Abstract
An increase in intracellular Ca2+ ([Ca2+]i) as a result of release of Ca2+ from intracellular stores or influx of extracellular Ca2+ contributes to the regulation of smooth muscle contractile activity. Human uterine smooth muscle cells exhibit receptor-, store-, and diacylglycerol (OAG)-mediated extracellular Ca2+-dependent increases in [Ca2+]i (SRCE) and express canonical transient receptor potential-like channels (TRPC) mRNAs (predominantly TRPC1, -4, and -6) that have been implicated in SRCE. To determine the role of TRPC6 in human myometrial SRCE, short hairpin RNA constructs were designed that effectively targeted a TRPC6 mRNA reporter for degradation. One sequence was used to produce an adenovirus construct (TC6sh1). TC6sh1 reduced TRPC6 mRNA but not TRPC1, -3, -4, -5, or -7 mRNAs in PHM1-41 myometrial cells. Compared with uninfected cells or cells infected with empty vector, the increase in [Ca2+]i in response to OAG was specifically inhibited by TC6sh1, whereas SRCE responses elicited by either oxytocin or thapsigargin were not changed. Similar findings were observed in primary pregnant human myometrial cells. When PHM1-41 cells were activated by OAG in the absence of extracellular Na+, the increase in [Ca2+]i was partially reduced. Furthermore, pretreatment with nifedipine, an L-type calcium channel blocker, also partially reduced the OAG-induced [Ca2+]i increase. Similar effects were observed in primary human myometrial cells. These findings suggest that OAG activates channels containing TRPC6 in myometrial cells and that these channels act via both enhanced Na+ entry coupled to activation of voltage-dependent Ca2+ entry channels and a nifedipine-independent Ca2+ entry mechanism to promote elevation of intracellular Ca2+.
Attenuation of TRPC6 expression specifically reduces OAG-mediated increases in intracellular Ca2+ in myometrial cells that is partially mediated by a Na+-dependent and nifedipine-sensitive mechanism.
An increase in intracellular Ca2+ ([Ca2+]i) contributes to the regulation of contraction in myometrium (1). Myometrial cells exhibit an increase in [Ca2+]i as a result of Ca2+ release from intracellular stores in response to G protein-coupled receptor stimulation or inhibition of the endoplasmic reticulum Ca2+-ATPase pump as well as by facilitation of extracellular Ca2+ entry (2,3,4). Extracellular Ca2+-dependent increases in [Ca2+]i, variously attributed to receptor- or store-operated or capacitative Ca2+ entry (5,6), are referred to here collectively for convenience as signal-regulated Ca2+ entry (SRCE). Canonical transient receptor potential (TRPC) are Ca2+-permeable, nonselective ion channels that have been implicated in SRCE. TRPCs have been divided into four subfamilies (TRPC1, TRPC2, TRPC3/6/7, and TRPC4/5) based on their amino acid sequences and functional similarities. They form homo- and heterotetrameric channels that are differentially regulated, depending on their subunit composition, concentration, and the cell type in which they are expressed (6,7,8). The cell-permeable diacylglycerol analog 1-oleoyl-2-acetyl-sn-glycerol (OAG) activates TRPC3/6/7 channels independent of protein kinase C (PKC) activation (9,10).
TRPC6 is expressed in tissues enriched in smooth muscle such as myometrium, vascular and pulmonary artery, colon, and stomach (11). TRPC6 has been implicated in depolarization and myogenic tone in small resistance arteries (12) and has been reported to respond to mechanical and osmotic stimulation (13). In human myometrium and myometrial cells, TRPC1, -4, and -6 are the most abundant mRNAs, although all TRPC mRNAs except TRPC2 (a pseudogene in humans) are expressed, and detection of all of the respective proteins except TRPC7 has been reported (2,14,15). We reasoned that, of these proteins, TRPC6 is the most likely to be activated by diacylglycerol in myometrium. OAG elicits a variety of patterns of [Ca2+]i transients in individual myometrial cells that are completely dependent on extracellular Ca2+ and are PKC independent (4).
The effect of TRPC6 on [Ca2+]i dynamics has largely been explored using overexpressed protein. TRPC6 has been implicated in receptor-operated Ca2+ entry in a simple or complex manner in these studies, some of which also implicate OAG-stimulated Na+ entry (10,16,17,18,19). Recently overexpression of Orai1, implicated in store-operated Ca2+ entry, has been shown to impair sensitivity to store depletion to cells overexpressing TRPC6 (20). However, because overexpression may change the nature and properties of the heterotetrameric TRP channels in a given cell type, it is important to study the effects of endogenous TRPC6 on [Ca2+]i dynamics in the cell type of interest.
The mechanism by which OAG produces an increase in [Ca2+]i is still not well understood. In A7r5 rat smooth muscle cells, TRPC6 has been implicated in mediating OAG-stimulated cation currents, and the OAG-stimulated increase in [Ca2+]i was inhibited by the L-type Ca2+ channel inhibitor nimodipine (21). Alternatively, ATP-induced Ca2+ entry in rat aorta smooth muscle cells has been attributed to localized Na+ entry that is inhibited by dominant negative TRPC6, with stimulation of Ca entry via reversal of the Na/Ca exchanger (22).
In the present study, we show that attenuation of TRPC6 expression specifically reduces OAG-mediated increases in [Ca2+]i in myometrial cells, and we provide evidence that this is partially mediated by a Na+-dependent and nifedipine-sensitive mechanism.
Materials and Methods
Materials
Fura 2-acetoxymethylester (AM) and pluronic acid F127 were obtained from Invitrogen (Carlsbad, CA). Thapsigargin, oxytocin, nifedipine, and other compounds were purchased from Sigma-Aldrich (St. Louis, MO). OAG was obtained from Calbiochem (La Jolla, CA). Cell culture medium and other tissue culture reagents were obtained from Invitrogen/Life Technologies, Inc. (Carlsbad, CA). Oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Restriction enzymes were purchased from New England Biolabs (Ipswich, MA). A7r5 cells were obtained from American Type Culture Collection (Manassas, VA). Uterine smooth muscle cells (UtSMCs) derived from nonpregnant human myometrium (catalog no. CC-2562, lot no. 17590) were purchased from Lonza (Walkersville, MD).
