Abstract
The steroidogenic acute regulatory (StAR) protein mediates the rate-limiting step of mitochondrial transport of cholesterol for steroid biosynthesis. To investigate the regulation of this protein in lower vertebrates, we cloned the StAR coding region from large-mouth bass for analysis. Induction of the mRNA corresponded with increasing levels of plasma sex steroids in vivo. Cultures of largemouth bass ovarian follicles were exposed to dibutyryl cAMP (dbcAMP), a potent signaling molecule for steroidogenesis. StAR mRNA expression was significantly up-regulated by dbcAMP signaling, suggesting that the 5′ regulatory region of the gene is functionally conserved. To further analyze its transcriptional regulation, a 2.9-kb portion of the promoter was cloned and transfected into Y-1 cells, a steroidogenic mouse adrenocortical cell line. The promoter activity was induced in a dose-responsive manner upon stimulation with dbcAMP; however, deletion of 1 kb from the 5′ end of the promoter segment significantly diminished the transcriptional activation. A putative retinoic acid-related receptor-α/rev-erbα element was identified between the −1.86- and −2.9-kb region and mutated to assess its potential role in dbcAMP regulation of the promoter. Mutation of the rev-erbα element significantly impeded dbcAMP-induced activation. Chromatin immunoprecipitation and EMSA results revealed rev-erbα and retinoic acid-related receptor-α enrichment at the site under basal and dbcAMP-induced conditions, respectively. These results implicate important roles for these proteins previously uncharacterized for the StAR promoter. Altogether these data suggest novel regulatory mechanisms for dbcAMP up-regulation of StAR transcription in the distal part of the largemouth bass promoter.
Orphan nuclear receptors regulate the steroidogenic acute regulatory protein.
Identification of the steroidogenic acute regulatory (StAR) protein in 1994 significantly advanced the field of cholesterol metabolism (1). It has now been well characterized in multiple mammalian species that StAR transports cholesterol across the mitochondrial membrane and controls the rate-limiting step for steroidogenesis (2,3,4,5,6). Regulation of steroidogenesis occurs in a tissue-specific manner and involves multiple signaling pathways, including protein kinase A (PKA) and protein kinase C (PKC), among others, and this appears to be conserved across most vertebrate species (2,7,8,9,10). It is known that ACTH, an upstream regulator of cAMP production, induces StAR mRNA expression in rainbow trout and eel (11,12,13). It has also been shown that exogenous exposure of Atlantic Croaker ovarian follicles to human chorionic gonadotropin (hCG; a potent LH receptor agonist) robustly stimulates gonadal StAR mRNA expression (14). Because StAR gene expression is highly responsive to cAMP and hCG in both higher and lower vertebrates, mammals and lower vertebrates such as fish are likely to exhibit similar transcriptional regulatory mechanisms for the StAR gene.
It is well established that the StAR gene is highly regulated by the cAMP/PKA pathway across multiple species and that this pathway is important in reproduction. Binding elements for transcription factors known to mediate cAMP responses, such as steroidogenic factor (SF)-1, activator protein (AP)-1, cAMP response element-binding protein (CREB), and others, have been extensively characterized in mammalian StAR gene promoters (15,16,17,18,19,20,21,22,23,24); however, there are no previous publications citing in silico or functional promoter analysis of the StAR gene in any fish model. In all vertebrates studied, the promoter for StAR is very complex with many transcriptional elements for which the functions are still unknown (25). The transcriptional mechanisms controlling the StAR gene in lower vertebrate animals such as fish have not been investigated and could provide much needed insight into the complex networks involved in the regulation of steroidogenesis.
Circadian rhythm plays an important role in reproduction in vertebrates, and it exists centrally, in peripheral tissues, and even within individual cells (26). Control of gene expression at the cellular level is important in regulating reproductive processes, including steroid hormone production. It is known that retinoic acid-related receptor (ROR)-α and rev-erbα are two signaling proteins that play integral roles in controlling genes central to the circadian cascade (27,28,29). RORα and rev-erbα both bind to similar core sequences [ROR element (RORE)]; however, they induce opposing effects on the transcription of target genes (30).
The goals of this study were to clone and characterize the largemouth bass (LMB) StAR gene and promoter at the tissue and cellular levels, respectively, and characterize a functional regulatory role for a RORE in LMB StAR transcription. Our results show that RORα and rev-erbα are both capable of binding a core sequence in the LMB StAR promoter and, interestingly, that rev-erbα also binds to a core sequence in the mouse StAR promoter. Altogether our study presents a novel mechanism through which StAR promoter activity is controlled.
Materials and Methods
Cloning of LMB StAR cDNA coding region
LMB StAR was PCR amplified from LMB ovarian cDNA using primers designed with the web program CODEHOP (http://blocks.fhcrc.org/codehop.html) and are listed in supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. The cDNA was amplified using 10 pmol of each primer and 1 U Amplitaq (PerkinElmer, Waltham, MA) according to the manufacturer’s protocol. The PCRs used a primer annealing temperature of 60.9 C for 45 cycles (PerkinElmer 9600 thermocycler). The remaining cDNA sequence for LMB StAR was obtained by rapid amplification of cDNA ends (RACE) using the SMART RACE protocol (CLONTECH, Mountain View, CA). Primers used for both 5′ and 3′ RACE are listed in listed in supplemental Table S1. The nucleotide sequence for the LMB StAR coding sequence has been deposited in the GenBank database under GenBank accession no. DQ166820.
