Abstract
Aromatase, the enzyme that catalyzes estrogen synthesis, is critical for the progression of estrogen receptor-positive breast cancer (BCa) in postmenopausal women. We show that calcitriol, the hormonally active form of vitamin D, regulates the expression of aromatase in a tissue-selective manner. Calcitriol significantly decreased aromatase expression in human BCa cells and adipocytes and caused substantial increases in human osteosarcoma cells (a bone cell model exhibiting osteoblast phenotype in culture) and modest increases in ovarian cancer cells. Calcitriol administration to immunocompromised mice bearing human BCa xenografts decreased aromatase mRNA levels in the tumors and the surrounding mammary adipose tissue but did not alter ovarian aromatase expression. In BCa cells, calcitriol also reduced the levels of prostaglandins (PGs), major stimulators of aromatase transcription, by suppressing the expression of cyclooxygenase-2 (which catalyzes PG synthesis) and increasing that of 15-hydroxyprostaglandin dehydrogenase (which catalyzes PG degradation). The mechanism of aromatase down-regulation by calcitriol in BCa cells is therefore 2-fold: a direct repression of aromatase transcription via promoter II through the vitamin D-response elements identified in this promoter and an indirect suppression by reducing the levels of PGs. Combinations of calcitriol with three different aromatase inhibitors (AIs) caused enhanced inhibition of BCa cell growth. The combination of calcitriol and an AI may have potential benefits for BCa therapy. In addition to augmenting the ability of AIs to inhibit BCa growth, calcitriol acting as a selective aromatase modulator that increases aromatase expression in bone would reduce the estrogen deprivation in bone caused by the AIs, thus ameliorating the AI-induced side effect of osteoporosis.
In addition to its known anti-cancer effects, calcitriol inhibits estrogen synthesis in breast cancer cells by directly repressing aromatase transcription and indirectly decreasing aromatase expression by reducing prostaglandin levels.
Aromatase (CYP19) catalyzes the synthesis of estrogens from androgenic precursors. The ovaries are the principal source of circulating estrogens in premenopausal women. However, many other tissues including the breast express aromatase and hence have the capacity to locally synthesize estrogens. Aromatase expression in these different sites is under the control of tissue-specific promoters differentially regulated by various transcription factors (1). After menopause when the circulating estrogen level from the ovaries dramatically declines, estrogens synthesized locally become the major source in the breast. Aromatase expression is higher in human breast cancer (BCa) than normal breast tissue (2). In postmenopausal women with BCa, estrogen levels within the breast tissue are severalfold higher than the serum levels indicating tumor accumulation or local synthesis of estrogens that can drive BCa growth (3). Aromatase inhibitors (AIs) have become major therapeutic agents to prevent BCa progression or recurrence in postmenopausal women after primary therapy (4,5,6). However, AIs inhibit estrogen synthesis globally and therefore have a detrimental effect at sites such as bone (7,8) in which normal estrogen function is required for the maintenance of bone mineralization. The development of selective aromatase modulators (SAMs) that inhibit aromatase expression in breast but allow unimpaired estrogen synthesis at other desirable sites such as bone would have great utility in BCa therapy (1).
Calcitriol (1,25-dihydroxyvitamin D3), the hormonally active form of vitamin D, suppresses proliferation and promotes apoptosis and differentiation of malignant cells and inhibits tumor angiogenesis and invasion, raising the possibility of its use as an anticancer agent (9,10,11,12,13). Calcitriol has been shown to increase aromatase expression in human osteoblasts and fibroblasts (14,15). Interestingly, our observations reveal that calcitriol down-regulates aromatase expression in BCa cells. In BCa cells, calcitriol also decreases the levels of biologically active prostaglandins (PGs), which are major stimulators of estrogen production, by suppressing the expression of the PG-synthesizing enzyme cyclooxygenase (COX)-2 and increasing that of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), which initiates PG catabolism. The mechanism of calcitriol-mediated aromatase down-regulation in BCa cells therefore appears to be 2-fold: 1) a direct transcriptional repression of the aromatase gene by calcitriol and 2) an indirect effect to reduce aromatase transcription by reducing the levels PGs, which are known stimulators of aromatase transcription in BCa cells (1). Importantly our data reveal that calcitriol regulation of aromatase expression is tissue specific with a significant suppression in BCa cells and adipocytes surrounding breast tumors, whereas a substantial increase is seen in osteosarcoma cells, used as a model for bone cells.
We and others have shown that calcitriol also inhibits estrogen signaling by down-regulating the expression of estrogen receptor (ER)-α in BCa cells (16,17,18). Due to its ability to suppress both the synthesis and biological activity of estrogens, calcitriol exhibits significant inhibitory effects on BCa cell growth. Furthermore, in this paper, we show that combinations of calcitriol and AIs are cooperative and cause enhanced inhibition of BCa cell growth. We therefore postulate that combining an AI (enzyme inhibitor) with calcitriol (transcriptional regulator) would be a useful therapeutic strategy in BCa. Calcitriol exhibits multiple beneficial actions including the following: 1) its action as a SAM to selectively decrease aromatase transcription in BCa cells, leading to a reduction in estrogen synthesis; 2) suppression of estrogen signaling by decreasing ERα levels; and 3) the inhibition of many other growth signaling pathways (13,19). We hypothesize that cumulatively these actions contribute to the beneficial effect of combining calcitriol with an AI to treat BCa patients.
Materials and Methods
Materials
Calcitriol, exemestane, letrozole, and anastrozole were kind gifts from BioXell (Segrate, Italy), Pfizer Inc. (Groton, CT), Norvartis Pharma AG (Basel, Switzerland), and AstraZeneca (Cheshire, UK), respectively. Tissue culture media, supplements, and fetal calf serum (FCS) were obtained from Life Technologies (Grand Island, NY), Lonza (Walkersville, MD), and Mediatech Inc. (Herndon, VA).
Cell culture
All cell lines were obtained from American Type Culture Collection (Manassas, VA). MCF-7 cells were cultured in DMEM-F12 supplemented with 5% FCS and MDA-MB-231 cells in MEM + 10% FCS. T47D, ZR-75-1, MG-63, and Saos-2 cells were grown in RPMI 1640 + 5% FCS, OVCAR-3, and SKOV-3 cells in RPMI 1640 + 10% FCS and 3T3-L1 cells in DMEM + 10% FCS. Cell cultures were maintained at 37 C with 5% CO2 in a humidified incubator.
RNA isolation, cDNA synthesis, and real-time PCR
Total RNA was isolated from cells treated with vehicle (0.1% ethanol) or calcitriol using Trizol (Invitrogen, Carlsbad, CA). 3T3-L1 cultures were also exposed to a differentiation-inducing medium containing insulin (5 μm), isobutyl methyl xanthine (0.5 mm), and dexamethasone (2.5 μm) for 10 d with and without calcitriol addition for the final 24 h and RNA was isolated. RNA was subjected to reverse transcription followed by PCR as described (20). Relative changes in mRNA were assessed by the 2−ΔΔC(T) method (21) after normalization to TATA binding protein expression. The primers used for total aromatase, COX-2, 15-PGDH, TATA binding protein, and adipocyte differentiation marker genes [adipocyte fatty acid binding protein 2 (AP2), peroxisome proliferator-activated receptor (PPAR)-γ, and perilipin] were as described before (20,22,23). Promoter-specific aromatase transcript levels were determined using primers designed for each of the promoter regions of aromatase exon I and PCR conditions as described (23).
