Abstract
Vitamin D metabolites are important effectors of bone and mineral homeostasis. Extrarenal conversion of 25-hydroxyvitamin D (25OHD) to the biologically active form of vitamin D, 1α,25-dihydroxyvitamin D [1,25(OH)2D] is catalyzed in several cell types by the 1α-hydroxylase (CYP27B1), but little is known about the expression or regulation of CYP27B1 in human bones. We examined whether human bone marrow stromal cells (hMSCs, also known as mesenchymal stem cells) participate in vitamin D metabolism and whether vitamin D hydroxylases in hMSCs are influenced by the vitamin D status of the individual from whom the hMSCs were obtained. We also investigated the effects of vitamin D metabolites on osteoblast differentiation and the role of IGF-I in the regulation of CYP27B1. In a series of 27 subjects, vitamin D hydroxylases in hMSCs were expressed at different levels and were correlated with serum 25OHD, 1,25(OH)2D, and PTH. In vitro treatment with 25OHD up-regulated CYP27B1 and IGF-I in hMSCs; IGF-I also up-regulated CY27B1 expression and stimulated osteoblast differentiation. When hydroxylation of 25OHD was blocked by ketoconazole, a cytochrome P450 inhibitor, 25OHD was no longer able to induce CYP27B1 expression. In summary, these findings show that human bone marrow stromal cells have the molecular machinery both to metabolize and respond to vitamin D. We propose that circulating 25OHD, by virtue of its local conversion to 1,25(OH)2D catalyzed by basal CYP27B1 in hMSCs, amplifies vitamin D signaling through IGF-I up-regulation, which in turn induces CYP27B1 in a feed-forward mechanism to potentiate osteoblast differentiation initiated by IGF-I.
Human bone marrow stromal cells include osteoblast progenitors and have the molecular machinery to metabolize, regulate, and respond to vitamin D, with amplification through IGF-I induction.
There are two major sources of vitamin D (cholecalciferol): dietary intake and conversion of 7-dehydrocholesterol in the skin to vitamin D by UV light. Vitamin D from both sources undergoes sequential steps of activation, a first hydroxylation in the liver to 25-hydroxyvitamin D (25OHD) and a second hydroxylation in the kidney to the active hormone 1,25-dihydroxyvitamin D [1,25(OH)2D] (1). The active metabolite, 1,25(OH)2D, is taken up by target cells that possess the vitamin D receptor (VDR). Vitamin D status is assessed by the circulating level of 25OHD. Vitamin D deficiency can lead to low bone density and, in severe instances, osteomalacia (2) and is associated with osteopenia and osteoporosis, muscle weakness, falls, and increased risk of fracture (3,4,5). We reported extreme vitamin D deficiency in U.S. women with hip fractures (6). Emerging evidence indicates that vitamin D deficiency is associated with many nonskeletal illnesses, including cancer, autoimmune diseases, infectious diseases, and cardiovascular disease (7).
Among the cytochrome P450 (CYP) isoforms that have been shown to hydroxylate vitamin D, CYP27B1/25OHD-1α-hydroxylase converts 25OHD into the active hormonal form, 1,25(OH)2D. CYP27B1 activity is tightly regulated through complex mechanisms that depend on the circulating levels of calcium, phosphorus, PTH, and 1,25(OH)2D3 (8) and by calcitonin (9). Previous studies suggest that IGF-I may regulate the renal production of 1,25(OH)2D3 (10,11,12,13,14). In addition to kidney tubule cells, other human cells have been demonstrated to produce 1,25(OH)2D, notably osteoblasts (15), activated macrophages (16), keratinocytes (17), endothelial cells (18), and cancer cells (19). Finding the VDR and vitamin D hydroxylases in many tissues suggests that the vitamin D hormone acts in an autocrine, paracrine, or intracrine fashion to affect the biology of nonclassical target tissues. Recent data from mouse studies appear to show more limited distribution of 1α-hydroxylase (8).