Construction and testing of TRPC6-shRNA plasmid vectors
The hTRPC6 RNA sequence (NM_004621) was analyzed for potential short hairpin RNA (shRNA) candidates using the Dharmacon siRNA Design Center (Lafayette, CO). Four potential shRNAs containing TRPC6 sequences that did not have significant homology to other TRPCs were selected for study. Oligonucleotide sequences of TRPC6shRNA (NM_004621) constructs were as follows; TC6sh1, 5′-GCGACAGAGCATCATTGACGCAAATCTGTGAAGCCACAGATGGGATTTGCGTCAATGATGCTCTGGTGCTTTTTT-3′; TC6sh2, 5′-GCGCTGCCACTCACTCAACGTTAACCTGTGAAGCCACAGATGGGGTTAACGTTGAGTGAGTGGCATTGCTTTTTT-3′; TC6sh3, 5′-GCGTCCCAAATCTCAGCCGTTTAAACTGTGAAGCCACAGATGGGTTTAAACGGCTGAGATTTGGGCTGCTTTTTT-3′; TC6sh4, 5′-GCGCATCGAGGACCAGCATACATGTCTGTGAAGCCACAGATGGGACATGTATGCTGGTCCTCGATTTGCTTTTTT-3′.
The shRNA constructs were designed to include a human premicroRNA stem sequence (23) between the sense and antisense sequences. Sense and antisense shRNA oligonucleotides were annealed and cloned into pSHAG vector (G. Hannon, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) under the control of a U6 promoter as described in detail elsewhere (24). Sequences were confirmed by direct sequencing. Efficacy of the TRPC6 shRNAs was tested using the psiCHECK-2 system (Promega, Madison, WI). A reporter construct was produced by inserting the TRPC6 coding sequence obtained by PCR (2) into the EcoRI site of pcDNA6/V5 (Invitrogen) and then excising with PmeI and NotI and cloning into the multiple cloning site located 3′ to the Renilla luciferase translation stop codon in the psiCHECK-2 vector. This reporter vector (1 μg) was cotransfected with pSHAG-hTRPC6shRNAs (1 μg) into AD293 cells and Renilla luciferase was measured 72 h after transfection. Measurement of firefly luciferase activity, also expressed in the vector, allowed normalization to correct for transfection efficiency.
Construction of TRPC6 shRNA-expressing adenovirus
As described in more detail in another knockdown study (24), the shRNA construct (TC6sh1 in this case) in pSHAG was subjected to recombination with modified pAdTrack-RfA(f) plasmid (C. Clay, Colorado State University, Fort Collins, CO). The integrity of the pAdT-TC6sh1 clone was checked by restriction enzyme digestion and by sequencing. The pAdTrack vector contains the sequence for green fluorescent protein under the control of a separate cytomegalovirus promoter, allowing identification of infected cells. The recombined vectors were linearized with PmeI and subjected to homologous recombination with pAdEasy-1 (Stratagene, La Jolla, CA) and used to prepare packaged virus in AD293 cells as described by the manufacturer. After viral stocks were amplified, they were purified with an adenovirus purification kit (CLONTECH, Mountain View, CA), aliquoted, and stored at −80 C until used.
Cell culture and viral infection
PHM1-41 immortalized myometrial cells were derived from late term myometrial tissue and retain many of the properties of primary myometrial cells and tissue (15,25,26,27,28). PHM1-41 cells were cultured in DMEM with 10% fetal calf serum, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.1 mg/ml G418 sulfate, and 2 mm l-glutamine and were used between passages 20 and 25 unless otherwise indicated. Primary human myometrial cells (HMC1) were isolated from late-term myometrial tissue from one patient, cultured in the media described above without G418 sulfate, and used at passages 4–8. The tissue was obtained under approved protocols (Colorado State University and University of Colorado Health Sciences Center) and with informed consent. Both PHM1-41 and primary myometrial cells were incubated with viral constructs at 1000 multiplicity of infection and harvested 48–72 h after infection. A7r5 cells and UtSMCs were cultured in the media used for the culture of HMC1 cells and used at passages 20–25 and 8–10, respectively.
Measurement of [Ca2+]i
Myometrial cells (0.3–1 × 105) were plated on 35-mm dishes (MatTek, Ashland, MA) in 1 ml culture medium. PHM1-41 cells were loaded with fura 2-AM (5 μm) in fluorescence buffer [FB; 145 mm NaCl, 5 mm KCl, 1 mm Na2HPO4, 0.5 mm MgCl2, 1 mm CaCl2, 10 mm HEPES, 5 mm glucose (pH 7.4)] at room temperature for 30 min. Primary myometrial cells were loaded with 5 μm fura 2-AM plus 0.1% Pluronic F-127 in FB. After loading, cells were washed and incubated for 45 min in FB to allow for ester hydrolysis. Ca2+-free FB contained 100 μm EGTA and no CaCl2. Changes in [Ca2+]i in individual cells were measured as the ratio of fluorescence at 340/380 nm excitation and 510 nm emission, using an InCyt2 imaging system (Intracellular Imaging, Cincinnati, OH). To test the effect of extracellular Na+ on the OAG-induced SRCE, Na+ was replaced with equimolar choline chloride.
The mean amplitude of the initial [Ca2+]i rise and integrated [Ca2+]i increase over baseline over a fixed time interval are reported. Integrated area was calculated using Kaleidagraph software (Synergy Software, Reading, PA) or numerical analysis software (CalciumComp) developed by an engineer consultant (K. J. Bois, Fort Collins, CO). CalciumComp aligns the initial [Ca2+]i peaks, removes noise, and calculates the extent of the increase [Ca2+]i (peak height) and integrated area under the [Ca2+]i transient curve.
Quantitative real-time RT-PCR
Real-time PCR studies were carried out as described in general elsewhere, using primers previously validated for amplification of the rat and human TRPC sequences (15,29). In these studies, identity of the quantitative RT-PCR products, all single bands of the expected size, was verified by direct sequencing. Total RNA was isolated from cells using the RNAeasy minikit, which includes a DNase step (QIAGEN, Valencia, CA). One-step RT-PCR was performed using the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA) and Bio-Rad iScript one-step RT-PCR kit. Specific conditions were 300 ng total RNA from PHM1-41 cells and 50 ng total RNA from A7R5 cells, cDNA synthesis at 50 C for 30 min, reverse transcriptase inactivation at 95 C for 10 min, 40 PCR cycles consisting of denaturation at 95 C for 15 sec, annealing at 60 C for 30 sec, and extension at 72 C for 1 min. Melting curves for all products showed single peaks. The relative target gene copy number was quantified by the ΔΔCt method (30), using β-actin for A7r5 cells and hydroxymethylbilane synthase for PHM1-41 cells as internal standards.