Measurement of aromatase activity
Aromatase enzyme activity has been assessed in cell cultures and recombinant enzyme preparations by measuring the conversion of androgenic substrates androstenedione or testosterone (T) into estrone or estradiol (E2) (24,25). We determined aromatase activity in cultures of BCa and osteosarcoma cells by measuring the conversion of T to E2. Briefly, cells were seeded in six-well tissue culture plates (3 × 105 cells/well) in their respective growth media. After 24 h, the growth media were replaced with serum-free medium (OptiMEM; Life Technologies) containing the aromatase substrate T (100 nm) and various concentrations of calcitriol or letrozol and incubated for 48 h. Aliquots of the culture media were used to measure E2 concentrations using an enzyme immunoassay (EIA) kit (Cayman Chemical Co., Ann Arbor, MI) and protein content by the Bradford method (26). Aromatase activity was expressed as picograms of E2 synthesized per hour per milligram protein.
Western blot analysis
Extracts from BCa cells treated with vehicle or calcitriol were subjected to Western blot analysis as described (20), using a monoclonal antibody against human COX-2 (Cayman Chemical) at 1:1000 dilution and against β-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA; dilution 1:500) as a control. COX-2 proteins was visualized as an approximately 70-kDa immunoreactive band.
Measurement of PGE2 levels
BCa cells were seeded in six-well tissue culture plates (3 × 105 cells/well). After 24 h the growth media were replaced with serum-free medium (OptiMEM; Life Technologies) containing vehicle or 10 or 100 nm calcitriol. After 72 h, PGE2 concentrations were measured in the conditioned media using an EIA kit (Cayman Chemical).
Transient transfections and luciferase assay
A plasmid containing the aromatase promoter I.3/II region (−561/+79, promoter II transcriptional start site as +1) inserted in pGL3-basic reporter vector was transiently transfected into MCF-7 cells using LipofectAmine reagent (Life Technologies/Invitrogen). A renilla luciferase plasmid (pRL null; Promega, Madison, WI) was cotransfected to control for transfection efficiency. After transfections the cells were treated with vehicle (0.1% ethanol) or 100 nm calcitriol in the absence or presence of 50 μm forskolin (Fsk) or 0.5 mm dibutyryl cAMP [(Bu)2cAMP] for 24 h. Reporter and renilla luciferase activities were measured using the dual-luciferase assay kit (Promega).
Chromatin immunoprecipitation (ChIP) assay
ZR-75-1 cells treated with vehicle or calcitriol were subjected to ChIP analysis as described (27). Immunoprecipitations of the chromatin fragments were carried out with IgG or a mixture of antibodies directed toward both the N and C terminus of the vitamin D receptor (VDR; N-20, C-20, and H-8; Santa Cruz Biotechnology). PCR was done on the eluted DNA using primers designed to amplify aromatase promoter II sequence from −477 to −282 containing the distal vitamin D-response element (VDRE; forward, 5′-GACAAACTGATGGAAGGCTCTGAG-3′; reverse, 5′-CCTGCTGATGAAGTCACA-3′) and primers designed to amplify promoter II sequence from −372 to −178 encompassing the proximal VDRE (forward, 5′-GGGGAGTTGGGAGATTGCCT-3′; reverse, 5′-CCACTCAAGGGCAAGATGA-3′).
Cell proliferation assay
MCF-7 cells were seeded (100,000 cells/well) in six-well dishes in medium containing 5% FCS, which was replaced after 24 h with phenol red-free RPMI + 5% charcoal-stripped FCS containing vehicle or 100 nm T for 6 d in the absence or presence of Casodex (Cdx; 10 μm); various concentrations of calcitriol (0.1–100 nm); the AIs exemestane, letrozole, and anastrozole (0.1 nm-1 μm); or combinations of different concentrations of calcitriol and various AIs. Fresh media and the drugs were replenished every other day and cell proliferation was assessed at the end of 6 d by measuring the DNA content (18).
Establishment of xenograft tumors in nude mice
A suspension of 10 million MCF-7 cells were mixed with an equal volume of Matrigel (BD Biosciences, San Jose, CA) and injected into the left mammary fat pads of 6- to 8-wk-old female, nude, intact, or ovariectomized (OVX) mice (Harlan Labs, Madison, WI). The OVX mice received daily ip injections of the estrogenic precursor androstenedione (100 μg/mouse). When tumors attained a size of about 70 mm3 or more, the mice were given ip injections of vehicle (0.1% ethanol in sterile saline) or a single high dose of calcitriol (0.75 μg/mouse per day) for 3 consecutive days and were euthanized 14 h after the final injections. This calcitriol dosing regimen has been established to be effective in eliciting changes in tumor gene expression in xenograft models of squamous cell carcinoma and prostate cancer in nude mice (28,29). Tumors, mammary fat, and ovaries were excised, RNA isolated, and total aromatase and COX-2 mRNA levels measured by real-time RT-PCR as described above.
Statistical analysis
All values are presented as mean ± sem. Relative mRNA levels are shown as a percent of the levels in vehicle-treated control set at 100%. Data were evaluated by ANOVA with Bonferroni-Dunn test as the post hoc analysis or the Student’s t test where appropriate using the Prism 5 software (GraphPad Inc., San Diego, CA). IC50 values for growth inhibition by individual drugs were calculated from dose-response data. Dose-response analyses were also carried out for the combinations of calcitriol and the various AIs. The interaction index (γ) for each drug combination was calculated from the dose-response data using the isobolar method as described (30).
Results
Aromatase mRNA down-regulation by calcitriol in BCa cells
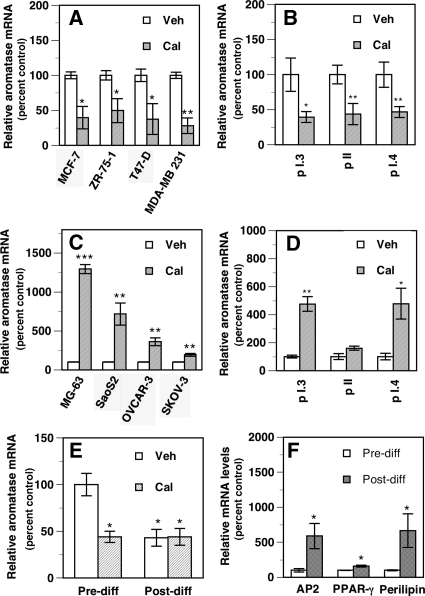
Calcitriol at 10 nm significantly decreased total aromatase mRNA levels in the ER-positive (ER+) human BCa cells MCF-7, ZR-75-1, and T47-D (∼50–60% decrease). Higher calcitriol concentrations (up to 100 nm) did not further decrease aromatase mRNA levels in these cells. In the ER-negative (ER−) MDA-MB 231 cells, a maximal decrease in aromatase mRNA was seen with 100 nm calcitriol (∼70% decrease, Fig. 1A). This calcitriol concentration (100 nm) was used to assess its effects in MDA-MB231 cells in various experiments described below. In all other cells 10 nm calcitriol was used as a single high dose unless otherwise stated. The levels of promoter-specific aromatase transcripts were determined in MCF-7 cells (Fig. 1B). Calcitriol decreased (by ∼50–60%) the levels of transcripts derived from promoters I.3 and II, the major promoters driving aromatase transcription in BCa and the stromal cells surrounding breast tumors (31) as well as transcripts from promoter I.4 (∼50% decrease), which is predominant in normal breast cells (1).
Figure 1.