Human marrow-derived stromal cells (hMSCs), also known as mesenchymal stem cells, include progenitors of several lineages, including osteoblasts, chondrocytes, and adipocytes (20,21,22). From studies with hMSCs isolated from marrow that was discarded during orthopedic surgery, we determined that there is an age-related decrease in their differentiation to osteoblasts (23,24). The differentiation of hMSCs to osteoblasts is enhanced by 1,25(OH)2D3 (25), but there is no information about vitamin D metabolism in hMSCs or the effects of 25OHD on these processes. In this series of investigations, we tested whether 25OHD3 stimulates hMSCs to differentiate to osteoblasts, whether hMSCs participate in vitamin D metabolism, whether expression of vitamin D hydroxylases in hMSCs in vitro are influenced by the vitamin D status of the individual from whom the hMSCs were obtained, and whether vitamin D metabolic enzymes in hMSCs are regulated in vitro.
Materials and Methods
Subjects
Bone marrow samples were obtained with Institutional Review Board approval and annual review as femoral tissue discarded during primary hip arthroplasty for osteoarthritis. Criteria for exclusion were rheumatoid arthritis, cancer, and other comorbid conditions that may influence skeletal metabolism, i.e. renal insufficiency, alcoholism, active liver disease, malabsorption, hyperthyroidism, ankylosing spondylitis, aseptic necrosis, hyperparathyroidism, morbid obesity, and diabetes. Also excluded were patients who were taking medications that may influence skeletal metabolism (e.g. thyroid hormone, glucocorticoids, bisphosphonates, and nonsteroidal antiinflammatory drugs). A set of 27 subjects scheduled for hip arthroplasty was consented for research studies, including measurement of serum 25OHD, 1,25(OH)2D, and PTH as well as body composition and bone mineral density (BMD). Although current estrogen use was not excluded in the screening criteria, none of the women in this series was receiving estrogen at the time of surgery. Another set of bone marrow samples that was used for osteoblast differentiation experiments was obtained as discarded tissue from 19 de-identified individuals with Institutional Review Board approval and the same preoperative exclusion screen.
Blood chemical assays
Blood chemistries and complete blood counts were performed in hospital clinical laboratories; the remaining tests were performed in the General Clinical Research Center laboratory unless otherwise specified. Serum 25OHD levels were assayed using an isotopic assay (DiaSorin RIA, Stillwater, MN), with a sensitivity of 1.5 ng/ml and an interassay coefficient of variation (CV) of less than 10.5%; sufficiency was defined as more than 32 ng/ml (26). Levels of 1,25(OH)2D were measured by an extraction and isotopic method (Diasorin) with a sensitivity of 2 pg/ml and an interassay CV of less than 14.7%; the normal range was 15–75 pg/ml. Serum intact PTH levels were measured with the sensitive chemiluminescent assay (Beckman Access II; Beckman Coulter, Inc., Fullerton, CA), with a sensitivity of 1 pg/ml and an interassay CV of less than 6.5%; the normal range of serum PTH was 10–65 pg/ml. Urinary N-telopeptide levels corrected for urinary creatinine, an index of bone resorption, were determined in a second morning spot urine collection by an ELISA that measures cross-linked collagen peptides (Osteomark Assay; Ostex International, Inc., Seattle, WA), the normal range was 13–65 nmol/mmol creatinine.
BMD and body composition
BMD of the spine (L1–L4) and proximal femur were measured with dual x-ray absorptiometry technique (Discovery; Hologic Inc., Bedford, MA). Least significant changes at the 95% confidence level for the spine and femoral neck bone density measurements were 0.017 and 0.014 g/cm2, respectively. Vertebrae with moderately severe osteoarthritic changes, disc space narrowing, or a fracture would be excluded from the analyses as those anatomic findings may elevate the spinal BMD. For these 27 subjects, none of the spine BMD measurements had to be excluded. If subjects had a hip replacement on the contralateral side, BMD was not measured at that site. Results were expressed as sd compared with BMD values for young normal controls (T-score). Body composition was also determined by dual x-ray absorptiometry (Discovery; Hologic) (27). Reproducibility for fat determination in our laboratory was 1.09 ± 0.15% (CV, mean ± sem).