In vivo LMB study
Adult LMB were purchased from American Sport Fish Hatchery (Montgomery, AL) and housed in fresh water ponds at the U.S. Geological Survey (Gainesville, FL) under ambient conditions. Females were collected every 2 wk by electroshock from October 1999 to April 2000. Fish were killed and the ovaries were stored in RNAlater solution (Ambion, Austin, TX) at −20 C until RNA isolation.
Quantitative real-time RT-PCR (Q-PCR)
LMB StAR mRNA levels were quantified using Q-PCR. In all experiments, total RNA was extracted using RNA STAT-60 reagent according to the manufacturer’s protocol (TEL-TEST, Friendswood, TX). Three μg of each total RNA sample was reverse transcribed into cDNA using 25 U of Stratascript reverse transcriptase according to the manufacturer’s protocol (Stratagene, La Jolla, CA). All Q-PCRs used 2× SYBR Green iQ Supermix (Applied Biosystems, Foster City, CA) and 10 pmol of forward and reverse primers (supplemental Table S1) to amplify 0.12 μg of cDNA using thermocycler parameters as recommended by Applied Biosystems. All Q-PCRs were normalized to 18S rRNA (Applied Biosystems). A standard curve for real-time PCR quantitation was developed using dilutions of a plasmid incorporated with a 300-bp fragment of the LMB StAR cDNA. StAR mRNA was quantified by extrapolation to the standard curve.
Ovarian follicle cultures
Adult LMB between 2 and 3 yr old were maintained at the Aquatic Toxicology Facility at the University of Florida. Ten ovarian follicles between 0.68 and 0.76 mm in diameter were cultured in 1 ml of DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 1.2 g of sodium bicarbonate and 1% antibiotic/antimycotic solution. Cultures were equilibrated in a chilled incubator at 21–22 C with 5% CO2 for 24 h before exposure to dibutyryl cAMP (dbcAMP) for 14 h. After exposure, tissues were snap frozen in liquid nitrogen and stored at −80 C until RNA was extracted using the STAT-60 protocol mentioned above (TEL-TEST).
Cloning of the LMB StAR promoter
Genomic DNA was isolated from LMB ovarian tissue using the Wizard genomic DNA isolation kit (Promega, Madison, WI). The LMB StAR promoter was cloned using the GenomeWalker kit (CLONTECH) according to the manufacturer’s protocol. All primer sequences for promoter cloning are listed in supplemental Table S1. PCR products were initially cloned into the TOPO2.1 vector (named pSP1) and then into the pGL3 basic plasmid (Promega) for transfection experiments. The promoter for the LMB StAR gene has been deposited in the GenBank database under GenBank accession no. DQ166819.
Transcriptional start site identification and in silico promoter analysis
The transcriptional start site for the LMB StAR promoter was identified by aligning the promoter sequence with the end of the 5′ untranslated region (UTR) obtained with RACE. Sequence upstream of the transcription start site was then analyzed with two different web search engines, MatInspector version 2.2 (http://searchlauncher.bcm.tmc.edu/gene-finder/examples/mat.html), and Genomatix MatInspector (http://www.genomatix.de/online_help/help_matinspector_help.html) to identify putative consensus binding sites.
Generation of deletion and site-directed mutagenesis promoter constructs
The 2.9-kb LMB StAR promoter vector was digested with EcoRV and BstEII to eliminate 1 kb of the distal sequence, producing a 1.86-kb promoter construct extending to the transcriptional start site. Additionally, the putative binding site for the ROR/−1969 in the 2.9-kb promoter was mutated using the QuikChange XL site-directed mutagenesis kit according to the manufacturer’s protocol (Stratagene). The primer sequences for the mutagenesis constructs are listed in supplemental Table S1.
Culturing of Y-1 and MA-10 cells
Y-1 mouse adrenocortical cells purchased from American Type Culture Collection (Manassas, VA) were cultured in Ham’s F12K culture medium containing 2 mm l-glutamine, supplemented with 1.5 g/liter sodium bicarbonate, 15% horse serum, 2.5% fetal bovine serum, and 1% penicillin-streptomycin mix. The MA-10 mouse Leydig tumor cell line was generously provided by Dr. Mario Ascoli (Department of Biochemistry, Vanderbilt University, Nashville, TN). MA-10 cells were cultured in RPMI 1640 culture medium supplemented with 15% horse serum, 20 mm HEPES (pH 7.2), and 50 μg/ml gentamicin (pH 7.7). All cells were grown at 37 C in a humidified 5% CO2 cell culture incubator.
Transient transfection assays
In transfections with Y-1 cells, 150,000 cells/well were plated in 24-well culture plates and allowed to grow for 24 h. Transfection reactions consisted of a 6:1 ratio of FuGENE 6 (Roche Diagnostics, Indianapolis, IN) to plasmid DNA (1.2 μl FuGENE 6 per 0.2 μg total DNA) suspended in 20 μl media with no serum or antibiotics. Twenty-four hours after transfection, Y-1 cells were exposed to dbcAMP for 20 h. In transfections done in MA-10 cells, 100,000 cells/well were plated in 24-well culture plates coated with 0.1% gelatin (dissolved in 1× PBS) and allowed to grow for 24 h. Transfection reactions consisted of a 4:1 ratio of FuGENE HD (Roche Diagnostics) to plasmid DNA (2 μl FuGENE HD/ 0.5 μg total DNA) suspended in 25 μl media with no serum or antibiotics. Thirty hours after transfection, MA-10 cells were exposed to 0.1% vehicle or 10 U/ml hCG in growth medium for 6 h. All transfections were normalized to Renilla luciferase. All Firefly and Renilla luciferase reactions were quantified using reagents from the dual luciferase kit (Promega).
