisions, the site of a majority of these neurons. In addition, even though there were fewer neuropeptide and PR cells in the male than female ARC, we observed the same high degree (>95%) of colocalization for each neuropeptide with PR in both males and females (Fig. 3B). In addition to the sex differences we observed for number of kisspeptin cells in the ARC, we also found sex differences in kisspeptin in the ovine POA (Fig. 4) with females having a significantly greater number of cells (44.4 ± 10.5, mean ± sem) than males (10.5 ± 2.7, mean ± sem).
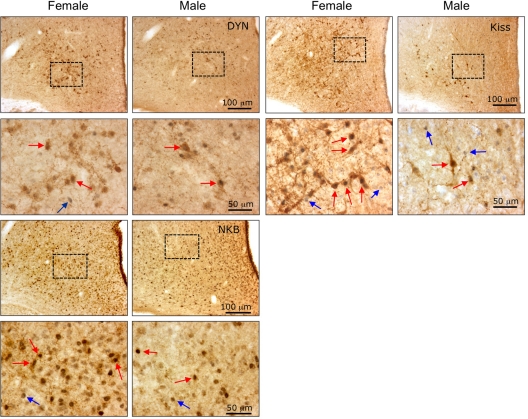
Figure 2.
Photomicrographs of sections through the middle division of the arcuate nucleus of the hypothalamus in adult female and male sheep that were dual-labeled for either DYN-, NKB-, or kisspeptin (Kiss)-positive cells and their coexpression of PR. The bottom panels in each section are the higher magnifications of the boxed areas shown in the top panels. Red arrows indicate examples of dual-labeled cells, and blue arrows indicate single-labeled PR-positive cells.
Figure 3.
A, Sex differences in the mean number (±sem) of DYN-, NKB-, kisspeptin (Kiss)- and PR-positive cells in the rostral, middle, and caudal divisions of the ARC between adult female (black bars, n = 4) and male (white bars, n = 4) sheep. *, P < 0.05. B, Mean percentage (±sem) of either DYN, NKB, or kisspeptin (Kiss) cells that colocalized PR in the rostral, middle, and caudal divisions of the ARC in female (black bars, n = 4) and male (white bars, n = 4) sheep.
Figure 4.
Mean number (±sem) of kisspeptin cells in the preoptic area of normal, adult female (n = 4) and male (n = 4) sheep (experiment 1) and control (n = 5) and prenatal T (n = 4) female sheep (experiment 2). Males had significantly fewer preoptic kisspeptin cells than females (*, P < 0.05), but prenatal T females did not differ from controls.
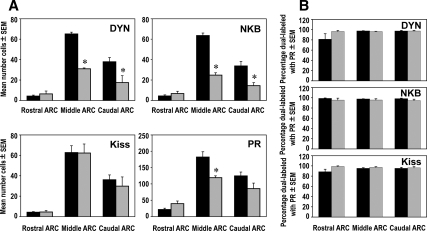
Prenatal T treatment resulted in fewer PR-, DYN-, and NKB-positive cells in the ARC, reducing the number of cells to levels comparable to that seen in normal male brains (Figs. 5 and 6A). Similar to the sex differences described above, the decreases in PR-, DYN-, and NKB-positive cells seen in prenatal T animals were regionally restricted to the middle and caudal regions of the ARC, with the exception that PR and NKB cell numbers did not significantly differ from controls in the caudal ARC (Fig. 6A). However, in contrast to PR, DYN, and NKB, the mean number of kisspeptin-positive cells did not change in any subdivision of the ARC and was not significantly different between control and prenatal T-treated ewes (Figs. 5 and 6A). Similarly, the number of kisspeptin cells in the POA did not differ between control and prenatal T-treated ewes (Fig. 4). Despite the differences in mean number of cells between kisspeptin and the other peptides in the ARC, the percentage of colocalization of each neuropeptide with PR remained high and did not differ between control and prenatal T-treated ewes (Fig. 6B).