Western blotting
PHM1-41 and A7R5 cells were scraped in homogenizing buffer [50 mm Tris-HCl, 5 mm MgCl2, 100 mm KCl, 1 mm EGTA, 250 mm sucrose, 1× protease inhibitor cocktail (Sigma-Aldrich) (pH 7.2)] and sonicated on ice. The supernatants after centrifugation for 15 min at 20,817 × g were termed cell lysates. Crude membrane preparations were obtained from PHM1-41 cell homogenates by centrifugation for 5 min at 2,655 × g, followed by centrifugation of the supernatant for 1 h at 100,000× g. The resulting pellets were resuspended in sample buffer (10 mm Tris-HCl, 250 mm sucrose, 1 mm EGTA, 1× protease inhibitor cocktail) and were quantified with the Bradford reagent (Bio-Rad). Twenty micrograms of crude membrane protein or 10 μg of cell lysate protein were subjected to electrophoresis in 6% sodium dodecyl sulfate-polyacrylamide gels and transferred onto nitrocellulose membranes (Whatman, Schleicher & Schuell, Florham Park, NJ). Membranes were blocked by incubation in 5% nonfat dry milk in PBS [2.68 mm KCl, 1.47 mm KH2PO4, 136.9 mm NaCl, and 8.1 mm Na2HPO4 (pH 7.2)] containing 0.05% Tween 20 for 1 h at room temperature. Membranes were exposed to anti-TRPC6 (1:200–300 in 5% milk/PBS containing 0.05% Tween 20; Alomone Labs, Jerusalem, Israel) overnight at 4 C and then to horseradish peroxidase-conjugated donkey antirabbit IgG (1:2000; Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 1 h at room temperature. For control immunoblots, antibodies (1 μg) were preabsorbed with an antigenic peptide (1 μg; Alomone Labs) 1 h before the overnight incubation. Detection was achieved using the ECL Plus system (Amersham Biosciences, Pittsburgh, PA) and quantitated using an Amersham Storm 860 instrument. Membranes were stripped and probed with antiphospholipase Cβ3 (1:750; Santa Cruz Biotechnology) as a loading control. The TRPC6 antibody is directed against the RRNESQDYLLMDELG sequence in rat/mouse TRPC6; human TRPC6 contains one additional S between residues D and E.
Statistics
Data are expressed as mean ± se. Data were analyzed using Student’s t test or one-way ANOVA and Tukey’s test using Prism software (GraphPad Software, La Jolla, CA) where indicated. A value of P < 0.05 was considered significant.
Results
Efficacy of TRPC6shRNA plasmid and viral constructs in altering myometrial cell TRPC6 expression
To examine the role of TRPC6 on the [Ca2+]i dynamics in human myometrial cells, four potential shRNAs targeting TRPC6 were designed and tested for their efficacy using the psiCHECK-2 system (Promega) with a Renilla luciferase-TRPC6 reporter. The four TRPC6 shRNA (TC6sh1-4) plasmid constructs reduced luminescence by 82, 85, 75, and 69%, respectively, compared with empty vector (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), suggesting that TRPC6 mRNA was targeted effectively for degradation. A TRPC4 shRNA construct generated for use in another study had no effect, indicating the specificity of the TRPC6 shRNA constructs.
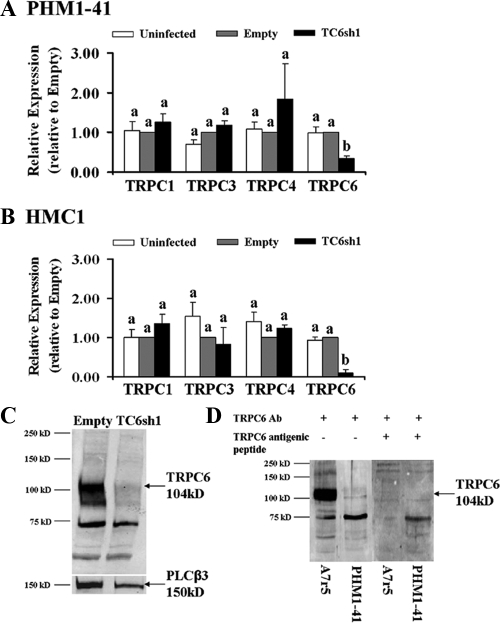
Prior experience established that PHM1-41 and primary myometrial cells are difficult to transfect. Therefore, a recombinant adenovirus was generated expressing TRPC6 shRNA no. 1 (TC6sh1) for use in myometrial cells. PHM1-41 cells were infected with adenovirus-expressing empty vector or TC6sh1 at greater than 95% efficiency 48 and 72 h after infection, as determined by visual green fluorescent protein expression. There were no differences between TRPC mRNA expression in uninfected cells and cells infected with adenovirus expressing empty vector (Fig. 1A), suggesting that viral infection itself has no significant effect on TRPC expression. Infection with virus expressing TC6sh1 produced 53–77% (mean 65%) knockdown of endogenous TRPC6 mRNA 72 h after infection. This effect was highly specific because there were no changes in TRPC1, TPRC3, or TRPC4 expression (Fig. 1A). TRPC5 and TRPC7 mRNAs are relatively nonabundant in myometrial cells and tissue (15); no changes in these mRNAs were detected within the limitations of the assay (data not shown). Infection of primary HMC1 cells with TC6sh1 resulted in 73–100% (mean 90%) knockdown of TRPC6 mRNA and no change in TRPC1, TPRC3, or TRPC4 mRNAs 72 h after infection (Fig. 1B).
Figure 1.