Tissue-selective regulation of aromatase mRNA by calcitriol. A, Calcitriol decreases total aromatase mRNA in BCa cells. Total aromatase mRNA levels were determined in BCa cells treated for 24 h with 0.1% ethanol vehicle (Veh) or calcitriol (Cal, 100 nm for MDA-MB 231 and 10 nm for all other cells). *, P < 0.05 and **, P < 0.01 compared with vehicle. B, Calcitriol decreases various promoter-specific aromatase transcript levels in BCa cells. The levels of promoter-specific aromatase transcripts were determined in MCF-7 cells treated as in A. *, P < 0.05 and **, P < 0.01 compared with vehicle. C, Calcitriol increases aromatase mRNA in human osteosarcoma and ovarian cancer cells. Total aromatase mRNA levels were measured in human osteosarcoma cells (MG-63 and SaoS-2) and human ovarian cancer cells (OVCAR-3 and SKOV-3) treated as in A. **, P < 0.01 and ***, P < 0.001 compared with vehicle. D, Calcitriol increases various promoter-specific aromatase transcript levels in osteosarcoma cells. MG-63 cells were treated as in A and the levels of various promoter-specific aromatase transcripts determined. *, P < 0.05 and **, P < 0.01 compared with vehicle. E and F, Calcitriol decreases aromatase expression in 3T3-L1 preadipocytes. 3T3-L1 mouse fibroblasts representing undifferentiated preadipocytes (E, Pre-diff) were treated with ethanol vehicle (Veh) or 10 nm (Cal) for 24 h, and aromatase mRNA was measured. The cultures were then exposed to a differentiation-inducing medium with and without calcitriol addition for the final 24 h and aromatase mRNA was measured (E, Post-diff). Aromatase mRNA levels are shown as a percent of vehicle-treated control in Pre-diff cells set at 100% (E). *, P < 0.05 compared with vehicle treatment in Pre-diff cells. AP2, PPAR-γ, and perilipin mRNA levels were measured before (Pre-diff) and after (Post-diff) the induction of differentiation of 3T3-L1 cells (F). Values are shown as a percent of levels in Pre-diff cells (control) set at 100%. *, P < 0.05 compared with Pre-diff.
Tissue selectivity of aromatase mRNA regulation by calcitriol
In contrast to BCa cells, in the human osteosarcoma cells MG-63 and SaoS-2, which exhibit osteoblastic features in culture, calcitriol induced an approximately 12-fold and approximately 7-fold increase in total aromatase mRNA levels, respectively (Fig. 1C). Calcitriol caused modest but significant increases (∼2- to 4-fold) in total aromatase mRNA in human ovarian cancer cell lines (Fig. 1C). The levels of transcripts derived from the bone-specific aromatase promoter I.4 were significantly increased (∼5-fold) in MG-63 cells by calcitriol (Fig. 1D). Calcitriol also increased promoter I.3-derived transcripts in MG-63 cells without altering promoter II-derived transcript levels (Fig. 1D).
To examine calcitriol effects in adipocytes, we used 3T3-L1 mouse fibroblasts (preadipocytes), which can be induced to undergo differentiation into mature adipocytes by manipulating culture conditions. Treatment of 3T3-L1 preadipocytes with calcitriol for 24 h caused an approximately 60% decrease in total aromatase mRNA (Fig. 1E, Pre-diff). After exposure to a differentiation-inducing medium for 10 d, the cells exhibited features characteristics of differentiated adipocytes, such as the loss of fibroblast morphology; increases in the expression of the adipocyte markers AP2, PPARγ, and perilipin (Fig. 1F); and concomitant accumulation of lipid droplets determined by Oil Red-O staining (75–80% of the cells exhibited positive staining, not shown). Calcitriol treatment of undifferentiated preadipocytes resulted in a significant decrease in aromatase mRNA (Fig. 1E, Pre-diff). The induction of differentiation (Fig. 1E, Post-diff) also led to a significant decrease (∼60%) in aromatase mRNA compared with the undifferentiated preadipocytes. Addition of 10 nm calcitriol to the differentiated cells for 24 h maintained the suppression of aromatase mRNA without causing a further decrease (Fig. 1E).
Tissue-selective regulation of aromatase activity by calcitriol
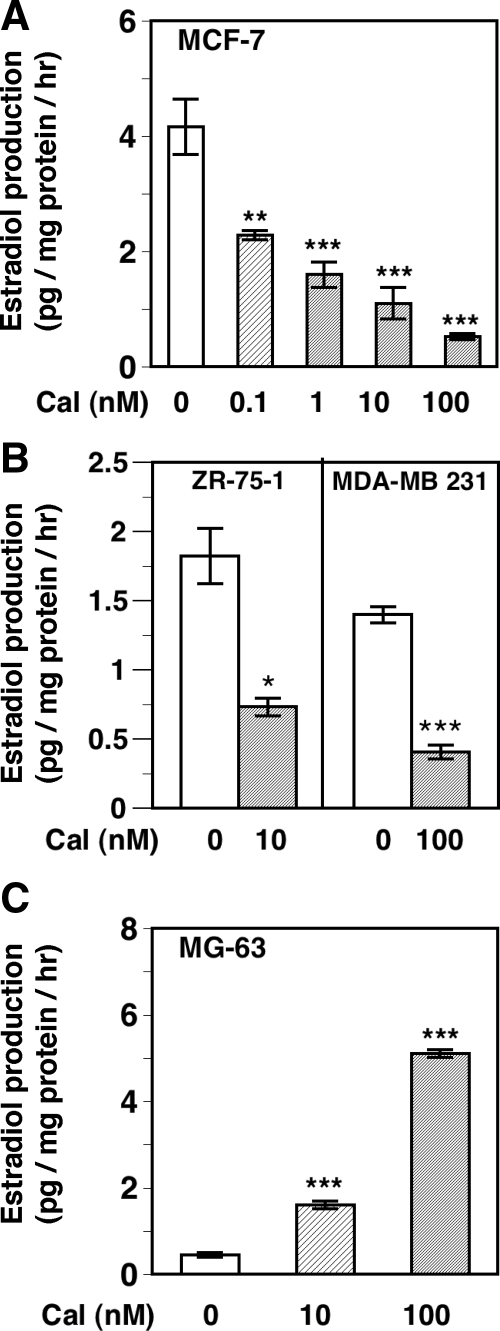
The effect of calcitriol on aromatase activity in BCa cells and osteosarcoma cells was examined by measuring E2 production after the addition of the substrate T. The changes in aromatase activity in the various cells due to calcitriol treatment reflected the changes seen in aromatase mRNA levels (Fig. 1, A and C) and again demonstrated the tissue specificity of aromatase regulation by calcitriol. Calcitriol decreased aromatase activity in a dose-dependent manner in MCF-7 cells with an IC50 of 1.9 nm (Fig. 2A). Treatment of cells with 1 nm letrozole was used as positive control for the inhibition of aromatase activity, which resulted in an approximately 75% decrease in E2 production (data not shown). Appreciable decreases were also seen in aromatase activity in ZR-75-1 cells (by ∼50%) and MDA-MB 231 cells (by ∼75%) after treatment with 10 and 100 nm calcitriol, respectively (Fig. 2B). In contrast, in MG-63 osteosarcoma cells, calcitriol at 10 and 100 nm caused an approximately 4-fold and an approximately 12-fold increase in aromatase activity respectively (Fig. 2C).
Figure 2.