Figure 5.
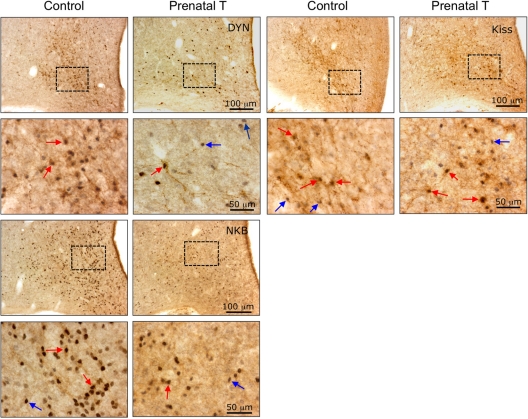
Photomicrographs of sections through the middle division of the ARC of the hypothalamus in control and prenatal T-treated ewes that were dual-labeled for either DYN-, NKB-, or kisspeptin (Kiss)-positive cells and their coexpression of PR. The bottom panels in each section are the higher magnifications of the boxed areas shown in the top panels. Red arrows indicate examples of dual-labeled cells, and blue arrows indicate single-labeled PR-positive cells.
Figure 6.
A, Differences in the mean number (±sem) of DYN-, NKB-, kisspeptin (Kiss)-, and PR-positive cells in the rostral, middle, and caudal divisions of the ARC in control (black bars, n = 5) and prenatal T-treated (gray bars, n = 4) ewes. *, P < 0.05. B, Mean percentage (±sem) of either DYN, NKB, or kisspeptin (Kiss) cells that colocalized PR in the rostral, middle, and caudal divisions of the ARC in control (black bars, n = 5) and prenatal T-treated (gray bars, n = 4) ewes.
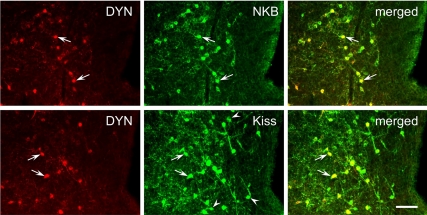
One possible explanation for the differences between kisspeptin and DYN or NKB cell number in the ARC of prenatal T-treated ewes was the possibility that separate cell populations expressed DYN, NKB, or kisspeptin in that group of animals. To examine this possibility, we performed dual-label immunofluorescence for DYN/NKB or DYN/kisspeptin in sections through the middle division of the ARC (Fig. 7), the site of the greatest number of KNDy cells, in prenatal T-treated sheep (n = 3). Even though the absolute number of DYN cells was reduced, we found that the mean percentage of DYN cells that colocalized NKB (98.9 ± 0.2%) or kisspeptin (97.5 ± 2.5%) remained very high and comparable to that seen in control animals (8). As expected, greater numbers of kisspeptin cells (62.5 ± 12.0, mean ± sem) were present than DYN cells (32.5 ± 7.7, mean ± sem), resulting in the appearance of numerous single-labeled kisspeptin cells (Fig. 7). Therefore, even though almost DYN cells colocalize kisspeptin (97.33 ± 1.5%, mean ± sem), only about half of kisspeptin cells (45.33 ± 2.2%, mean ± sem) were double labeled with DYN in prenatal T animals.
Figure 7.
Dual-label immunofluorescent detection of DYN and either NKB (top panels) or kisspeptin (Kiss, lower panels) in the middle division of the ARC of prenatal T-treated ewes. Greater than 95% of DYN cells coexpressed NKB and Kiss (arrows); also note the presence of many single-labeled Kiss cells (arrowheads) reflecting the imbalance in neuropeptide expression seen in prenatal T-treated ewes. Bar, 50 μm.