Preparation of hMSCs
Low-density marrow mononuclear cells were isolated by centrifugation on Ficoll/Histopaque 1077 (Sigma Chemical Co., St. Louis, MO) (28). This procedure removes differentiated cells and enriches for undifferentiated, low-density marrow mononuclear cells that include a population of nonadherent hematopoietic cells and a fraction capable of adherence and differentiation into musculoskeletal cells. Adherent hMSCs were expanded in monolayer culture with phenol red-free α-MEM, 10% fetal bovine serum-heat inactivated (FBS-HI), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). All samples were used at passage 2.
Alkaline phosphatase (AlkP) enzyme assay
A set of MSCs that were obtained from 19 de-identified subjects was used to assess osteoblast differentiation in vitro. Cells were cultured in triplicate in 12-well plates in α-MEM with 10% FBS-HI until confluence; this required different times depending upon rates of proliferation. Upon confluence, medium was changed to osteogenic medium (α-MEM with 1% FBS-HI, 100 U/ml penicillin, 100 μg/ml streptomycin plus 10 nm dexamethasone, 5 mm β-glycerophosphate, and 50 μg/ml ascorbate-2-phosphate) for 7 d. Reduction of serum to 1% for differentiation was designed to minimize possible differences in proliferation that could confound interpretation of effects of vitamin D3 metabolites or other agents on osteoblastogenesis. AlkP enzyme activity was measured as previously described (24). Cells from an enrolled 72-yr-old woman were used to measure the effect of IGF-I on osteoblastogenesis.
RNA isolation and RT-PCR
Total RNA was isolated from hMSCs with Trizol reagent (Invitrogen). For RT-PCR, 2 μg total RNA was reverse-transcribed into cDNA with SuperScript II (Invitrogen), following the manufacturer’s instructions. One twentieth of the cDNA was used in each 50-μl PCR (30–40 cycles of 94 C for 1 min, 55–60 C for 1 min, and 72 C for 2 min) as described (28). The gene-specific primers for human CYP27B1 (29), CYP24A1 (30), VDR (31), and IGF-I (32) were used for amplification. CYP27A1/25OHase primers were modified from Seifert et al. (30): forward 5′-GGAAAGTACCCAGTACGG-3′ and reverse 5′-AGCAAATAGCTTCCAAGG-3′ (289-bp product). Concentration of cDNA and amplification conditions were optimized to reflect the exponential phase of amplification. Quantitative data were expressed by normalizing the densitometric units to GAPDH (internal control) as described (24,28).
In vitro biosynthesis of 1,25(OH)2D3 by hMSCs
hMSCs were cultivated in 12-well plates until confluence and then treated with or without 1 μm 25OHD3 (Sigma) with or without 10 μm CYP inhibitor ketoconazole (Sigma) or 100 ng/ml IGF-I (R&D Systems, Minneapolis, MN) in serum-free α-MEM supplemented with 1% ITS+1 (Sigma). 1,2-Dianilinoethane (N,N′-diphenylethylene-diamine) (10 μm) (Sigma) was added into the cultures as an antioxidant as described (18). After 24 h treatment, the media were collected from each well. The 1,25(OH)2D3 levels in media were quantitatively determined with a 1,25(OH)2D3 ELISA kit (USA Immunodiagnostic Systems Ltd., Fountain Hills, AZ), according to the manufacturer’s instructions. The hMSCs were lysed with a buffer containing 150 mm NaCl, 3 mm NaHCO3, 0.1% Triton X-100, and a mixture of protease inhibitors (Roche Diagnostics, Pleasonton, CA). Protein concentration was determined with the BCA system (Pierce, Rockford, IL). The 1α-hydroxylase activity was expressed as biosynthesized 1,25(OH)2D3 in medium per milligram protein per hour of 25OHD3 treatment (femtomoles per milligram protein per hour).
Statistical analyses
All experiments were performed at least in triplicate. Group data are presented as mean values ± sd. Unless otherwise indicated, quantitative data were analyzed with nonparametric tools, either the Mann-Whitney U test for group comparisons or Spearman correlation test. If data allowed, parametric tools were used, either t test for two-group or one-way ANOVA for multiple-group comparisons or Pearson correlation test. A value of P < 0.05 was considered significant.