Tissue-selective regulation of aromatase activity by calcitriol. A, Calcitriol decreases aromatase activity in MCF-7 cells. MCF-7 cells were treated with vehicle (Cal, 0) or various concentrations of calcitriol (Cal, 0.1–100 nm) in serum-free medium containing the aromatase substrate T (100 nm) for 48 h. Aromatase activity was determined by measuring the concentrations of E2 formed in the conditioned media by an EIA and expressed as picograms of E2 formed per hour per milligram protein. **, P < 0.01 and ***, P < 0.001 compared with vehicle. B, Calcitriol decreases aromatase activity in other BCa cells. ZR-75-1 and MDA-MB 231 cells were treated with 10 or 100 nm calcitriol and aromatase activity was determined as in A. *, P < 0.05 and ***, P < 0.001 compared with vehicle. C, Calcitriol increases aromatase activity in MG-63 cells. MG-63 human osteosarcoma cells were treated with 10 and 100 nm calcitriol and aromatase activity was determined as in A. ***, P < 0.01 compared with vehicle.
Down-regulation of aromatase by calcitriol in MCF-7 xenograft tumors and surrounding mammary adipose tissue in mice
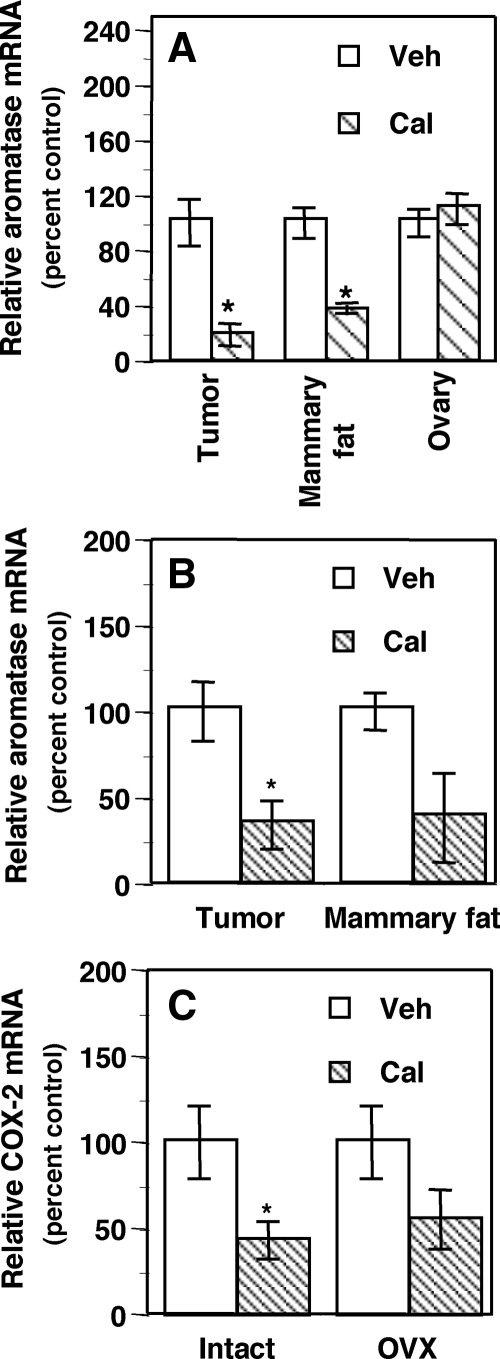
Calcitriol administration to nude mice carrying MCF-7 xenografts in their mammary fat pads decreased total aromatase mRNA in the xenograft tumors in both intact mice (Fig. 3A, ∼80% decrease) and OVX mice (Fig. 3B, ∼65% decrease). Calcitriol also decreased aromatase mRNA in the mammary fat pads surrounding the tumors in intact mice (∼65% decrease, P < 0.05) as well as OVX mice, in which, however, the decrease did not attain statistical significance (Fig. 3B). No significant change was observed in ovarian aromatase mRNA in the intact mice (Fig. 3A). COX-2 mRNA expression in the tumors also showed a significant decrease after calcitriol treatment in the intact mice (Fig. 3C). The same negative trend in COX-2 expression was also seen in OVX mice treated with calcitriol, although the decrease did not attain statistical significance (Fig. 3C). Serum calcium levels in the mice receiving calcitriol at a high dose for 3 d assayed approximately 14 h after the final calcitriol injection registered only a slight increase, which, however, was not statistically significant.
Figure 3.
Tissue-selective regulation of aromatase mRNA by calcitriol in mice bearing human BCa xenografts. Vehicle (Veh) or calcitriol (Cal, 0.75 μg/mouse per day for 3 d) was administered to intact (A) or OVX (B) female nude mice bearing MCF-7 xenografts. Total aromatase mRNA was measured in tumors, mammary fat tissue surrounding the tumors, and ovaries. COX-2 mRNA was measured in the tumors from intact and OVX mice (C). *, P < 0.05 compared with vehicle.
Suppression of the PG pathway by calcitriol in BCa cells
We examined calcitriol regulation of the expression of COX-2 and 15-PGDH, two key enzymes involved in PG metabolism. Calcitriol caused significant decreases (∼35–65%) in COX-2 mRNA in various BCa cells (Fig. 4A) and reduced COX-2 protein levels in MDA-MB 231 cells (Fig. 4B). On the other hand, calcitriol increased 15-PGDH mRNA in three (MCF-7, ZR-75-1, and MDA-MB 231) of the four BCa cell lines tested (Fig. 4C). As a result of these regulatory actions, calcitriol significantly decreased PGE2 levels secreted into the conditioned media from MCF-7 (∼75% decrease) and MDA-MB 231 cells (∼30% decrease, Fig. 4D).
Figure 4.
Calcitriol decreases PG synthesis in BCa cells. A, Calcitriol decreases COX-2 mRNA levels in BCa cells. BCa cells were treated with calcitriol as described in Fig. 1A and COX-2 mRNA levels were determined. *, P < 0.05 and ***, P < 0.001 compared with vehicle. B, Calcitriol decreases COX-2 protein levels. Western blot showing COX-2 expression in MDA-MB 231 cells treated with vehicle (Cal−) or 100 nm calcitriol (Cal+) for 48 h. COS-7 cell extracts overexpressing COX-2 and COX-1 protein served as a positive and negative control, respectively. β-Actin was used as a loading control. C, Calcitriol increases 15-PGDH mRNA levels in BCa cells. BCa cells were treated as described in Fig. 1A and 15-PGDH mRNA levels were determined. *, P < 0.05 compared with vehicle. D, Calcitriol decreases PGE2 levels in BCa cells. MCF-7 and MDA-MB 231 cells were treated with vehicle (Veh) or calcitriol (Cal) at 10 and 100 nm, respectively, in serum-free culture media for 72 h. PGE2 levels in conditioned media were measured. **, P < 0.01 and ***, P < 0.001 compared with vehicle.
Transcriptional repression of the aromatase promoter II by calcitriol in BCa cells
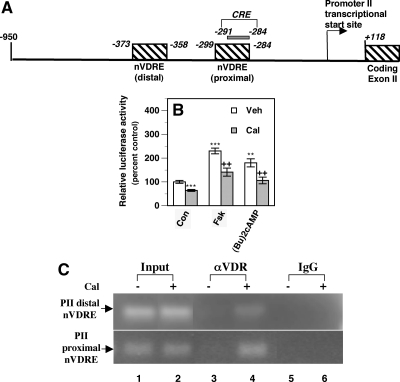
In silico analysis of the aromatase promoter I.3/II sequence revealed the presence of two putative VDREs at −373 to −358 bp (distal VDRE) and at −299 to −284 bp (proximal VDRE, promoter II transcriptional start site as +1, Fig. 5A). Promoter II has a cAMP response element (CRE) residing at −291 and −284 bp (32). The proximal VDRE overlaps this CRE sequence (Fig. 5A), potentially providing a mechanism for calcitriol to counter the stimulation of this promoter due to PG induction of cAMP. We transiently transfected a plasmid containing an aromatase promoter I.3/II fragment (−561/+79) inserted into pGL3-basic reporter vector into MCF-7 cells. After calcitriol treatment, we observed a significant decrease in basal reporter luciferase activity (∼40% decrease, Fig. 5B). We also observed similar decreases in reporter activity in MDA-MB 231 cells (data not shown). In MCF-7 cells, addition of Fsk and (Bu)2cAMP caused an approximately 2-fold increase reporter activity, which was significantly attenuated by calcitriol cotreatment (Fig. 5B).