Discussion
Our results show that the KNDy cell subpopulation of the ovine ARC in sheep is sexually dimorphic, with greater numbers of kisspeptin-, NKB-, -DYN-, and PR-positive cells present in the adult female hypothalamus than in the male brain. Although either DYN or NKB expression has been shown previously to be sexually dimorphic in the ARC of rodents (29) and sheep (9), this study is the first demonstration of sex differences in kisspeptin in the ARC of any species. Surprisingly, although prenatal T treatment reduced the number of cells producing immunodetectable DYN, NKB, and PR cells in the ewe ARC to levels comparable to that seen in normal male brains, the number of kisspeptin cells was not significantly changed. In both male and prenatal T-treated animals, the decreased number of DYN, NKB, and PR cells were regionally restricted in the ARC, predominantly in its middle and/or caudal subdivisions. In addition, the vast majority (>95%) of DYN and NKB cells in prenatal T-treated animals still colocalized kisspeptin, and the total number of kisspeptin cells in the ARC remained the same as in control females. Thus, the results are consistent with divergent effects of prenatal T excess on DYN and NKB, distinct from that on kisspeptin, all within the same ARC neurons.
The effects of prenatal T treatment on PR are more difficult to interpret because approximately half the PR-positive nuclei in the ARC are found in non-KNDy neurons, unlike the changes in expression of the three neuropeptides, which are limited to the same set of ARC neurons. The decrease in PR-containing cells in the middle and caudal ARC in prenatal T-treated females suggests that these may be occurring in KNDy neurons. However, if this were the case, then the percentage of kisspeptin neurons containing PR should be suppressed by prenatal T treatment. Because this was clearly not the case, we propose that prenatal T decreases PR in a population of ARC neurons that do not contain DYN, NKB, and kisspeptin. It should be noted that this logic does not apply to the sexual dimorphism in PR-containing neurons, which may well include both non-KNDy and KNDy neurons in the middle and caudal ARC.
Because sex differences in this study were examined in gonadally intact females and males, we cannot exclude the possibility that these differences reflect the presence and activational influence of E2, P4, and/or T. However, several pieces of evidence suggest that sex differences in neuropeptide/steroid receptors in the KNDy population are not due to circulating gonadal steroids. First, as noted above, we found that prenatal T treatment in females produced a similar decrease in DYN, NKB, and PR cells as seen in males. In this study, both control and prenatal T-treated females were ovariectomized during adult life and given exogenous E2 and P4 before perfusion, so that endogenous hormone levels were the same in both groups of animals. Second, ovarian steroids decrease kisspeptin peptide and mRNA levels in the ovine ARC, in contrast to the POA where they increase expression (30). Thus, sex differences in kisspeptin, at least in the ARC, are unlikely to be due to differences in circulating levels of steroids. Nonetheless, it is possible that other differences in endogenous hormonal milieu may have contributed to the adult sex differences observed in this study, and direct comparison of gonadectomized ewes and rams will be needed to address this possibility.
The sex differences we observed in kisspeptin cells of the ARC in sheep stand in sharp contrast to observations in rats and mice; in those species, sexual dimorphism is limited to kisspeptin cells located in the anteroventral periventricular preoptic area (AVPV-POA) and is not seen in kisspeptin cells of the ARC (21,22). Although there is no distinct anteroventral periventricular preoptic area (AVPV-POA) in the sheep hypothalamus, there is a rostral population of kisspeptin cells in the medial POA, of which approximately half express estrogen receptor-α (15). In the present study, we found that POA kisspeptin cells in the sheep are also sexually dimorphic with more cells present in the female than male. However, the preoptic kisspeptin cells, unlike those in the ARC, do not coexpress either DYN or NKB (8), and although they have been implicated in E2 positive feedback in rodents (31,32), their functional role in sheep has yet to be determined. The meaning of the difference between sheep and rodents in the sexual dimorphism of the KNDy population is not clear but may be related to species differences in the importance of this population in P4 negative feedback. Supporting this is evidence that neonatal T does not alter P4 negative feedback in rats (33), so that the absence of sexual dimorphism in rodent ARC KNDy neurons is consistent with our model.