Oligonucleotide annealing reactions
Sense and antisense oligonucleotides designed against the RORE (supplemental Table S1) were based on bioinformatic analysis of the LMB StAR promoter. Positive control probes were generated from published sequences. All probes were prepared and obtained commercially (Eurofins MWG Operon, Huntsville, AL). The probes were biotinylated at one end of each sense strand; cold probes lacked biotinylation. Sense and antisense oligos were annealed by adding equimolar amounts of each probe to a reaction containing 10 mm Tris (pH 8.0), 1 mm EDTA, 20 mm NaCl, 10 mm MgCl2, and 5 mm dithiothreitol. The reactions were boiled for 10 min at 95 C followed by gradual cooling to room temperature.
EMSA
Fluorescence-based EMSAs were conducted using a modification of the commercially available EMSA gel shift kit protocol (Panomics, Fremont, CA) and an RORα antibody designed against human RORα4 supplied from Steinhilber and colleagues (31). One microgram of RORα protein was used per reaction, and in the instances where antibody was added, 1 μg of RORα antibody was used. Ten nanograms of probe were added to each reaction, and if a competition assay was performed, cold probe was added in 100× excess. The binding reaction conditions are outlined in the manufacturer’s instructions. The binding reactions were incubated at 17.5 C for 30 min. A 6% nondenaturing Tris-borate EDTA polyacrylamide gel was then prepared and prerun in prechilled 0.5× Tris-borate EDTA for 10 min at 120 V at 4 C. Once loaded, gels were electrophoresed for 20 min at 60 V at 4 C, followed by 80 min at 100 V at 4 C. The EMSA gel-shift kit protocol and reagents from the manufacturer were used for the remainder of the EMSA.
Chromatin immunoprecipitation (ChIP) assay
Y-1 cells were transfected with the 2.9-kb LMB StAR promoter plasmid using FuGENE HD (Roche Diagnostics). After transfecting overnight, medium was changed and half of the transfected plates were treated with 1 mm dbcAMP for 20 h. Samples were processed for ChIP analysis using a modified version of the ChIP-IT kit protocol (Active Motif, Carlsbad, CA). Once cells were fixed and scraped, chromatin was isolated and sheared using a Fisher sonic dismembrator. Cross-linked sheared chromatin obtained from both treatments was immunoprecipitated at 4 C overnight with antibodies against normal IgG, RORα (Santa Cruz Biotechnology, Santa Cruz, CA), or rev-erbα (Cell Signaling Technology, Beverly, MA). After immunoprecipitation and protein G collection, cross-links were reversed and protein and RNA were digested from each sample. DNA was column purified and Q-PCR was run on each sample as described above and data were normalized to 1:10 input control. The primers used in Q-PCR are listed in supplemental Table S1. The mouse primers were designed against a putative RORE (mRORE/−634) identified in the mouse StAR promoter using the Genomatix MatInspector software as described in the in silico analysis section above. The putative site was located between bp −619 and −641 relative to the transcriptional start site of the gene.
Statistical testing
Student paired t test was used for statistical analysis of control vs. treatments, and significant differences of P < 0.05 between the groups are noted by an asterisk in the figure.
Results
Characterization of the coding sequence for LMB StAR protein
To assess how StAR is regulated in LMB, a partial 345-bp sequence was amplified using degenerate primer-based PCR and then completed with 5′ and 3′ RACE. Sequence alignment of LMB StAR with other species, including, human, pig, horse, zebrafish, brook and rainbow trout, revealed 52% similarity with mammals and 72% similarity with other fish species (supplemental Fig. S1).
A sequence comparison of the LMB StAR protein with StAR protein sequences from several species revealed several important residues, including a glutamic acid residue at amino acid 170 within the START domain, the hydrophobic region in which cholesterol binds. Analysis with the ScanProsite web program (http://www.expasy.ch/tools/scanprosite) revealed multiple putative PKA and PKC phosphorylation sites, which are known to be critical for mammalian StAR function. A potential PKA site was identified at amino acids 193–196 as well as four potential PKC sites at amino acids 5–7, 13–15, 60–62, and 187–189. Collectively the protein sequence information suggested that LMB StAR protein may be regulated by similar mechanisms and signal transduction pathways as seen in higher vertebrates and mammals (25), such as regulation by the cAMP/PKA signaling cascade.
In vivo expression of StAR mRNA and serum 17β-estradiol (E2) levels
To determine the basal expression of LMB StAR mRNA in vivo, tissue samples were collected from pond-raised fish throughout the months of October to April, which spans the typical reproductive cycle for LMB. Quantitation of StAR message by Q-PCR revealed that StAR mRNA levels fluctuate in correlation with steroid production throughout the reproductive cycle (Fig. 1). LMB StAR mRNA levels started increasing in late December and then peaked between February and March before declining by April, and the message levels paralleled serum 17β-estradiol levels throughout the reproductive season. Histology of the ovarian follicles revealed that vitellogenin, the egg yolk protein necessary for the nourishment of newly hatched fry, was present in ovarian follicles with increased levels of StAR mRNA (Fig. 2A). These data clearly demonstrate that LMB StAR is acutely regulated, probably through signaling initiated by LH and FSH, in the same period as the production of steroid hormones, as in mammalian models.
Figure 1.

Seasonal expression of LMB StAR mRNA and plasma E2. RNA was isolated from ovarian tissue of LMB previously collected every few weeks through the reproductive cycle (n = 7 for the first 10 time points and n = 6 for April 11, 2000) and in vivo expression of StAR mRNA was analyzed by Q-PCR. Data are reported on a logarithmic scale as mean copy number ± mean se. E2 levels were quantified from the plasma of the same individuals by RIA as described previously (43).