Effect of exposure to TC6sh1 on TRPC mRNA expression in PHM1-41 cells (mean ± se, n = 3–7) (A) and HMC1 cells (mean ± se, n = 3–5) (B), expressed relative to empty vector. Significant differences between groups are indicated by different lower-case letters. C, Reduction of expression TRPC6 protein in PHM1-41 crude membrane (20 μg) from cells infected with adenovirus-expressing TC6sh1 for 72 h, compared with membrane from cells infected with empty adenovirus. The membrane was stripped and reprobed for phospholipase Cβ3 as a loading control. D, Demonstration that preabsorbing the anti-TRPC6 antibody (Ab) with antigenic peptide eliminated the TRPC6 band in cell lysates (10 μg) from A7r5 and PHM1-41 cells.
Exposure to TC6sh1 significantly reduced expression of TRPC6 protein by 64% compared with empty vector (Fig. 1C); in this experiment TRPC6 mRNA was reduced 73%. Mean protein knockdown in seven experiments was 57 ± 12% compared with empty vector. Figure 1D shows that the 104 kDaTRPC6 band was not detected with antibody preabsorbed with TRPC6 antigenic peptide.
TrpC6 shRNA specifically suppresses OAG-stimulated SRCE in PHM1 and primary myometrial cells
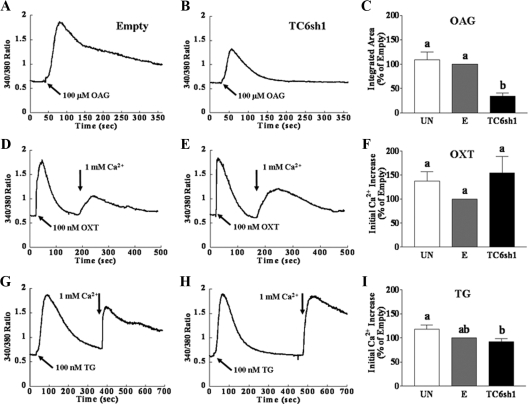
Consistent with our previous observations (4), individual uninfected PHM1-41 cells showed a variety of patterns of [Ca2+]i increase in response to 100 μm OAG in the presence of extracellular Ca2+. These increases were independent of PKC inhibition by the combination of staurosporin (1 μm) and Gö6983 (1 μm) (data not shown). Because of the variation in the [Ca2+]i responses, data are reported as mean integrated [Ca2+]i over a fixed time interval. Figure 2A shows an example of the effect of OAG on [Ca2+]i in one group of PHM1-41 cells infected with empty vector, plotted as mean [Ca2+]i, and the partial attenuation of this response in cells infected with virus expressing TC6sh1 (Fig. 2B). Figure 2C summarizes the mean responses observed in multiple experiments, analyzed as mean integrated [Ca2+]i over baseline in 6 min. The response to OAG was not affected by infection with empty virus. Expression of TC6sh1 suppressed the OAG-stimulated increase in [Ca2+]i by 39–88% (mean of 66%) at 72 h after infection, compared with the response in cells exposed to empty vector. In contrast, the SRCE responses elicited by the addition of extracellular Ca2+ after exposure of PHM1-41 cells to oxytocin (receptor stimulated) or thapsigargin (store depletion stimulated) in the absence of extracellular Ca2+ were not affected, compared with empty vector, by exposure to virus expressing TC6sh1 (Fig. 2, D–F, and Fig. 2, G–I, respectively). Qualitatively similar results were also observed 48 h after infection.
Figure 2.
TC6sh1 attenuates the increase in [Ca2+]i in response to OAG but not the SRCE response elicited by oxytocin (OXT) and thapsigargin (TG) in PHM1-41 cells. Representative [Ca2+]i traces of the averaged response of 20–25 cells to SRCE stimuli in dishes exposed to empty virus or virus expressing TC6sh1 are shown in panels A and B, D and E, and G and H and the corresponding mean responses (mean ± se, n = 8–9 dishes) in uninfected cells (UN), cells infected with empty virus (E), and cells infected with adenovirus expression TC6sh1 are shown in panels C, F, and I. Cells were exposed to empty vector or to adenovirus expressing TC6sh1 for 72 h before loading with fura 2-AM. Cells were stimulated with 100 μm OAG in the presence of 1 mm extracellular Ca2+ (panels A–C) or with 100 nm OXT or TG in the absence of extracellular Ca2+, followed by addition of 1 mm extracellular Ca2+ (arrow) to elicit SRCE (panels D–F and G–I, respectively). Responses to OAG are reported as integrated [Ca2+]i increase over baseline for 6 min, and responses to OXT and TG are reported as mean amplitude of initial [Ca2+]i rise. Significant differences between groups are indicated by different lowercase letters.
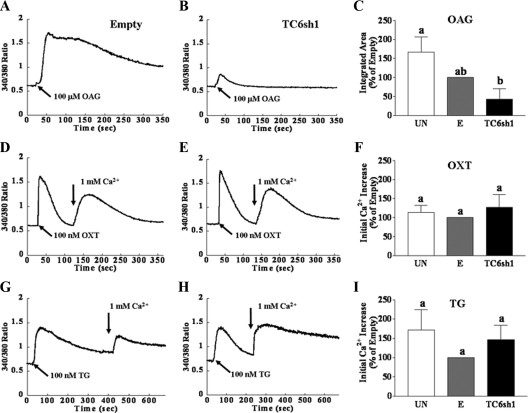
Similar responses were also observed in primary pregnant human myometrial cells. Figure 3 shows representative [Ca2+]i transients in HMC1 cells and a summary of the means from multiple experiments. There were no differences between SRCE responses in uninfected cells and cells infected with adenovirus expressing empty vector (Fig. 3, C, F, and I). TC6sh1 specifically suppressed the OAG-stimulated response to OAG by 71–99% (mean of 85%) at 72 h after infection compared with cells infected with adenovirus expressing empty vector. As observed in PHM1-41 cells, the SRCE responses elicited by oxytocin (Fig. 3, D–F) and thapsigargin (Fig. 3, G–I) were not affected by infection of HMC1 cells with TC6sh1. Qualitatively similar results were also observed 48 h after infection.
Figure 3.