Figure 5.
Direct repression of aromatase transcription through promoter II by calcitriol. A, A schematic diagram of the aromatase promoter I.3/II. We identified two putative nVDREs (designated as proximal and distal nVDRE) in the aromatase I.3/II promoter. The putative proximal nVDRE sequence overlaps a CRE that mediates the cAMP stimulation of promoter II. B, Calcitriol suppresses basal and cAMP stimulation of aromatase I.3/II promoter activity in BCa cells. An aromatase I.3/II promoter-luciferase reporter and renilla luciferase plasmids were transiently transfected into MCF-7 cells. Cells were treated with vehicle (Veh) or 100 nm calcitriol (Cal) in the absence (control, Con) or presence 50 μm Fsk or 0.5 mm (Bu)2cAMP for 24 h. Results are expressed as a percent of relative luciferase activity in vehicle-treated control set at 100%. **, P < 0.01 and ***, P < 0.001 compared with vehicle. ++, P < 0.01 compared with Veh + Fsk or Veh + (Bu)2cAMP. C, Calcitriol recruits the VDR to the VDREs in the aromatase II promoter. ZR-75-1 cells were exposed to vehicle (Cal−) or 10 nm calcitriol (Cal+) for 2 h, and the cell extracts were subjected to ChIP analyses. Immunoprecipitations were carried out with IgG or a mixture of three αVDR antibodies, followed by PCR using primers designed to amplify fragments of the aromatase promoter II encompassing the proximal nVDRE and the distal nVDRE.
To demonstrate calcitriol-induced direct binding of the VDR to the putative negative VDRE sequences in the aromatase II promoter, extracts of ZR-75-1 cells treated with vehicle or calcitriol were subjected to ChIP analysis. When PCR was performed with the chromatin DNA immunoprecipitated with the VDR antibodies using primers designed to amplify the sequence encompassing the distal VDRE in the aromatase promoter II, a 195-bp band was produced in cells treated with calcitriol but not vehicle (Fig. 5C, top panel, lane 4), indicating calcitriol-induced VDR binding to the distal VDRE. Similarly primers encompassing the proximal VDRE amplified a 194-bp product in calcitriol-treated cells subjected to immunoprecipitation with the αVDR antibodies (Fig. 5C, bottom panel, lane 4).
Cooperative effects of calcitriol and AIs to suppress BCa cell proliferation
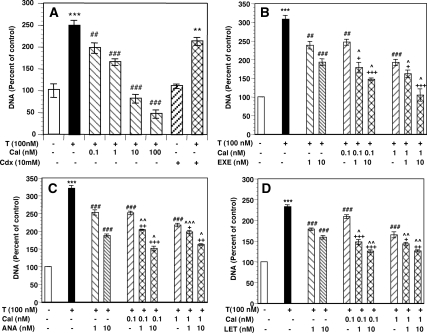
We next examined the effects of calcitriol and three different AIs individually and in combination on the growth of ER+ MCF-7 cells. Addition of the aromatase substrate T to cell cultures in media depleted of endogenous steroids caused a 2- to 3-fold increase in cell growth (Fig. 6, A–D). The growth stimulation by T was not abolished by the addition of the androgen receptor (AR) antagonist bicalutamide (Cdx, Fig. 6A). Addition of graded concentrations of calcitriol inhibited T stimulation of cell growth (Fig. 6A) with an IC50 of 5.3 nm. Complete inhibition down to the growth level in basal media without T was achieved at 10 nm calcitriol. A further increase in calcitriol concentration (100 nm) caused a significant reduction in cell growth below basal levels. Similarly when tested individually, the AIs letrozole, anastrozole, and exemestane inhibited T stimulation of cell growth in a dose-dependent manner with IC50 values of 10–15 nm (data not shown).
Figure 6.
Enhanced inhibition of BCa cell growth by calcitriol-AI combinations. A, Calcitriol inhibits T stimulation of MCF-7 growth. Cells were grown in steroid-depleted media containing vehicle (control, open bar) or 100 nm T in the absence (solid bar) or presence of the indicated concentrations of calcitriol or 10 μm Cdx for 6 d. DNA contents were determined and shown as a percentage of vehicle-treated control set at 100%, which corresponded to 22.1 ± 1 μg/well. **, P < 0.01 and ***, P < 0.001 compared with control; ##, P < 0.01 and ###, P < 0.001 compared with T. B–D, Enhanced inhibition of cell growth by calcitriol-AI combinations. Cells were grown as described in A in the absence or presence of calcitriol (Cal), exemestane (Exe, 6b), anastrozole (Ana, 6c), letrozole (Let, 6d), or combinations of calcitriol with each AI at the indicated concentrations. DNA contents are shown as a percentage of vehicle-treated control set at 100%, which corresponded to 20 ± 1.2 μg/well. ***, P < 0.001 compared with control; ##, P < 0.01, ###, P < 0.001 compared with T; +, P < 0.05; ++, P < 0.01; and +++, P < 0.001 compared with the corresponding doses of calcitriol; ^, P < 0.05; ^, P < 0.01; and ^^, P < 0.01 compared with the corresponding doses of exemestane, letrozole, or anastrozole.
We tested the growth-inhibitory effects of a series of concentrations of calcitriol (0.1–10 nm) and AIs (1–100 nm) individually and in various combinations. Figure 6, B–D, illustrates the effect of combinations of suboptimal concentrations of calcitriol (0.1 and 1 nm) and the three AIs (each at 1 and 10 nm). Calcitriol alone at 0.1 nm caused modest but statistically significant inhibition (∼11–21%) of T-stimulated cell growth (Fig. 6, B–D). The steroidal AI exemestane (Fig. 6B) and the nonsteroidal AIs anastrozole (Fig. 6C) and letrozole (Fig. 6D) when used individually at 1 nm also modestly but significantly inhibited T-stimulated growth (∼20–30% inhibition). The combinations, however, were more effective (∼40–55% inhibition, Fig. 6, B–D) and caused significantly greater growth inhibition when compared with 0.1 nm calcitriol alone (P < 0.05 to P < 0.001) or the individual AIs (P < 0.05 to P < 0.01), indicating cooperative effects of these agents to inhibit cell growth. Similarly combinations of 1 nm calcitriol and 10 nm of each AI produced significantly higher growth inhibition (∼50–65%) than the individual agents (∼30–35% growth inhibition). Based on dose-response data for the combinations (not shown), we calculated the interaction index (γ) (30) for each drug combination, which was less than 1, indicating a synergistic (superadditive) effect when suboptimal concentrations of calcitriol and the AI were used. For example as shown in Fig 6, B–D, when we used 1 nm of an AI in combination with 0.1 or 1 nm calcitriol, we could obtain approximately the same degree of growth inhibition elicited by a 10-fold higher concentration of the AI (10 nm) used alone. The values of the interaction index-γ for calcitriol-letrozole, calcitriol-anastrozole, and calcitriol-exemestane combinations at suboptimal concentrations were 0.33 ± 0.07, 0.29 ± 0.09, and 0.24 ± 0.09, respectively. However, the γ-values for combinations of higher doses approaching near maximal (for growth inhibition) concentrations of calcitriol or the AIs were closer to 1, indicating that the effects of the combinations were additive at higher concentrations.