Figure 2.
dbcAMP induction of StAR mRNA in LMB ovarian follicle cultures. A, Hematoxylin-eosin histology of a vitellogenic LMB ovarian follicle. The following are labeled: germinal epithelium (GE), germinal vesicle (GV), yolk globules (YG), and oil droplets (OD). B, Representative ovarian follicle culture. Ten follicles were cultured per well in a 24-well culture plate and exposed to three doses of dbcAMP for 14 h. RNA was isolated from the follicles and reverse transcribed for analysis by real-time Q-PCR. This graph represents data obtained from one fish, with each treatment cultured in triplicate. Error bars, se between wells. This experiment was repeated three times with follicles from three individual fishes. Student’s t test was used to test for significance. *, P < 0.05.
Regulation of endogenous LMB StAR by dbcAMP in ovarian follicle cultures
Based on the in vivo pattern of StAR expression, vitellogenic ovarian follicles with a defined diameter range of 0.68–0.76 mm were cultured for in vitro analysis. This experiment was repeated with four individual fish. Ovarian follicles were exposed to 0.25, 0.5, or 1 mm dbcAMP for 14 h to investigate the potential for regulation of StAR in lower vertebrates by the PKA/cAMP pathway. RNA was subsequently extracted from the follicles for quantitation of StAR mRNA expression by Q-PCR. A 4-fold induction of StAR mRNA was seen with the 0.25 and 0.5 mm doses, and a 10-fold stimulation occurred with 1 mm dbcAMP (Fig. 2B). The ovarian follicle data show that LMB StAR transcription is responsive to cAMP signaling.
Cloning of the LMB StAR promoter
To ascertain the location of cAMP regulatory regions in the LMB StAR gene, we cloned the promoter using the GenomeWalker kit (CLONTECH) and primers designed within the coding sequence previously discussed. PCR products were obtained from all four of the restriction digested genomic libraries that were generated. 5′ RACE of the cDNA transcript was used to locate the boundaries of the 142-bp 5′ UTR and, by inference, the transcriptional start site. Additionally, the transcriptional start site identified from 5′ RACE matched the site predicted by a web-based program called Neural Network Promoter Prediction (http://www.fruitfly.org/seq_tools/promoter.htm). A TATA box is located about 23 bp upstream from the start site, and putative transcriptional elements were identified via bioinformatics by the MatInspector and TFsearch (http://www.cbrc.jp/research/db/TFSEARCH.html) programs. A 2.9-kb portion (upstream from the transcriptional start site) of the LMB StAR promoter was cloned into the pGL3 basic vector for functional studies in Y-1 mouse adrenocortical cells.
The 2.9-kb LMB StAR promoter is responsive to cAMP and hCG-mediated signaling when transfected into mammalian steroidogenic cells
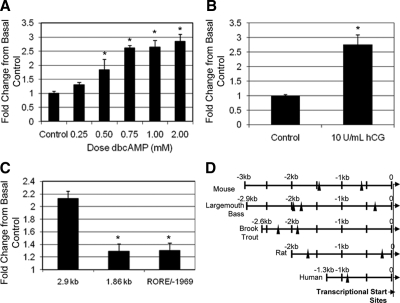
We next examined the response of the 2.9-kb promoter construct to dbcAMP in Y-1 cells. Transfected cells were exposed to a range of dbcAMP between 0 and 2 mm for 20 h. Maximal induction of LMB StAR promoter activity occurred between 0.75 and 2 mm dbcAMP, by an average of about 2.6–2.8-fold above basal control levels (Fig. 3A). Indeed, the 2.9-kb promoter could also be stimulated by about 2.8-fold with hCG in MA-10 mouse Leydig tumor cells, another mammalian steroidogenic cell line (Fig. 3B). Thus, the promoter is responsive to both cAMP and hCG, supporting that the LMB StAR gene is regulated by established steroidogenic signaling pathways.
Figure 3.
A, Dose-response exposure of LMB StAR promoter to dbcAMP. Y-1 cells were transfected with the 2.9-kb LMB StAR promoter and exposed to increasing doses of dbcAMP from 0 to 2 mm dbcAMP. Graph is representative of one experiment done in triplicate that was repeated at least three individual times. B, Exposure of LMB StAR promoter to hCG. To further verify responses observed in Y-1 cells, MA-10 cells were transfected with the 2.9-kb LMB StAR promoter and exposed to 10 U/ml hCG. C, LMB StAR promoter constructs containing a deletion (∼1 kb from distal end of promoter) or a site-directed mutation (RORE/−1969) were evaluated for basal and dbcAMP stimulation of StAR promoter activity. C, Data collected from three to five individual experiments. In all experiments, values are normalized to renilla luciferase (internal control) and are reported in fold change from basal control. Student’s t test was used to determine significance. *, P < 0.05. D, In silico comparison of the StAR promoter across species. Promoter sequences (including the 5′ UTR) for the StAR gene promoters from LMB (accession no. DQ166819), brook trout (accession no. AY308064), rat (accession no. AB006007), mouse (39), and human (accession no. U29098) were aligned based on the transcriptional start sites for the gene. Putative ROREs were identified using Genomatix MatInspector online software. The sites selected for mapping had 80% or greater homology to the core mammalian transcription factor sequence.