TC6sh1 attenuates the increase in [Ca2+]i in response to OAG but not oxytocin (OXT) and thapsigargin (TG) in primary HMC1 cells. Representative [Ca2+]i traces of the averaged response of 20–25 cells to SRCE stimuli are shown in panels A and B, D and E, and G and H and the corresponding mean responses (mean ± se, n = 3 dishes) responses in uninfected cells (UN), cells infected with empty virus (E), and cells infected with adenovirus expression TC6sh1 are shown in panels C, F, and I. Cells were exposed to empty vector or adenovirus-expressing TC6sh1 for 72 h before loading with fura 2-AM. Cells were stimulated with 100 μm OAG in the presence of 1 mm extracellular Ca2+ (panels A–C) or with 100 nm OXT or TG in the absence of extracellular Ca2+, followed by addition of 1 mm extracellular Ca2+ (arrow) to elicit SRCE (panels D–F and G–I, respectively). Responses to OAG are reported as integrated [Ca2+]i increase over baseline for 6 min, and responses to OXT and TG are reported as mean amplitude of initial [Ca2+]i rise. Significant differences between groups are indicated by different lowercase letters.
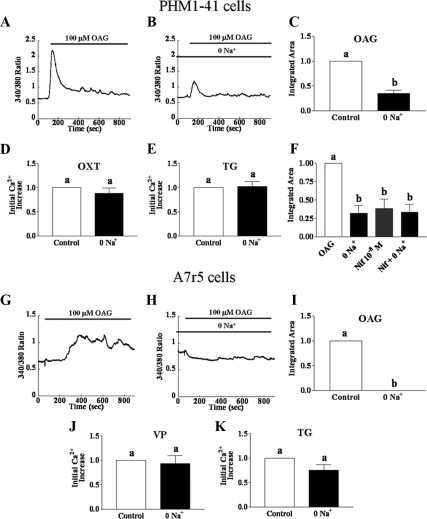
The OAG-stimulated increase in intracellular Ca2+ is attenuated in the absence of extracellular Na+ and inhibited by an L-type channel blocker
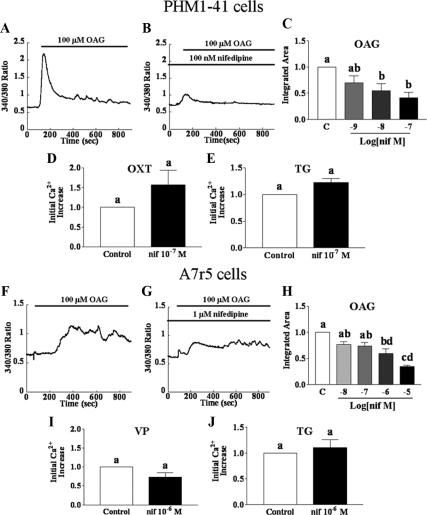
In A7r5 rat aortic smooth muscle cells, TRPC6 knockdown attenuated OAG-induced cation currents but, paradoxically, did not affect OAG-stimulated increases in [Ca2+]i (21), The OAG effect on [Ca2+]i involved the action of L-type Ca channels, as evidenced by sensitivity to nimodipine, an L-type channel blocker, and this was postulated to be secondary to Na+ entry (21). We explored whether a similar mechanism might pertain in PHM1-41 cells. Treatment of PHM1-41 cells with nifedipine, an L-type calcium channel blocker, reduced the OAG-induced [Ca2+]i increase, with significant inhibition at 0.01 μm. (Fig. 4, A–C). We found that the OAG response in A7r5 cells (Fig. 4F) was also inhibited by pretreatment with nifedipine and was significant at 1 μm (Fig. 4, G and H).
Figure 4.
Comparison of the effects of L-type channel blocker nifedipine on the effects of OAG-, oxytocin (OXT)-, vasopressin (VP)-, and thapsigargin (TG)-stimulated [Ca2+]i in PHM1-41 (panels A–E) and A7r5 cells (panels F–J). Where indicated, cells were pretreated with nifedipine for 5 min before the start of the experiments. Cells were stimulated with 100 μm OAG (panels A–C and F–H) in the presence of 1 mm extracellular Ca2+ or with 100 nm OXT (panel D, PHM1-41 cells) or VP (panel I; A7r5 cells) or 100 nm TG (panels E and J) in the absence of extracellular Ca2+, followed by addition of 1 mm extracellular Ca2+. Traces are the mean responses in 30–35 cells. Responses to OAG are reported as integrated [Ca2+]i increases over baseline for 15 min to take into account the delayed response in A7r5 cells. Responses to vasopressin, OXT, and TG were measured as mean amplitude of initial [Ca2+]i rise and expressed as percent of control (mean ± se, n = 3–4 dishes). Significant differences between groups are indicated by different lowercase letters.
In contrast to its effect on OAG-stimulated SRCE, at a concentration eliciting 59% inhibition of OAG-mediated SRCE in PHM1-41 cells (0.1 μm), nifedipine did not affect the SRCE stimulated by oxytocin or by thapsigargin (Fig. 4, D and E, respectively). Similarly, nifedipine (1 μm) did not affect vasopressin (receptor mediated) SRCE or thapsigargin (store depletion mediated) SRCE in A7r5 cells (Fig. 4, I and J, respectively). Nifedipine also did not significantly affect the [Ca2+]i response in the absence of extracellular Ca2+ elicited by oxytocin or thapsigargin in PHM1-41 cells or vasopressin or thapsigargin in A7r5 cells (data not shown).
To test the role of Na+ entry in the increase in [Ca2+]i, we examined the effect of removing extracellular Na+ on SRCE responses. In PHM1-41 cells, substitution of choline chloride for extracellular NaCl partially attenuated OAG-activated increases in [Ca2+]i (Fig. 5, A–C) but had no effect on oxytocin- (Fig. 5D) or thapsigargin-stimulated (Fig. 5E) increases in [Ca2+]i. The effects of removal of extracellular Na+ or inhibition by nifedipine (0.01 μm) on OAG-mediated SRCE were not additive (Fig. 5F). In A7r5 cells, the absence of extracellular Na+ also essentially eliminated the OAG-stimulated increase in [Ca2+]i (Fig. 5, G–I). Removing extracellular Na+ had no effect on vasopressin- (Fig. 5J) or thapsigargin-mediated SRCE (Fig. 5K). In both A7r5 and PHM1-41 cells, removing extracellular Na+ did not significantly affect the [Ca2+]i response to these agents in the absence of extracellular Ca2+ (data not shown).
Figure 5.