The divergent effects of prenatal T on DYN, NKB, and kisspeptin expression suggest that the sex differences in kisspeptin observed in the sheep ARC, unlike that of DYN and NKB, are not programmed solely by prenatal T during d 30–90 of embryonic development. Instead, the critical period of programming of sex differences in kisspeptin expression may reside postnatally or, alternatively, require extended exposure to T that span the pre- and postnatal periods. Another possibility is that sex differences in kisspeptin, unlike DYN or NKB, could be due to genetic factors, such as differences in X and Y chromosomes (34,35). Regardless of mechanism, one important implication of these observations is that the effects of prenatal T on DYN- and NKB-containing neurons most likely reflects a loss of peptide expression rather than developmental apoptosis of KNDy neurons, as is seen in some other sexually dimorphic neural populations (36). Moreover, these results raise the interesting possibility that different critical periods/mechanisms for sex differences exist for multiple peptides coexpressed in the same neuron. To our knowledge, this is the first experimental evidence to support this possibility and, as such, it suggests a new level of potential complexity to the mechanisms underlying the development of sex differences in the brain.
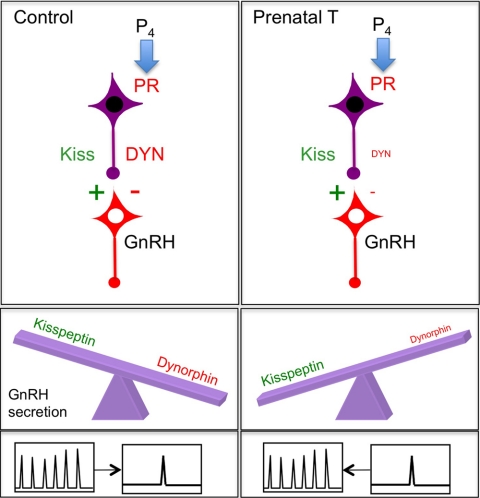
The divergent effects of prenatal T on DYN and kisspeptin expression lead to a hypothesis for the decrease in P4 negative feedback produced by prenatal T exposure in sheep. Namely, we propose that this decrease is due to changes in the balance of neuropeptide expression within a single cell population of the ARC (Fig. 8). Because DYN inhibits pulsatile GnRH secretion (5), whereas kisspeptin stimulates GnRH release (37), a relative decrease in inhibitory neuropeptide coupled with no change in stimulatory peptide would shift the net balance of peptidergic input to the GnRH neuron toward the excitatory side, and impair the ability of P4 to inhibit GnRH pulses (Fig. 8). The decrease in number of PR-containing cells in the ARC could also contribute to the loss of inhibition of GnRH pulses. However, the T-induced decrease in PR apparently did not occur in the KNDy neurons that mediate P4 negative feedback so the significance of this decrease remains to be determined. Thus, the simplest explanation for the decrease in P4 negative feedback in prenatal T animals is that the persistence of normal levels of kisspeptin expression outweighs any inhibitory influence of DYN input to GnRH neurons that remains.
Figure 8.
Proposed model to account for the effects of prenatal T on P4 negative feedback. Schematic diagram showing the decrease in DYN expression without a change in kisspeptin in KNDy neurons of prenatal T-treated ewes, which is proposed to shift the balance of excitatory (green) and inhibitory (red) neuropeptide inputs to GnRH neurons, and the consequent ability of P4 to inhibit GnRH/LH pulses. For simplicity, and because it is unlikely that changes in NKB contribute to P4 negative feedback (see Discussion), NKB is not included in the diagram.