Deletion of the distal region of the 2.9-kb promoter diminishes dbcAMP response
To begin to identify potentially critical regions involved in the cAMP response of the LMB StAR promoter, 1 kb was deleted from the 5′ end using EcoRV and BstEII restriction sites to yield a shorter 1.86-kb sequence. The response of the transfected 1.86-kb construct to 1 mm dbcAMP was significantly diminished by greater than 80% in comparison with the response seen with the 2.9-kb construct (Fig. 3C). These data suggest the presence of transcriptional elements important for cAMP activation between the −1.86- and −2.9-kb region.
Site-directed mutagenesis reveals a novel dbcAMP-responsive RORE
The acute dbcAMP regulation of the distal region of the LMB StAR promoter was further examined by mutating a putative site for RORE/−1969. The nucleotide mutations yielded a NotI restriction site, which web-based programs predicted no transcription factors can bind. The site-specific mutations were confirmed by digestion with NotI and sequence verified.
The mutated RORE/−1969 construct and the intact 2.9-kb construct were exposed in parallel to 1 mm dbcAMP. Indeed, mutation of the RORE/−1969 site substantially reduced the dbcAMP induction from that observed in the 2.9-kb construct by approximately 85% (Fig. 3C). The reduced response implicates a regulatory role for the RORE in activating the promoter.
In silico analysis of the 2.9-kb LMB StAR promoter sequence revealed several well-characterized putative transcriptional elements that have been previously described in mammalian systems (6,20,21,25). In addition, several putative ROREs were identified throughout the 2.9-kb sequence, with only one putative RORE (RORE/−1969) lying in the distal 1 kb. The StAR promoter sequences from multiple species were analyzed for putative ROREs and in all species examined, ROREs were present in the StAR promoters (Fig. 3D).
ChIP verification of RORα/rev-erbα proteins binding to the RORE/−1969 element in the LMB StAR promoter and to the mRORE/−634 element in the murine StAR promoter
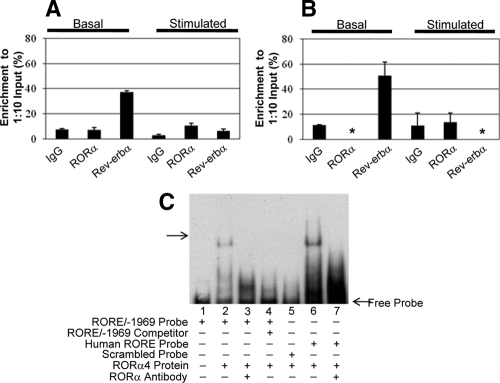
To verify that RORα and rev-erbα bind to the RORE/−1969 element in the LMB StAR promoter, ChIP assays were run using chromatin fixed from Y-1 cells transfected with the LMB StAR promoter and cultured under both basal and dbcAMP-induced conditions. After immunoprecipitation with a polyclonal antibody against mouse IgG (nonspecific control), RORα, or rev-erbα, DNA was purified and Q-PCR was run on each sample using primers encompassing either the RORE/−1969 element in the LMB StAR promoter (Fig. 4A) or the mRORE/−634 element in the murine StAR promoter (Fig. 4B) present in the same cells. Note that asterisks in Fig. 4B indicate that DNA was below detection limits.
Figure 4.
Functional analysis of RORα and rev-erbα by ChIP and EMSA. ChIP assays were run using chromatin fixed from Y-1 cells transfected with the LMB StAR promoter and cultured under both basal and dbcAMP-induced conditions. ChIP was run on each sample with antibodies specific to mouse IgG (nonspecific control), RORα, or rev-erbα. Immunoprecipitation was accomplished by the addition of protein G agarose beads. The pull-downs were washed, complexes were eluted, and DNA was purified. Q-PCR was run on each sample and results are reported graphically as percent enrichment to a 1:10 dilution of each input control. Primers used in the Q-PCR encompassed the RORE/−1969 element in the LMB StAR promoter (A) or the RORE/−634 element in the mouse promoter (B), immunoprecipitated from the same transfected cells. Each figure is representative of one of three replicated experiments. Asterisks (B) indicate that DNA levels were below detection limits. To further investigate the capacity for the LMB RORE/−1969 element to bind RORα, an EMSA was run using recombinant human RORα4 protein (C). A probe encompassing the RORE/−1969 sequence was added to recombinant human RORα4 protein in 1× binding buffer. The reactions were separated on a native gel and transferred to a membrane for EMSA analysis using chemiluminescence. Addition of an antibody specific to RORα to the reactions diminished the banding pattern observed in the lanes containing the probe and protein only, verifying specificity of the protein-DNA interaction in vitro. The following are the sequences for the probes used: RORE/−1969 probe (5′-AAT AGG CAT ATG ACC TAC TTT GGC TC); perfect (human consensus) RORE probe (5′-TCG AGT CGT ATA ACT AGG TCA AGC GCT GGA C-3′); scrambled probe (5′-CCT CTA TAA CGG GTC GGA TAC TAT TA-3′). Lane 1, RORE/−1969 probe only; lane 2, RORE/−1969 probe with RORα4 protein; lane 3, RORE/−1969 probe, RORα4 protein, and RORα4 antibody; lane 4, RORE/−1696 probe, RORE/−1969 cold unlabeled probe, and RORα4 protein; lane 5, scrambled probe and RORα4 protein; lane 6, perfect (human consensus) RORE probe and RORα4 protein; and lane 7, perfect (human consensus) RORE probe, RORα4 protein, and RORα4 antibody.