Comparison of the effect of removal of extracellular Na+ on the OAG-, oxytocin (OXT)-, vasopressin (VP)-, and thapsigargin (TG)-stimulated increases in [Ca2+]i in PHM1-41 cells (A–F) and A7r5 cells (G–K). For zero Na+, NaCl in the buffer was replaced with equimolar choline chloride. Cells were stimulated with 100 μm OAG (A–C and F–I) in the presence of 1 mm extracellular Ca2+ or with 100 nm OXT (D; PHM1-41 cells) or VP (J; A7r5 cells) or 100 nm TG (E and K) in the absence of extracellular Ca2+, followed by addition of 1 mm extracellular Ca2+. Traces are the mean responses in 30–35 cells. Responses to OAG are reported as integrated [Ca2+]i increases over baseline for 15 min. Responses to VP, OXT, and TG were measured as mean amplitude of initial [Ca2+]i rise and expressed as percent of control (mean ± se, n = 3–4 dishes). Significant differences between groups are indicated by different lowercase letters.
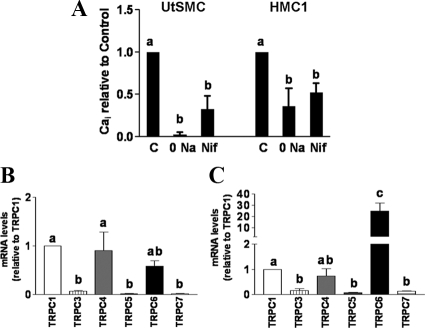
Qualitatively similar attenuation of OAG-stimulated SRCE was also noted in primary myometrial cells from nonpregnant (UtSMC) and pregnant (HMC1) patients (Fig. 6A). These data implicate Na+ entry linked to activation of L-type Ca channels in the SRCE elicited by OAG in human myometrial as well as in A7r5 smooth muscle cells and imply that receptor- and store-operated SRCE use different mechanisms than does OAG to achieve an increase in [Ca2+]i in both cell types.
Figure 6.
A, Comparison of the effect of removal of extracellular Na+ or nifedipine (Nif) on the OAG-stimulated increases in [Ca2+]i in UtSMC and HMC1 cells (mean ± se, n = 3). Responses are reported as integrated [Ca2+]i increases of baseline for 15 min. B and C, TRPC mRNA profiles in PHM1-41 and A7r5 cells, respectively. TRPC mRNAs were determined using quantitative real-time RT-PCR. In PHM1-41 cells, TRPC mRNAs were normalized to hydroxymethylbilane synthase and expressed relative to TRPC1 (mean ± se, n = 5–8). In A7r5 cells, TRPC mRNAs were normalized to β-actin and expressed relative to TRPC1 (mean ± se, n = 5, log transformed data). Significant differences between groups are indicated by different lowercase letters.
Despite these similarities, in PHM1-41 cells TRPC6 knockdown attenuated OAG- but not receptor-stimulated SRCE, whereas in A7r5 cells OAG-stimulated SRCE was not affected but receptor-stimulated SRCE was partially attenuated by TRPC6 knockdown. TRPC proteins form homo- and heterotetramers and the relative expression of TRPC isoforms could result in differing properties of TRPC channels in different cell types (6,7,8). Consistent with an earlier observation (15), PHM1-41 cells expressed TRPC1, TRPC4, and TRPC6 mRNAs in greatest relative abundance (Fig. 6B). UtSMCs and HMC1 cells exhibit the same relative TRPC isoform expression profile as PHM1-41 cells (15,24). In the A7r5 cells used in this study, TRPC6 mRNA exhibited greater relative abundance than all other TRPC isoforms (Fig. 6C). The TRPC6 antibody used in the Western blot in Fig. 2C targets a common 15 amino acid epitope in human and rat TRPC6, except that the human sequence has one additional amino acid after position 12. Assuming that the affinity of the antibody for both proteins is reasonably similar, A7r5 membranes contains significantly more TRPC6 immunoreactivity than an equal amount of PHM1-41 membrane protein (Fig. 1D).
Discussion
In the present study, attenuation of TRPC6 mRNA and protein expression inhibited OAG-induced SRCE in both PHM1-41 and human primary cells but did not affect G protein-coupled receptor-induced or store depletion-induced SRCE. OAG directly stimulates the activity of TRPC3/6/7 (9,10). In human myometrial cells, TRPC6 mRNA is significantly more abundant than TRPC3 and TRPC7 mRNAs, which are barely detectable [(15) and this study], suggesting that TRPC6 is a major responder to OAG in this cell type.
Diacylglycerol can act as a signaling molecule, both in itself and as an activator of PKC, in various cell types. OAG has been implicated in affecting [Ca2+]i dynamics, independent of PKC activation (9,10). In myometrial cells, OAG induced an increase in [Ca2+]i that was enhanced by diacylglycerol lipase inhibition but was not affected by PKC inhibition (4). We noted no effect of PKC inhibition on the OAG effects reported here. Nonetheless, effects of PKC on Ca2+ pumps or the Na+/Ca2+ exchanger that could affect intracellular Ca2+ dynamics cannot be ruled out entirely in these studies.
The effect of TRPC6 on [Ca2+]i dynamics has primarily been explored by overexpression (9,10,16,17,18,19). TRPC6 overexpression led largely to enhancement of OAG- and receptor-mediated but not store depletion-mediated SRCE. Nonetheless, overexpression may alter the composition of the predominant tetrameric TRP channels in a given cell type, thus changing their properties. Moreover, a recent study showing that overexpression of Orai1 can increase sensitivity of overexpressed TRPC6 to store depletion (20) suggests that stoichiometry in relation to other cellular components can also be a factor in determining the nature of the stimuli to which TRPC6 responds.
Reduction of endogenous TRPC6 has a variety of effects on [Ca2+]i dynamics in different cell types. Endogenous TRPC6 activity is apparently inhibited by phosphatidylinositide 4,5-bisphosphate and therefore may be indirectly stimulated by receptor-mediated phospholipase C activation (31). Knockdown of TRPC6 decreased store-operated SRCE in pulmonary artery smooth muscle cells (32) and phenylephrine-induced SRCE in prostate cancer epithelial cells (33). In HEK293 cells, stable knockdown of endogenous TRPC6 inhibited OAG-stimulated and carbachol-stimulated but not store depletion-stimulated Ba2+ influx (34). In a study using A7r5 smooth muscle cells, knockdown of TRPC6 inhibited OAG- and vasopressin-stimulated but not thapsigargin-stimulated cation currents but, paradoxically, partially inhibited vasopressin-stimulated but not OAG-stimulated increases in [Ca2+]i (21).