We would note that although our data provide an explanation for differences in P4 negative feedback between prenatal T-treated ewes and control females, they do not address the differences in this feedback control between males and females. In this regard, although previous work has demonstrated that normal females are more responsive to P4 negative feedback than androgenized females or males, the latter still do respond to higher physiological concentrations of P4 (38), and it may be that differences in dose responsiveness distinguish prenatal T-treated ewes from males. Furthermore, the observation that a combination of P4 and T can inhibit LH in males (39) is consistent with the quantitative aspect of this sexually differentiated feedback control.
The functional consequences of the decrease in NKB expression in KNDy cells is more difficult to interpret given contradictory evidence as to the actions of NKB. Inhibitory effects have been reported in rodents (11), whereas we have observed that an NKB agonist dramatically increases LH secretion in ewes (10). The recent report that loss-of-function mutations in either the gene encoding NKB, or its receptor, produce gonadotropin deficiencies in humans (40) raises the possibility that there may be marked species differences in the role of NKB between rodents and larger mammals. In sheep, NKB appears to stimulate LH secretion in the follicular phase of the cycle but not during the luteal phase when P4 is high (10). Thus, it is unlikely that changes in NKB contribute functionally to P4 negative feedback, although NKB may play a role in facilitating the GnRH/LH surge (10), control of which is sexually differentiated in sheep (41).
In addition to its role in P4 negative feedback, there is evidence that the KNDy cell subpopulation may also play a role in other types of steroid feedback control of GnRH secretion. For example, recent studies have shown striking seasonal changes in kisspeptin peptide and mRNA in the sheep ARC that parallel robust seasonal shifts in the responsiveness of GnRH neurons to E2 negative feedback (30,42). These changes are limited to kisspeptin cells in the ARC but not in the POA (30), suggesting further functional heterogeneity among these cell populations. In addition, seasonal changes in ARC kisspeptin persist in ovariectomized ewes bearing implants that produced constant low levels of E2 throughout the year; thus, changes in kisspeptin levels, like those in E2 negative feedback, are a reflection of season independent of alterations in endogenous steroid levels (30). There is also evidence in sheep suggesting the involvement of KNDy cells in the positive feedback effects of E2 leading to the preovulatory GnRH/LH surge. First, kisspeptin mRNA in the middle portion of the ARC reaches peak levels late in the follicular phase in gonadally intact ewes (43). Second, E2 implants in the MBH close to the location of the kisspeptin ARC neurons, but not the POA, induce a GnRH surge in ovariectomized sheep (44). Thus, KNDy neurons of the ARC may serve as a common pathway for both negative and positive feedback control of GnRH secretion, and the balance of synthesis and/or release of each peptide may be an important determinant of the effectiveness of this type of feedback control of GnRH secretion.
Finally, it should be noted that prenatal T treatment in monkeys and sheep leads to a constellation of abnormalities that closely resemble those seen in human endocrine disorders, including polycystic ovarian syndrome (PCOS) patients (45). These abnormalities include the reduced ability of gonadal steroids, including E2 and P4 to exert normal feedback control of GnRH and LH secretion. Because kisspeptin, NKB, and DYN cells are each present in the human infundibular (arcuate) nucleus and show morphological changes in postmenopausal women associated with loss of steroid negative feedback (17,46,47,48), it is tempting to speculate that alterations in the balance of neuropeptide expression within this single subpopulation of neurons underlie defects in feedback sensitivity associated with PCOS and perhaps other clinical disorders.
In summary, KNDy cells of the ARC in the sheep represent a sexually dimorphic subpopulation comprised of multiple neuropeptides and gonadal steroid receptors, which likely play a key role in hormonal feedback control of GnRH secretion. Prenatal T treatment only partially mimicked these sex differences in KNDy neurons, decreasing DYN and NKB expression although not affecting kisspeptin, suggesting that multiple neuropeptides expressed by the same neuron may have different critical periods for sexual differentiation. Finally, the imbalance of neuropeptide expression within KNDy cells in prenatal T-treated ewes provides a potential explanation for defects in gonadal hormone feedback control of GnRH secretion, including P4 negative feedback, seen as a consequence of prenatal T excess in sheep and other species.