In both species, the DNA encompassing each of the ROREs was highly enriched under basal conditions when pulled down with the antibody for rev-erbα, implicating that rev-erbα binds to the LMB RORE/−1969 element and to the mRORE/−634 element in the StAR promoter of each species under basal conditions. Upon treatment with dbcAMP, the enrichment of DNA bound to rev-erbα observed under basal conditions diminished to levels observed with the nonspecific IgG antibody with both the LMB and mouse elements. Concomitantly with this decrease, a slight increase was seen for the LMB element when the RORα antibody was used, whereas there was no detectable enrichment above the nonspecific IgG control with the mRORE/−634 element.
EMSA analysis of RORα4 binding to the RORE/−1969 site in the LMB StAR promoter
To assess whether recombinant human RORα4 protein could bind to the RORE/−1969 transcriptional site in the LMB StAR promoter, an EMSA was performed using the specific probes for this promoter site and recombinant human RORα4 protein (Fig. 4C). The recombinant protein bound to the RORE/−1969 probe, producing a single band. Addition of both the RORα antibody and the cold unlabeled probe verified the specificity of the RORE/−1969-RORα4 interaction. Further verification of specificity was exhibited by the use of a scrambled probe, which did not bind the protein. A RORE probe designed against a consensus human RORE sequence was run as a positive control, yielding a band around the same size as that seen with RORE/−1969.
Discussion
We report the first in-depth study on transcriptional regulation of the StAR gene in a fish model, LMB. The StAR cDNA and a large portion of its promoter were cloned to study its regulation in vivo and ex vivo in LMB ovarian follicle cultures and then, ultimately, for more comprehensive examination of regulation of StAR transcription with transfection assays. Our data show that LMB StAR is transcriptionally regulated by dbcAMP and that rev-erbα/RORα play critical roles in the activation of the LMB StAR promoter.
In vivo and ex vivo examination of LMB StAR established a temporal correlation of StAR mRNA expression with steroidogenesis in LMB. An increase in StAR mRNA levels in vivo paralleled the levels of 17β-estradiol detected in the plasma (Fig. 1), suggesting that the connection between StAR mRNA synthesis and the biosynthesis of steroids from cholesterol occurs in LMB as it does in mammals. The regulation and metabolism of steroid hormones is very complex in all vertebrates, and although we associate an increase in StAR mRNA abundance with increased plasma 17β-estradiol, the activities of other key enzymes, such as P450 aromatase present predominantly in the gonad and brain and at lower levels in peripheral tissues, and enzymes involved in phase II metabolism in the liver, are also important factors that control plasma levels of steroid hormones. The up-regulation of LMB StAR mRNA by dbcAMP in ex vivo cultures of LMB ovarian follicles (Fig. 2B) implicates that important signaling mechanisms that transactivate StAR may be conserved across species.
To identify the transcriptional elements involved in the regulation of StAR transcription in lower vertebrates, a 2.9-kb portion of the LMB StAR promoter was cloned for sequence analysis and used in transfection assays. In silico analysis revealed multiple putative ROREs, which appeared to be conserved in the promoters of fish and mammals, although the specific positions in the sequences did not always correspond. The putative elements predicted by the bioinformatic programs are not necessarily biological regulators of the promoter; therefore, determination of their functionality requires further experimentation.
Critical elements involved in dbcAMP regulation of LMB StAR transcription are located in the distal portion of the promoter. The 2.9-kb LMB StAR promoter was induced greater than 2.7-fold in response to both 1 mm dbcAMP (Fig. 3A) and 10 U/ml hCG (Fig. 3B), which mirrored results published for the human StAR promoter in Y-1 cells (32). However, deletion of 1 kb from the 5′ end of the LMB 2.9-kb promoter significantly diminished the induction by dbcAMP (Fig. 3C). There are putative AP-1, SF-1, ROR/rev-erb, and estrogen response element sites as well as others within this region, which could be critically involved in regulating StAR transcription. Functional roles of SF-1- and AP-1-mediating StAR promoter transactivation have previously been reported (17,32,33), and these sites were not tested in this study. Instead our research was focused on the RORE site.
Recent studies implicated RORα and rev-erbα in regulating the activity of genes involved in circadian rhythm (34). Because steroidogenesis in fish is seasonally regulated, we focused our investigation on characterizing the potential roles of RORα and rev-erbα in transcriptional regulation of the StAR promoter.
Site-directed mutation of the putative RORE/−1969 element (located between the −1.86- and −2.9-kb region of the LMB StAR promoter) robustly diminished the response of the promoter to dbcAMP (Fig. 3C), suggesting potentially new signaling mechanisms for dbcAMP regulation of StAR transcription. RORα is an important nuclear factor involved in the transcriptional activation of genes that control multiple physiological processes, including those central to controlling peripheral circadian rhythm (35). There are four isoforms of RORα that arise from alternative promoter usage and alternative splicing. RORα1 and -4 are ubiquitously expressed. It is notable that cholesterol has been identified as a ligand for RORα in recent studies (36,37), suggesting that it may be a key sensor for steroid production. In addition, rev-erbα has been reported to competitively bind the same element as RORα, disallowing the activation of target genes by RORα (38). The possibility that RORα and rev-erbα regulate steroidogenesis is strongly supported by the presence of multiple ROR elements in the LMB StAR promoter, in particular the RORE at −1969 bp upstream from the transcriptional start site located in the 5′ distal promoter segment. Indeed, bioinformatic analysis also revealed high-affinity sites for RORα/rev-erbα in several mammalian species, including human, rat, and mouse, but the roles that these proteins may play in regulating the mammalian StAR gene have not yet been investigated.