Results
In vitro stimulation of osteoblast differentiation by both 25OHD3 and 1,25(OH)2D3
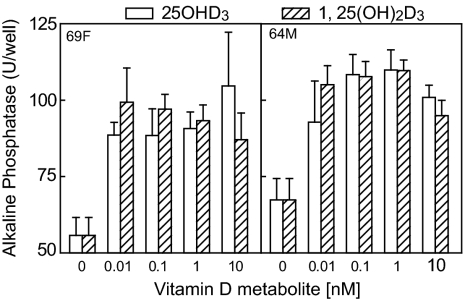
A set of MSCs that were obtained from 19 de-identified subjects was used to assess osteoblast differentiation in vitro. There were samples from 10 men and nine women between 64 and 83 yr of age. Cultures of these hMSCs were treated with either 1,25(OH)2D3 or 25OHD3 (0.01–10 nm) for 7 d in osteogenic medium. Osteoblast differentiation, assessed with AlkP enzyme activity assays, was stimulated by 1,25(OH)2D3 in all but two of the 19 samples (89%), with peak stimulation between 1 and 10 nm. Two thirds of the samples were stimulated by both 1,25(OH)2D3 and 25OHD3. In some cases, there was equivalent dose-response stimulation of osteoblast differentiation in hMSCs by both 1,25(OH)2D3 and 25OHD3 (Fig. 1). In those examples, both 1,25(OH)2D3 and 25OHD3 (0.01–10 nm) significantly stimulated AlkP activity, compared with vehicle controls (P < 0.001), and there was no significant difference between 1,25(OH)2D3 and 25OHD3.
Figure 1.
Effects of vitamin D metabolites on osteoblast differentiation in hMSCs from a 69-yr-old female (69F) and a 64-yr-old male (64M) subject. Osteoblast differentiation of hMSCs was assessed with AlkP enzyme activity assays (n = 6) after 7 d culture with or without 25OHD3 or 1,25(OH)2D3 (0.01–10 nm) in osteogenic medium.
Characteristics of the study subjects
Clinical data were available for 27 consented subjects, from whom MSCs were isolated from bone marrow discarded during orthopedic surgery. The mean age was 66 ± 10 yr, ranging from 41–81 yr. There were 14 men and 13 women. There was a wide range of values for serum 25OHD, 1,25(OH)2D, PTH, cross-linked N-telopeptides of type I collagen (NTX), urine creatinine levels, percent fat, body mass index (BMI), and BMD T-scores (Table 1). We found that 28% of the subjects were vitamin D deficient (<20 ng/ml serum 25OHD), that 36% were insufficient with level between 20 and 32 ng/ml, and that 36% were vitamin D sufficient (>32 ng/ml). There were no differences in serum 25OHD between genders or with age. Serum 25OHD was inversely correlated with serum PTH (r = −0.49; P = 0.015), with BMI (r = −0.41; P = 0.035), and with percent body fat (Spearman r = −0.50; P = 0.0077) (Table 2). There were no correlations with 1,25(OH)2D, urine NTX, or T-score of spine and total hip. There was a trend for an inverse correlation between serum 25OHD3 and urinary creatinine (r = −0.43; P = 0.058).
Table 1.
Characteristics of the study subjects (n = 27, except as noted)
| Mean ± sd | Range | |
|---|---|---|
| Age (yr) | 66 ± 10 | 41–81 |
| 25OHD (ng/ml) | 27.5 ± 10.8 | 7.6–48.8 |
| 1,25(OH)2D (pg/ml) (n = 23) | 40.3 ± 12.5 | 20.0–71.0 |
| PTH (pg/ml) (n = 24) | 37.6 ± 16.6 | 12.1–83.7 |
| Urine creatinine (mg/dl) | 97.7 ± 47.6 | 22.5–213.3 |
| Urine NTX (nmol/mmol creatinine) (n = 25) | 43.6 ± 18.8 | 14.0–84.0 |
| Spine T-score | 0.54 ± 1.74 | −2.8 to 3.7 |
| Left total hip T-score (n = 22) | −0.45 ± 1.12 | −1.9 to 1.9 |
| BMI (kg/m2) (n = 26) | 29.1 ± 6.6 | 19.9–49.3 |
| % Fat | 35.6 ± 8.9 | 19.1–49.1 |
Table 2.