Discussion
Calcitriol inhibits BCa cell growth and retards the growth of xenografts of human tumors in immunocompromised hosts by multiple pathways (9,10,13,33). In this study we explored additional new mechanisms by which calcitriol can inhibit the growth of ER+ BCa by suppressing the expression of aromatase and thereby reducing estrogen synthesis. Calcitriol reduced total aromatase mRNA levels and decreased aromatase enzymatic activity in BCa cells. Interestingly, the regulation of aromatase expression by calcitriol is tissue specific. Our data show significant increases in aromatase mRNA and estrogen synthesis in human osteosarcoma cells, confirming earlier reports of calcitriol-mediated up-regulation of aromatase in osteoblasts (14,15). Other studies in keratinocytes (34) and prostate cancer cells (35) found no effect of calcitriol on aromatase expression. Kinuta et al. (36) reported histological abnormalities and decreases in aromatase activity and expression in the gonads of both female and male VDR null mice. Their results indicated that calcitriol plays a role in gonadal estrogen synthesis to a large extent by maintaining calcium homeostasis. However, aromatase deficiency in the VDR null mice was not totally corrected by normalization of serum calcium levels, suggesting that the direct regulation of aromatase by calcitriol might also play a role (36). We observed a modest increase in aromatase mRNA in human ovarian cancer cells after calcitriol treatment, whereas in mice bearing BCa tumors, calcitriol administration did not alter ovarian aromatase mRNA. Studies on calcitriol effects in human ovarian granulosa cells report either no change (37) or an inhibition of aromatase expression (38). Furthermore, as discussed below, calcitriol decreased aromatase expression in mouse preadipocytes and the mammary adipose tissue surrounding human BCa tumors in mice. Collectively our data demonstrate that calcitriol acts as a SAM and its ability to do so may have important implications for BCa treatment as discussed below.
The tissue-specific expression of aromatase is based on the use of tissue-specific promoters that give rise to aromatase transcripts with unique 5′-untranslated sequences. The majority of the transcripts present in osteoblastic cells and the stromal mesenchymal cells of the adipose tissue in the normal breast contain promoter I.4-derived sequences (1,39). However, in BCa aromatase expression is induced via promoters I.3 and II, whereas transcripts derived from promoter I.4 become minor in both the malignant epithelial cells and mammary adipose tissue surrounding them (1,31,40,41,42). In MCF-7 cells calcitriol significantly decreased the levels of the cancer predominant promoter I.3- and II-derived transcripts as well as transcripts from I.4 promoter used in normal breast. In contrast, calcitriol substantially increased total aromatase mRNA as well as I.4- and I.3-derived transcripts in human osteosarcoma cells. The tissue-specific regulation of aromatase expression by calcitriol could be explained by either the differential use of aromatase promoters induced by calcitriol treatment of the different cell types and/or by the differences in the cellular factors such as the various comodulators recruited by calcitriol-bound VDR to the aromatase promoter. Our data indicate that aromatase promoter use is not always different in the different tissues exposed to calcitriol, suggesting that the differences in the cellular context and comodulator recruitment might be important in the differential regulation by calcitriol.
Based on the identification of putative VDREs in promoter II, we hypothesized that calcitriol directly regulated the transcription of aromatase in a tissue-specific manner via VDREs present in the various aromatase promoters. Data from our promoter-reporter assay and ChIP analysis support this hypothesis and demonstrate that calcitriol directly represses aromatase transcription through promoter II in BCa cells through the proximal and distal VDREs we identified in this promoter. Direct effects of calcitriol to regulate aromatase promoter activity have been demonstrated in other target cells (43,44). A functional analysis of aromatase promoters concluded that calcitriol stimulated the activity of an aromatase I.3 promoter- luciferase construct in human osteoblastic cells (43). An imperfect palindromic sequence in the placental promoter I.1 mediates the regulatory effects of both calcitriol and retinoid X receptor ligands in human choriocarcinoma cells (44). Aromatase promoter I.3/II is a cAMP-responsive promoter, which has a CRE (32) that overlaps the proximal VDRE. This overlap provides a potential mechanism for calcitriol to counter the stimulatory effect of cAMP on this promoter. Our reporter assay demonstrates the ability of calcitriol to suppress cAMP stimulation of promoter II activity in MCF-7 cells. The finding raises the possibility that the occupancy of the VDRE by a VDR-retinoid X receptor heterodimer may competitively inhibit the positive regulator CRE-binding protein-1 from binding to the CRE, leading to a decrease in aromatase transcription. Competition for binding with other transcription factors has been implicated in the repressive effects of calcitriol on the promoters of several target genes including the human PTH gene (45,46).
COX-2-derived PGE2, which signals through cAMP, is a major stimulator of aromatase transcription in BCa via promoters I.3 and II (1,47). Several studies have shown that COX-2 expression is positively correlated with aromatase expression in human breast tumors (48,49). 15-PGDH initiates PG inactivation and thereby acts as a tumor suppressor in BCa (50). We previously found that calcitriol regulated the expression of genes involved in PG metabolism in normal and malignant human prostate cells (20). We now show that calcitriol reduces the levels of biologically active PGs in BCa cells as well by decreasing COX-2 and increasing 15-PGDH. Because tumor-derived PGE2 is an important stimulator of aromatase transcription through promoter II in BCa cells and the surrounding adipose tissue (1), calcitriol-mediated reduction of biologically active PG levels provides an important second, indirect mechanism for its down-regulatory effect on aromatase expression in BCa.
In the breast, a major source of aromatase is the breast stromal-adipose-fibroblast cells referred to as breast adipose fibroblasts (BAFs) (41,51,52,53), which are preadipocytes capable of differentiation into mature adipocytes (52). In the breast adipose tissue, aromatase is mainly expressed in the BAFs and is undetectable in mature, lipid-filled adipocytes (1,51,54). In BCa the BAFs provide structural support for tumors, whereas the malignant epithelial cells secrete factors such as PGE2 and cytokines that act on the BAFs, preventing their differentiation and thereby increasing aromatase expression and local estrogen synthesis (1,41,52,55). The rise in aromatase in the BAFs surrounding a breast tumor is accompanied by a switch in aromatase promoter usage from promoter I.4, which is normally used in adipose tissue to promoters I.3 and II (31,40,42). In our 3T3-L1 preadipocyte model, calcitriol significantly decreased aromatase mRNA in the undifferentiated preadipocytes. As expected, we found a reduction in aromatase mRNA when the cells were induced to undergo differentiation into lipid-laden adipocytes. Interestingly, calcitriol itself is known to cause the differentiation of 3T3-L1 cells into mature adipocytes (56). Therefore, we hypothesize that calcitriol decreases aromatase expression in the preadipocytes/BAFs by three mechanisms: 1) inducing the differentiation of preadipocytes into mature adipocytes with reduced aromatase expression, 2) directly suppressing aromatase transcription through promoter II in the tumor-adjacent preadipocytes, and 3) an indirect mechanism of decreasing the secretion by the malignant epithelial cells of PGE2, which is a major inducer of aromatase transcription through promoter II/I.3 in the surrounding BAFs (1,53,55).