Noting that RORα and rev-erbα bind to the same core sequence, mutation of the RORE/−1969 site in the LMB StAR promoter could attenuate the binding of the two proteins under dbcAMP-stimulated and basal conditions, respectively. This may account for the loss of response to dbcAMP in the LMB StAR promoter observed upon mutation, particularly if RORα mediates dbcAMP activation of the promoter. This site, with both positive and negative regulatory potential (e.g. through RORα/rev-erbα signaling pathways), may be especially important in regulating fish steroidogenesis and may be the link to circadian control of this process.
Endogenous binding of RORα and rev-erbα to the LMB RORE/−1969 element (Fig. 4A) and also of rev-erbα to the mouse mRORE/−634 element (Fig. 4B) were verified by ChIP, implicating that these proteins play significant roles in controlling the transcriptional activation of the promoters in both species. The mouse StAR promoter has been very well characterized (20), and it has been reported that negative regulatory elements may lie between base pairs −966 and −254 relative to the transcriptional start site (39,40). Our studies have shown that rev-erbα binds to the mouse RORE/−634 element (located ∼−634 bp upstream of the transcriptional start site) under basal conditions and that the protein does not associate with the element under dbcAMP-stimulated conditions, signifying that rev-erbα may play an important role in the basal control of the promoters in LMB and mice.
Because the enrichment of RORα in the ChIP studies on the LMB RORE/−1969 element was not as robust when compared with the results with rev-erbα, we chose to conduct EMSA experiments to further investigate the binding of RORα to the LMB promoter. Indeed, the human RORα4 protein is capable of binding to the LMB RORE/−1969 site (Fig. 4C). Interestingly, one study investigated gene expression profiles in staggerer mice (which do not express the RORα gene) and reported that key enzymes in the steroid biosynthetic pathway were down-regulated in the mutant phenotype, indicating that RORα may play a role in controlling genes involved in steroid hormone biosynthesis (41). These studies further suggest that the ROR and rev-erb families of orphan nuclear receptors may play an integral role in modulating steroidogenesis.
Several studies have shown that the proximal several hundred base pair segment of the mammalian StAR promoter is sufficient in mediating maximal cAMP-induced transactivation (19,39). The results observed in the current study suggest that the functional RORE found in the distal segment of the LMB StAR promoter is important in mediating cAMP-induced promoter transactivation. It has been reported that RORα/rev-erbα elements located nearly −7 kb upstream of the transcriptional start site of a gene can play critical roles in enhancing or repressing gene transcription (42). This phenomenon could be due to the recruitment of essential cofactors, the presence of enhancers in distal regions, or altered DNA folding and structure. It is possible that the distal segment of the LMB StAR promoter functions in one of these ways to regulate the LMB StAR gene, although further studies are warranted to investigate this hypothesis.
The combination of ovarian follicle, promoter deletion, and site-directed mutation data implies that transcriptional elements between −1.86 and −2.9 kb of the LMB StAR promoter are required for cAMP-induced activation. The mutation data revealed that more than 80% loss in transcriptional activity of the LMB StAR promoter can be attributed to an RORE upstream from −2 kb of this region. ChIP and EMSA analyses in our studies reveal that both rev-erbα and RORα may play integral roles in the activation of the LMB StAR promoter. Additionally, it is likely that regulation of StAR transcription is conserved from mammals to lower vertebrates and that nonclassical species such as LMB are increasingly pertinent model systems for comparative studies.
Supplementary Material
Acknowledgments
We thank Dr. Tara Sabo-Attwood for generating the cDNA for the in vivo studies and Dr. Mario Ascoli for the gift of the MA-10 cells.
Footnotes
This work was supported by the Superfund Basic Research Program Grant P42 ES 07375 from the National Institute of Environmental Health Sciences (NIEHS) and NIEHS Grant RO1 ES015449.
Current address for J.K.: Department of Neuroscience, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 11, 2009
Abbreviations: AP, Activator protein; ChIP, chromatin immunoprecipitation; dbcAMP, dibutyryl cAMP; E2, 17β-estradiol; hCG, human chorionic gonadotropin; LMB, largemouth bass; PKA, protein kinase A; PKC, protein kinase C; Q-PCR, quantitative real-time RT-PCR; RACE, rapid amplification of cDNA ends; SF, steroidogenic factor; ROR, retinoic acid-related receptor; RORE, ROR element; StAR, steroidogenic acute regulatory; UTR, untranslated region.