TRPC6 knockdown exclusively attenuated OAG-stimulated SRCE in myometrial cells, which is only partially consistent with observations in other smooth muscle cell types studied to date. This may reflect different properties of the heterotetrameric TRPC channels present in different cell types. In myometrial cells, oxytocin receptor-mediated activation of phospholipase C produces both diacylglycerol and 1,4,5-phosphatidylinositide trisphosphate (1,35). Therefore, it is not clear why there was not an observable effect of TRPC6 knockdown on oxytocin-induced SRCE in PHM1-41 and HMC1 cells. Possibly the effect of locally generated OAG on channels containing TRPC6 comprise such a small proportion of the oxytocin-mediated SRCE response in myometrial cells that this goes undetected. Alternatively, OAG may not be generated close enough or in high enough concentrations to stimulate OAG-responsive channels under these conditions.
The mechanisms underlying the OAG-induced SRCE responses in PHM1-41 and A7r51 cells appear to be qualitatively similar. Data obtained in A7r5 cells prompted the suggestion that OAG-stimulated TRPC6 currents evoke primarily Na+ entry, resulting in [Ca2+]i increases through activation of L-type voltage activated channels, whereas the TRPC6 channels activated by vasopressin appeared to carry larger amounts of Ca2+ (21). Overexpressed TRPC6 channels were originally reported to have a permeability ratio of 6:1 for Ca2+ vs. Na+ (36). However, more recent analysis indicates that the kinetics and permeability ratio are influenced by membrane potential changes and the complement of other channels such as TRPC3/7 and may be closer to 1:1 under some conditions (37).
The effects of TRPC6 knockdown as well as the requirement for extracellular Na+ and sensitivity to nifedipine are restricted to OAG-stimulated SRCE in myometrial cells. The fact that only partial inhibition of OAG-induced SRCE was induced by removal of extracellular Na+ or in the presence of nifedipine in PHM1-41 and HMC1 cells, and the lack of additive effects of these two treatments in PHM1-41 cells, suggests that a proportion of the response may also be due to a Ca2+ entry mechanism independent of L-type Ca channel activation in these cells. Importantly, the findings that neither oxytocin- nor thapsigargin-mediated SRCE are attenuated by TRPC6 knockdown, the removal of extracellular Na+ or by nifedipine suggest that these stimuli target channels with very different properties in PHM1-41 cells than those activated by OAG and probably do not involve TRPC6 to a major extent because its knockdown did not affect these responses. In agreement with this suggestion, we recently found that knockdown of TRPC4 specifically attenuates G protein-coupled receptor-stimulated SCRE but does not affect store depletion- or OAG-stimulated SRCE (24). The lack of an effect of nifedipine on receptor- or store depletion-stimulated SRCE is not consistent with a prior suggestion, based on measurement of Ca2+-activated K+ channel activity, that nifedipine inhibits store-operated capacitative Ca2+ entry in myometrial cells (38). The data presented here illustrate the specificity of the OAG response in human myometrial cells.
TRPC6 knockout mice showed elevated blood pressure and enhanced vascular smooth muscle contractility attributed to a compensatory up-regulation of TRPC3 expression (39). No effect on parturition was reported or systematically examined. Notably, in the knockdown experiments we performed in PHM1-41 and HMC1 cells, there were no compensatory increases in TRPC3, TRPC7 or any other TRPC mRNAs within 48–72 h after infection with virus expressing TC6sh1.
In rat and mouse myometrium, TRPC4 mRNA is the predominant isoform and TRPC6 mRNA is present in considerably less relative abundance (Ref. 29 and our unpublished observations). Therefore, phenotype in the knockout mouse model may not provide substantial insight into the physiological importance of TRPC6 in human myometrium. To our knowledge, shRNA technology has not been applied to intact myometrium, and it may be technically difficult to find conditions under which TRPC6 knockdown using viruses or oligonucleotides is reasonably uniform throughout the tissue under conditions that retain contractile activity. Furthermore, interpretation of effects of OAG on contractile activity is complicated because OAG activates PKC independent of its effects on [Ca2+]i (9,10), and it may be difficult to achieve uniform PKC inhibition throughout the uterine strips. PKC, in turn, affects a number of processes through regulatory phosphorylation, including ion channel function, G protein receptor function and coupling, phospholipase Cs, 1,4,5-phosphatidylinositide trisphosphate receptors, and calcium pumps as well as a number of components of the contractile apparatus (reviewed in Refs. 1 and 40). Therefore, although physiological relevance may be difficult to assess in intact myometrial tissue or model animals, hints may come from studies in other smooth muscle more amenable to in vitro study. TRPC6 channels may be responding primarily to stretch, as reported for intact cerebral arteries (13), rather than to locally generated OAG in the physiological context of the tissue.
The importance of myometrial TRPC6 in relation to pregnancy is unknown at present. TRPC6 has been implicated in growth and proliferation of smooth muscle (41), an effect that might be important in the uterus early in pregnancy. TRPC6 might contribute via Ca2+ signaling to enhanced uterine tone during pregnancy and possibly to Ca2+-dependent gene regulation in preparation for parturition. TRPC6 mRNA and protein were unchanged with the onset of labor in term fundal myometrial samples in our previous study (15). Another study reported an increase in TRPC6 protein but not mRNA in labor (42) and subsequently reported an increase in TRPC3 (also responsive to OAG) but not TRPC6 in myometrial cells stretched in culture (43). TRPC6 has been reported to be activated by hypoxia in pulmonary arterial smooth muscle (44), and this could be important in myometrium during labor as well.
In summary, these findings implicate TRPC6 in OAG-stimulated increases in [Ca2+]i in myometrial cells. The data suggest that these channels act both via enhanced Na+ entry coupled to activation of voltage-dependent Ca2+ entry channels and by a nifedipine-independent Ca2+ entry mechanism to promote elevation of intracellular Ca2+. Attenuation of TRPC6 expression does not affect receptor- or store depletion-mediated increases in [Ca2+]i, indicating that specific signals can affect specific types of signal-regulated Ca2+ entry in the myometrium.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the gifts of the pSHAG vector from G. Hannon (Cold Spring Harbor Lab, Cold Spring Harbor, NY) and the modified pAdTrack-RfA(f) vector from Dr. C. Clay and J. Cantlon (Colorado State University, Fort Collins, CO).