Footnotes
This work was supported by NIH R01 HD39916 (M.N.L. and R.L.G.), R01 HD41098 (V.P.), P01 HD44232 (V.P. and M.N.L.), and Canadian Institutes of Health Research Operating Grant 86744 (M.N.L.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 30, 2009
Abbreviations: ARC, Arcuate nucleus; CIDR, controlled internal drug release P4 implant; CV, coefficient of variation; DYN, dynorphin; E2, estradiol; MBH, mediobasal hypothalamus; NKB, neurokinin B; P4, progesterone; PR, P4 receptor; PB, phosphate buffer; POA, preoptic area; T, testosterone.
References
- Goodman RL, Inskeep EI 2006 Neuroendocrine control of the ovarian cycle of the sheep. In: Knobil and Neill’s physiology of reproduction. 3rd ed. New York: Academic Press; 2389–2447 [Google Scholar]
- Zeleznik AJ, Pohl CR 2006 Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine control of the menstrual cycle in higher primates. In: Knobil and Neill’s physiology of reproduction. 3rd ed. New York: Academic Press; 2449–2510 [Google Scholar]
- Skinner DC, Caraty A, Allingham R 2001 Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN 2002 Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN 2004 Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN 2005 Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN 2006 Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin cells in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE 2000 Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- McManus CJ, Valent M, Connors JM, Goodman RL, Lehman MN, A neurokinin B agonist stimulates LH secretion in follicular, but not luteal phase, ewes. Program of the 35th Annual Meeting of the Society for Neuroscience, Washington, DC, 2005 (Abstract 760.8) [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, and NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA 2006 Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE 2006 Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- Rance NE 2009 Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA 1999 In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology 140:5797–5805 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK 2002 Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK 2008 Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology 149:3054–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss 1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I'Anson H, Bucholtz DC, Yellon SM, Foster DL 1991 Prenatal androgens time neuroendocrine sexual maturation. Endocrinology 128:2457–2468 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V 2009 Developmental programming: contribution of prenatal androgen and estrogen in organizing estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod 80:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson Jr RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert Jr LE, Midgley Jr AR, Nalbandov AV 1969 Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 84:1166–1173 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ 1995 Evidence for short or ultrashort loop negative feedback of GnRH secretion. Neuroendocrinology 62:248–258 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Karsch FJ 1980 Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G 2006 Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141:1731–1745 [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN 2008 Variation in kisspeptin and gonadotropin-inhibitory hormone expression and terminal connections to GnRH neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda KI 2005 Involvement of central metastin in the regulation of preovulatory LH surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, McDevitt MA, Acosta-Martínez M, Horton TH, Levine JE 2008 Neuroendocrine consequences of androgen excess in female rodents. Horm Behav 53:673–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP 2002 A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Grisham W, Arnold AP 2009 X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci 29:768–776 [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ 2004 Delection of Bax eliminates sex differences in the mouse forebrain. Proc Nat Acad Sci USA 101:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Grindrod JA, Taylor JA, Unsworth WP 2003 Sexual differentiated regulation of GnRH release by gonadal steroid hormones in sheep. Reprod Suppl 61:299–310 [PubMed] [Google Scholar]
- Turner AI, Tilbrook AJ, Clarke IJ, Scott CJ 2001 Progesterone and testosterone in combination act in the hypothalamus of castrated rams to regulate the secretion of LH. J Endocrinol 169:291–298 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TAC3R mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DL, Padmanabhan V, Wood RI, Robinson JE 2002 Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: concepts derived from sheep models. Reprod Suppl 59:83–99 [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ 2007 KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ 2006 Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory GnRH/LH surge in the ewe suggests a stimulatory role for kisspeptin in estrogen-positive feedback. J Neuroendocrinol 18:806–809 [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A 1998 Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin-releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V 2007 Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Rance NE, Young 3rd WS 1991 Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE 2008 Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]