The results of our in vivo study on the effect of short-term calcitriol treatment in mice bearing MCF-7 xenograft tumors also support our hypothesis and demonstrate that calcitriol causes aromatase down-regulation in the tumors as well as in the mammary fat tissue surrounding tumors. The down-regulation of COX-2 expression in the tumors after calcitriol administration suggest that a decrease in the levels COX-2-derived PGs could also contribute to the decreased expression of aromatase in the tumors. In our short-term study, the administration of a single high concentration of calcitriol for a short duration of 72 h did not significantly increase serum calcium levels in the mice, indicating that this dosing regimen was not toxic to the mice. In studies investigating calcitriol effects on tumor growth, which typically use longer treatment periods (4 wk or more), more modest doses of calcitriol or the use of calcitriol analogs with reduced calcemic activity would be more appropriate.
The stimulation of MCF-7 cell proliferation by T was likely due to its conversion to E2 rather than its actions through the AR because T stimulation of cell growth was not abolished by the AR antagonist bicalutamide. Calcitriol produced a dose-dependent inhibition of T-stimulated cell growth due to its ability to inhibit both estrogen synthesis (aromatase down-regulation) and estrogen signaling (ERα down-regulation), completely abolishing T-stimulated growth by 10 nm. Further increases in calcitriol concentration caused a reduction in cell growth below basal levels suggesting that all the other known calcitriol-mediated antiproliferative mechanisms (11,13) were at play. AIs decrease estrogen synthesis by inhibiting the enzymatic activity of aromatase. We expected that the action of calcitriol to reduce aromatase expression at the genomic level would complement the AI effect and hypothesized that a combination of calcitriol and AIs would cause enhanced inhibition of BCa cell growth. This hypothesis is supported by our data showing synergistic (at low doses) and additive (at high doses) cooperativity of the combinations of calcitriol and three different AIs to inhibit BCa cell growth.
In summary, calcitriol regulates aromatase expression in a tissue-selective manner. Acting as a SAM, calcitriol decreases aromatase expression in BCa cells and breast adipose tissue surrounding BCa and increases aromatase expression in bone-derived cells. The mechanism of the down-regulatory effect of calcitriol on BCa aromatase expression is 2-fold: a direct repression of aromatase transcription via promoter II through the VDREs present in this promoter and an indirect effect (due to COX-2 suppression and 15-PGDH up-regulation) to reduce the levels of PGE2, which is a major stimulator of aromatase transcription through promoter II in BCa. We propose that the combination of calcitriol and an AI would have beneficial effects in BCa therapy. In addition to augmenting the ability of AIs to inhibit estrogen synthesis and BCa cell proliferation, calcitriol acting as a SAM would increase aromatase expression in bone. AI therapy has been reported to worsen bone health, leading to increased occurrence of osteopenia, osteoporosis, and bone fractures as a result of estrogen deprivation induced by the treatment (7,8). We hypothesize that calcitriol, acting as a SAM, would increase local estrogen synthesis in bone, thus ameliorating the AI-induced side effect of osteoporosis. A recent study on a transgenic mouse model expressing human aromatase mRNA specifically in bone (57) reports stimulatory estrogenic effects to improve bone health without increasing serum E2 levels, lending support to our hypothesis. Interestingly, a recent clinical study also shows that vitamin D supplementation in women undergoing adjuvant letrozole therapy for BCa results in a significant reduction in the musculoskeletal symptoms associated with the AI therapy (58). Our findings suggest that administering calcitriol in combination with an AI would be a beneficial therapeutic approach in postmenopausal women with BCa.
Acknowledgments
We thank Dr. Shiuan Chen (Beckman Research Institute of the City of Hope, Duarte, CA) for providing the aromatase promoter I.3/II-luciferase reporter plasmid. We also thank Dr. Milan Uskokovic (BioXell), Dr. Donnie W. Owens, Pfizer Inc., Dr. Dean Evans (Norvartis Pharma AG), and Dr. Gary Nunn and Dr. David Scanlan (AstraZeneca) for their kind gifts of calcitriol, exemestane, letrozole, and anastrozole, respectively.
Footnotes
Current address for J.M.: Department of Biological Sciences, University of Alabama-Huntsville, Huntsville, Alabama 35899.
This work was supported by National Cancer Institute Grant CA130991 and Komen Foundation Grant 070101 (to D.F.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 11, 2009
Abbreviations: AI, Aromatase inhibitor; AP2, adipocyte fatty acid binding protein 2; AR, androgen receptor; BAF, breast adipose fibroblast; BCa, breast cancer; (Bu)2cAMP, dibutyryl cAMP; Cdx, Casodex; ChIP, chromatin immunoprecipitation; COX, cyclooxygenase; CRE, cAMP response element; E2, estradiol; EIA, enzyme immunoassay; ER, estrogen receptor; FCS, fetal calf serum; Fsk, forskolin; OVX, ovariectomized; PG, prostaglandin; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; PPAR, peroxisome proliferator-activated receptor; SAM, selective aromatase modulator; T, testosterone; VDR, vitamin D receptor; VDRE, vitamin D-response element.
References
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M 2002 Aromatase—a brief overview. Annu Rev Physiol 64:93–127 [DOI] [PubMed] [Google Scholar]
- Chen S 1998 Aromatase and breast cancer. Front Biosci 3:d922–d933 [DOI] [PubMed] [Google Scholar]
- Brodie A, Long B, Lu Q 1998 Aromatase expression in the human breast. Breast Cancer Res Treat 49(Suppl 1):S85–91; discussion S109–S119 [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW 2006 Update on the use of aromatase inhibitors in breast cancer. Expert Opin Pharmacother 7:1919–1930 [DOI] [PubMed] [Google Scholar]
- Geisler J, Lønning PE 2005 Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res 11:2809–2821 [DOI] [PubMed] [Google Scholar]
- Wheler J, Johnson M, Seidman A 2006 Adjuvant therapy with aromatase inhibitors for postmenopausal women with early breast cancer: evidence and ongoing controversy. Semin Oncol 33:672–680 [DOI] [PubMed] [Google Scholar]
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD 2007 Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone 41:346–352 [DOI] [PubMed] [Google Scholar]
- Mincey BA, Duh MS, Thomas SK, Moyneur E, Marynchencko M, Boyce SP, Mallett D, Perez EA 2006 Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer 7:127–132 [DOI] [PubMed] [Google Scholar]
- Colston K, Welsh J 2005 Vitamin D and breast cancer. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. San Diego: Elsevier Academic Press; 1663–1677 [Google Scholar]
- Gombart AF, Luong QT, Koeffler HP 2006 Vitamin D compounds: activity against microbes and cancer. Anticancer Res 26:2531–2542 [PubMed] [Google Scholar]
- Krishnan AV, Moreno J, Nonn L, Swami S, Peehl DM, Feldman D 2007 Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. J Bone Miner Res 22(Suppl 2):V74–V80 [DOI] [PubMed] [Google Scholar]
- Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS 2006 Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res 26:2551–2556 [PubMed] [Google Scholar]
- Welsh J 2007 Targets of vitamin D receptor signaling in the mammary gland. J Bone Miner Res 22(Suppl 2):V86–V90 [DOI] [PubMed] [Google Scholar]
- Enjuanes A, Garcia-Giralt N, Supervia A, Nogués X, Mellibovsky L, Carbonell J, Grinberg D, Balcells S, Díez-Pérez A 2003 Regulation of CYP19 gene expression in primary human osteoblasts: effects of vitamin D and other treatments. Eur J Endocrinol 148:519–526 [DOI] [PubMed] [Google Scholar]
- Yanase T, Suzuki S, Goto K, Nomura M, Okabe T, Takayanagi R, Nawata H 2003 Aromatase in bone: roles of Vitamin D3 and androgens. J Steroid Biochem Mol Biol 86:393–397 [DOI] [PubMed] [Google Scholar]
- Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J 1997 Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat 42:31–41 [DOI] [PubMed] [Google Scholar]
- Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB 1999 Regulation of estrogen receptor-α gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem 75:640–651 [PubMed] [Google Scholar]
- Swami S, Krishnan AV, Feldman D 2000 1α,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res 6:3371–3379 [PubMed] [Google Scholar]
- Krishnan AV, Peehl DM, Feldman D 2003 Inhibition of prostate cancer growth by vitamin D: Regulation of target gene expression. J Cell Biochem 88:363–371 [DOI] [PubMed] [Google Scholar]
- Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D 2005 Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 65:7917–7925 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Chen TL, Shen WJ, Qiu XW, Li T, Hoffman AR, Kraemer FB 2007 Generation of novel adipocyte monolayer cultures from embryonic stem cells. Stem Cells Dev 16:371–380 [DOI] [PubMed] [Google Scholar]
- Díaz-Cruz ES, Shapiro CL, Brueggemeier RW 2005 Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 90:2563–2570 [DOI] [PubMed] [Google Scholar]
- Ohno K, Araki N, Yanase T, Nawata H, Iida M 2004 A novel nonradioactive method for measuring aromatase activity using a human ovarian granulosa-like tumor cell line and an estrone ELISA. Toxicol Sci 82:443–450 [DOI] [PubMed] [Google Scholar]
- Trösken ER, Fischer K, Völkel W, Lutz WK 2006 Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 219:33–40 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Peng L, Malloy PJ, Feldman D 2004 Identification of a Functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol 18:1109–1119 [DOI] [PubMed] [Google Scholar]
- Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS 1999 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res 59:2644–2649 [PubMed] [Google Scholar]
- Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL 2001 Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 7:1043–1051 [PubMed] [Google Scholar]
- Tallarida RJ 2002 The interaction index: a measure of drug synergism. Pain 98:163–168 [DOI] [PubMed] [Google Scholar]
- Harada N, Utsumi T, Takagi Y 1993 Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA 90:11312–11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Chen S 1999 Identification and characterization of a cAMP-responsive element in the region upstream from promoter 1.3 of the human aromatase gene. Arch Biochem Biophys 371:179–190 [DOI] [PubMed] [Google Scholar]
- Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, Welsh JC, Byrne B, Narvaez CJ 2002 Impact of the Vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J Steroid Biochem Mol Biol 83:85–92 [DOI] [PubMed] [Google Scholar]
- Hughes SV, Robinson E, Bland R, Lewis HM, Stewart PM, Hewison M 1997 1,25-Dihydroxyvitamin D3 regulates estrogen metabolism in cultured keratinocytes. Endocrinology 138:3711–3718 [DOI] [PubMed] [Google Scholar]
- Lou YR, Murtola T, Tuohimaa P 2005 Regulation of aromatase and 5α-reductase by 25-hydroxyvitamin D(3), 1α,25-dihydroxyvitamin D(3), dexamethasone and progesterone in prostate cancer cells. J Steroid Biochem Mol Biol 94:151–157 [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y 2000 Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 141:1317–1324 [DOI] [PubMed] [Google Scholar]
- Yanase T, Mu YM, Nishi Y, Goto K, Nomura M, Okabe T, Takayanagi R, Nawata H 2001 Regulation of aromatase by nuclear receptors. J Steroid Biochem Mol Biol 79:187–192 [DOI] [PubMed] [Google Scholar]
- Brian H, Bano G, Mason HD, Nussey SS, 1,25-Dihydroxyvitamin D3 has a direct effect on steroid production by human granulosa cells. Proc 192nd Annual Meeting of the Society for Endocrinology, London, UK, 2001 (Abstract P2-73) [Google Scholar]
- Agarwal VR, Bulun SE, Simpson ER 1995 Quantitative detection of alternatively spliced transcripts of the aromatase cytochrome P450 (CYP19) gene in aromatase-expressing human cells by competitive RT-PCR. Mol Cell Probes 9:453–464 [DOI] [PubMed] [Google Scholar]
- Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER 1996 Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab 81:3843–3849 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S 2005 Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- Zhou D, Zhou C, Chen S 1997 Gene regulation studies of aromatase expression in breast cancer and adipose stromal cells. J Steroid Biochem Mol Biol 61:273–280 [PubMed] [Google Scholar]
- Enjuanes A, Garcia-Giralt N, Supervía A, Nogués X, Ruiz-Gaspà S, Bustamante M, Mellibovsky L, Grinberg D, Balcells S, Díez-Pérez A 2005 Functional analysis of the I. 3, I. 6, pII and I. 4 promoters of CYP19 (aromatase) gene in human osteoblasts and their role in vitamin D and dexamethasone stimulation. Eur J Endocrinol 153:981–988 [DOI] [PubMed] [Google Scholar]
- Sun T, Zhao Y, Mangelsdorf DJ, Simpson ER 1998 Characterization of a region upstream of exon I. 1 of the human CYP19 (aromatase) gene that mediates regulation by retinoids in human choriocarcinoma cells. Endocrinology 139:1684–1691 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen T, Huhtakangas J, Mäenpää PH 2005 Negative regulation of human parathyroid hormone gene promoter by vitamin D3 through nuclear factor Y. Biochem Biophys Res Commun 328:831–837 [DOI] [PubMed] [Google Scholar]
- Koszewski NJ, Alimov AP, Park-Sarge OK, Malluche HH 2004 Suppression of the human parathyroid hormone promoter by vitamin D involves displacement of NF-Y binding to the vitamin D response element. J Biol Chem 279:42431–42437 [DOI] [PubMed] [Google Scholar]
- Richards JA, Petrel TA, Brueggemeier RW 2002 Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol 80:203–212 [DOI] [PubMed] [Google Scholar]
- Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, DeJong PC, Blankenstein MA, Nortier JW, Slee PH, van de Ven J, van Gorp JM, Elbers JR, Schipper ME, Blijham GH, Thijssen JH 2001 Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol 79:41–47 [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM 1999 Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett 140:27–35 [DOI] [PubMed] [Google Scholar]
- Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, Tai HH, Karlan BY, Koeffler HP 2006 15-Hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res 66:7818–7823 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Sharda G, Rink J, Sharma S, Simpson ER 1996 Distribution of aromatase P450 transcripts and adipose fibroblasts in the human breast. J Clin Endocrinol Metab 81:1273–1277 [DOI] [PubMed] [Google Scholar]
- Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE 2001 Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: mechanism of desmoplastic reaction. Cancer Res 61:2250–2255 [PubMed] [Google Scholar]
- Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE 2001 Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein β. Cancer Res 61:2328–2334 [PubMed] [Google Scholar]
- Price T, Aitken J, Head J, Mahendroo M, Means G, Simpson E 1992 Determination of aromatase cytochrome P450 messenger ribonucleic acid in human breast tissue by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab 74:1247–1252 [DOI] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE 2007 Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res 67:8914–8922 [DOI] [PubMed] [Google Scholar]
- Vu D, Ong JM, Clemens TL, Kern PA 1996 1,25-Dihydroxyvitamin D induces lipoprotein lipase expression in 3T3-L1 cells in association with adipocyte differentiation. Endocrinology 137:1540–1544 [DOI] [PubMed] [Google Scholar]
- Sjögren K, Lagerquist M, Moverare-Skrtic S, Andersson N, Windahl SH, Swanson C, Mohan S, Poutanen M, Ohlsson C 2009 Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J Bone Miner Res 24:1263–1270 [DOI] [PubMed] [Google Scholar]
- Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O'Dea AP, Klemp JR, Fabian CJ 2009 Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat 10. 1007/S 10549-009-0495-x [DOI] [PMC free article] [PubMed] [Google Scholar]