References
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- Manna PR, Stocco DM 2005 Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metab Disord 5:93–108 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Stocco DM 1995 Expression of the steroidogenic acute regulatory (StAR) protein: a novel LH-induced mitochondrial protein required for the acute regulation of steroidogenesis in mouse Leydig tumor cells. Endocr Res 21:243–257 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 51:197–205 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1997 The role of the steroidogenic acute regulatory protein in steroidogenesis. Steroids 62:29–36 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ, Reinhart AJ, Williams SC, Dyson M, Dassi B, Walsh LP, Manna PR, Wang XJ, Zeleznik AJ, Orly J 2001 Elements involved in the regulation of the StAR gene. Mol Cell Endocrinol 177:55–59 [DOI] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM 2005 Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod 73:244–255 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, Jo Y, Stocco DM 2006 cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37:81–95 [DOI] [PubMed] [Google Scholar]
- Aesøy R, Mellgren G, Morohashi K, Lund J 2002 Activation of cAMP-dependent protein kinase increases the protein level of steroidogenic factor-1. Endocrinology 143:295–303 [DOI] [PubMed] [Google Scholar]
- Clem BF, Hudson EA, Clark BJ 2005 Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology 146:1348–1356 [DOI] [PubMed] [Google Scholar]
- Hagen IJ, Kusakabe M, Young G 2006 Effects of ACTH and cAMP on steroidogenic acute regulatory protein and P450 11β-hydroxylase messenger RNAs in rainbow trout interrenal cells: relationship with in vitro cortisol production. Gen Comp Endocrinol 145:254–262 [DOI] [PubMed] [Google Scholar]
- Li YY, Inoue K, Takei Y 2003 Steroidogenic acute regulatory protein in eels: cDNA cloning and effects of ACTH and seawater transfer on its mRNA expression. Zoolog Sci 20:211–219 [DOI] [PubMed] [Google Scholar]
- Aluru N, Vijayan MM 2006 Aryl hydrocarbon receptor activation impairs cortisol response to stress in rainbow trout by disrupting the rate-limiting steps in steroidogenesis. Endocrinology 147:1895–1903 [DOI] [PubMed] [Google Scholar]
- Nunez BS, Evans AN 2007 Hormonal regulation of the steroidogenic acute regulatory protein (StAR) in gonadal tissues of the Atlantic croaker (Micropogonias undulatus). Gen Comp Endocrinol 150:495–504 [DOI] [PubMed] [Google Scholar]
- Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P 1997 DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature 390:311–315 [DOI] [PubMed] [Google Scholar]
- Christenson LK, Osborne TF, McAllister JM, Strauss 3rd JF 2001 Conditional response of the human steroidogenic acute regulatory protein gene promoter to sterol regulatory element binding protein-1a. Endocrinology 142:28–36 [DOI] [PubMed] [Google Scholar]
- Shea-Eaton W, Sandhoff TW, Lopez D, Hales DB, McLean MP 2002 Transcriptional repression of the rat steroidogenic acute regulatory (StAR) protein gene by the AP-1 family member c-Fos. Mol Cell Endocrinol 188:161–170 [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM 2003 Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol 30:381–397 [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Stocco DM 2004 Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol 18:558–573 [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM 2003 Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids 68:1125–1134 [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Stocco DM 2009 Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Strauss 3rd JF 2004 Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: temporal and spatial changes in transcription factor binding and histone modification. Mol Cell Endocrinol 215:119–126. [DOI] [PubMed] [Google Scholar]
- Silverman E, Yivgi-Ohana N, Sher N, Bell M, Eimerl S, Orly J 2006 Transcriptional activation of the steroidogenic acute regulatory protein (StAR) gene: GATA-4 and CCAAT/enhancer-binding protein β confer synergistic responsiveness in hormone-treated rat granulosa and HEK293 cell models. Mol Cell Endocrinol 252:92–101 [DOI] [PubMed] [Google Scholar]
- Martin LJ, Boucher N, Brousseau C, Tremblay JJ 2008 The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol Endocrinol 22:2021–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR 2005 Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- Dolatshad H, Davis FC, Johnson MH 2009 Circadian clock genes in reproductive tissues and the developing conceptus. Reprod Fertil Dev 21:1–9 [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Ikeda M, Kimura T, Honma S, Ohmiya Y, Honma K 2004 Bidirectional role of orphan nuclear receptor RORα in clock gene transcriptions demonstrated by a novel reporter assay system. FEBS Lett 565:122–126 [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U 2002 The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260 [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB 2004 A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43:527–537 [DOI] [PubMed] [Google Scholar]
- Giguère V 1999 Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725 [DOI] [PubMed] [Google Scholar]
- Lechtken A, Zündorf I, Dingermann T, Firla B, Steinhilber D 2006 Overexpression, refolding, and purification of polyhistidine-tagged human retinoic acid related orphan receptor RORα4. Protein Expr Purif 49:114–120 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Saito M, Fujimoto S 2000 Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology 141:2895–2903 [DOI] [PubMed] [Google Scholar]
- Lopez D, Nackley AC, Shea-Eaton W, Xue J, Schimmer BP, McLean MP 2001 Effects of mutating different steroidogenic factor-1 protein regions on gene regulation. Endocrine 14:353–362 [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Dardente H, Giguère V, Cermakian N 2005 Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20:391–403 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S 2005 System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 37:187–192 [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B 2004 Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem 279:14033–14038 [DOI] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B 2002 X-ray structure of the hRORα LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORα. Structure 10:1697–1707 [DOI] [PubMed] [Google Scholar]
- Moraitis AN, Giguère V 1999 Transition from monomeric to homodimeric DNA binding by nuclear receptors: identification of RevErbAα determinants required for RORα homodimer complex formation. Mol Endocrinol 13:431–439 [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ 1997 Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol 11:138–147 [DOI] [PubMed] [Google Scholar]
- Clem BF, Clark BJ 2006 Association of the mSin3A-histone deacetylase 1/2 corepressor complex with the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol 20:100–113 [DOI] [PubMed] [Google Scholar]
- Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM 2007 Gene expression profiling reveals a regulatory role for ROR α and ROR γ in phase I and phase II metabolism. Physiol Genomics 31:281–294 [DOI] [PubMed] [Google Scholar]
- Bois-Joyeux B, Chauvet C, Nacer-Chérif H, Bergeret W, Mazure N, Giguère V, Laudet V, Danan JL 2000 Modulation of the far- upstream enhancer of the rat α-fetoprotein gene by members of the ROR α, Rev-erb α, and Rev-erb beta groups of monomeric orphan nuclear receptors. DNA Cell Biol 19:589–599 [DOI] [PubMed] [Google Scholar]
- Sabo-Attwood T, Kroll KJ, Denslow ND 2004 Differential expression of largemouth bass (Micropterus salmoides) estrogen receptor isotypes α, β, and γ by estradiol. Mol Cell Endocrinol 218:107–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.