Footnotes
This work was supported by March of Dimes Grant 6-FY05-77, National Institutes of Health Grant HD38970, the Lalor Foundation (to Y.-S.K.), and National Institutes of Health Grant T32-HD0703 (to A.U.).
Disclosure Summary: The authors have nothing to declare.
First Published Online November 25, 2009
Abbreviations: AM, Acetoxymethylester; [Ca2+]I, intracellular free Ca2+; FB, fluorescence buffer; OAG, 1-oleoyl-2-acetyl-sn-glycerol; PKC, protein kinase C; shRNA, short hairpin RNA; SRCE, extracellular calcium-dependent signal-regulated increase in intracellular Ca2+; TRPC, canonical transient receptor potential-like channels; UtSMC, uterine smooth muscle cells.
References
- Sanborn BM 2007 Hormonal signaling and signal pathway crosstalk in the control of myometrial calcium dynamics. Semin Cell Dev Biol 18:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM 2002 Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 67:988–994 [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Yang M, Alcorn JL, Sanborn BM 2003 Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod 69:647–655 [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Sanborn BM 2004 Stimulation of interacellular Ca 2+ oscillations by diacylglycerol in human myometrial cells. Cell Calcium 36:157–164 [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Boulay G, Brown D, Jiang M, Dietrich A, Mikoshiba K, Zhu X, Qin N 2000 Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Horm Res 55:127–161; discussion 161–162 [PubMed] [Google Scholar]
- Putney Jr JW 2005 Physiological mechanisms of TRPC activation. Pflugers Arch 451:29–34 [DOI] [PubMed] [Google Scholar]
- Albert AP, Large WA 2006 Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular yachts. J Physiol 570:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE 2006 An introduction to TRP channels. Annu Rev Physiol 68:619–647 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G 1999 Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263 [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Rost BR, Gudermann T 2005 The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch 451:72–80 [DOI] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R 2004 Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol 559:685–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE 2002 Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90:248–250 [DOI] [PubMed] [Google Scholar]
- Spassova M, Hewavitharana T, Xu W, Soboloff J, Gill DL 2006 A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103:16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Beech D, Poston L, Tribe RM 2002 Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod 8:946–951 [DOI] [PubMed] [Google Scholar]
- Ku CY, Babich L, Word RA, Zhong M, Ulloa A, Monga M, Sanborn BM 2006 Expression of transient receptor channel proteins in human fundal myometrium in pregnancy. J Soc Gynecol Investig 13:217–225 [DOI] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L 1997 Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem 272:29672–29680 [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y 2001 The transient receptor potential protein homologue TRP6 is the essential component of vascular α(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88:325–332 [DOI] [PubMed] [Google Scholar]
- Boulay G 2002 Ca(2+)-calmodulin regulates receptor-operated Ca(2+) entry activity of TRPC6 in HEK-293 cells. Cell Calcium 32:201–207 [DOI] [PubMed] [Google Scholar]
- Estacion M, Li S, Sinkins WG, Gosling M, Bahra P, Poll C, Westwick J, Schilling WP 2004 Activation of human TRPC6 channels by receptor stimulation. J Biol Chem 279:22047–22056 [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L 2007 Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA 104:4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL 2005 Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 280:39786–39794 [DOI] [PubMed] [Google Scholar]
- Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C 2007 Transient receptor potential channel 6-mediated, localized cytosolic[Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101:1030–1038 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wagner EJ, Cullen BR 2002 Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol Cell 6:1327–1333 [DOI] [PubMed] [Google Scholar]
- Ulloa A, Gonzales AL, Zhong M, Kim YS, Cantlon J, Clay C, Ku CY, Earley S, Sanborn BM 2009 Reduction in TRPC4 expression specifically attenuates G-protein coupled receptor-stimulated increases in intracellular calcium in human myometrial cells. Cell Calcium 46:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M, Ku CY, Dodge K, Sanborn BM 1996 Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod 55:427–432 [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Barhoumi R, Stickney M, Monga M, Ku CY, Sanborn BM 1996 Correlation between connexin43 expression, cell-cell communication, and oxytocin-induced Ca2+ responses in an immortalized human myometrial cell line. Biol Reprod 55:433–438 [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Awooda I, Mouneimne Y, Safe S, Burghardt RC 2006 Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol Lett 165:133–141 [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Wiater E, Vale W 2008 Activin-A in myometrium: characterization of the actions on myometrial cells. Endocrinology 149:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM 2004 Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod 70:919–924 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Saleh SN, Large WA 2008 Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J Physiol 586:3087–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX 2003 PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284:C316–C330 [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen'kyi V, Slomianny C, Beck B, Mariot P, Bonnal JL, Mauroy B, Shuba Y, Capiod T, Skryma R, Prevarskaya N 2006 Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res 66:2038–2047 [DOI] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Villereal ML 2005 Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem 280:29559–29569 [DOI] [PubMed] [Google Scholar]
- Sanborn BM 2001 Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol 86:223–237 [DOI] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, Bird GS, Putney Jr JW 2003 The TRPC3/6/7 subfamily of cation channels. Cell Calcium 33:451–461 [DOI] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP 2006 Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol 572:359–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Schumann R, Zhang P 2001 Nifedipine block of capacitative calcium entry in cultured human uterine smooth-muscle cells. J Soc Gynecol Investig 8:210–215 [DOI] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Hoffmann M, Müller C, Stolz S, Scheunemann J, Weissgerber P, Flockerzi V 2004 Functional role of TRPC proteins in vivo: lessons from TRPC-deficient mouse models. Biochem Biophys Res Commun 322:1352–1358 [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Morgan KG 2007 Regulation of the uterine contractile apparatus and cytoskeleton. Semin Cell Dev Biol 18:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi L, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX 2004 Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101:13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Poston L, Tribe RM 2004 Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1β. J Clin Endocrinol Metab 89:1291–1300 [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe R 2007 Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod 13:31–39 [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T 2006 Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 103:19093–19098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.