Correlations between serum 25OHD and other clinical parameters
| Serum PTH | Serum 1,25(OH)2D | BMI | % Fat | |
|---|---|---|---|---|
| r | −0.49 | 0.016 | −0.41 | −0.50 |
| n | 24 | 23 | 26 | 27 |
| P | 0.015 | 0.94 | 0.035 | 0.0077 |
n, Number of subjects; r, Spearman correlation coefficient.
Relationships between serum parameters and constitutive expression of vitamin D hydroxylases in hMSCs
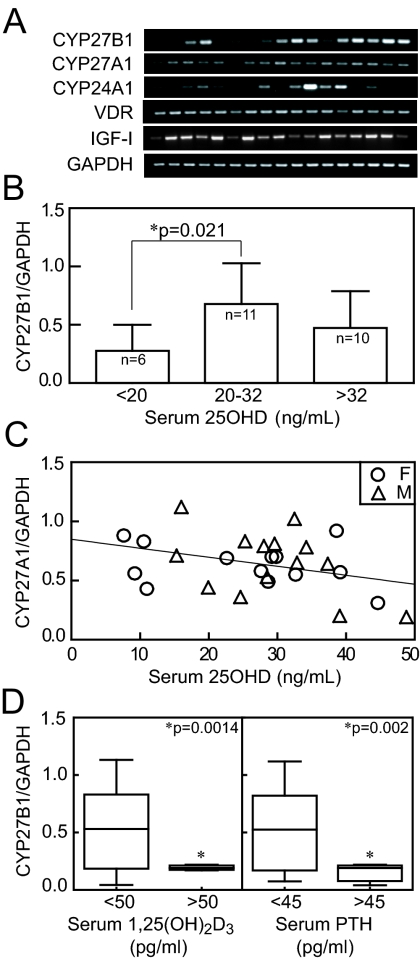
Gene expression was assessed in hMSCs from 27 subjects for whom clinical information was available. In this series, there was a wide range of constitutive expression of 1α-hydroxylase (CYP27B1), with no differences between genders or with age (for clarity, 17 representative samples are shown in Fig. 2A). All 27 samples expressed the VDR, IGF-I, and CYP27A1/25-hydroxylase, whereas 18 of 27 had detectable CYP24A1/24-hydroxylase (Fig. 2A). There were relationships between CYP27B1/1αOHase gene expression in hMSCs and serum 25OHD, some of which depended on the level of serum 25OHD. There was significantly lower expression of CYP27B1 in hMSCs from subjects with serum 25OHD less than 20 ng/ml (0.28 ± 0.23, n = 6; P = 0.021), compared with those from subjects with serum 25OHD between 20 and 32 ng/ml (0.67 ± 0.34, n = 12) (Fig. 2B). There was a significant correlation between serum 25OHD and CYP27B1 gene expression in hMSCs from subjects with serum 25OHD from 7–20 ng/ml (r = 0.893; P < 0.0001; n = 7). In addition, there was a trend for an inverse correlation between CYP27A1/25-hydroxylase and serum 25OHD (P = 0.065, Pearson correlation) (Fig. 2C). There were no associations found between serum parameters and CYP24A1/24OHase gene expression in hMSCs. Of the 24 subjects, 9% had serum PTH levels greater than 45 pg/ml, and 10% had serum 1,25(OH)2D levels greater than 50 pg/ml. There was lower expression of CYP27B1 in hMSCs from subjects with serum PTH higher than 45 pg/ml (P = 0.002, Mann-Whitney U test) and for subjects with serum 1,25(OH)2D higher than 50 pg/ml (P = 0.0014, Mann-Whitney U test) (Fig. 2D).
Figure 2.
Relationship between serum parameters with vitamin D metabolic enzyme gene expression in hMSCs. A, The expression of CYP27B1/1αOHase, CYP27A1/25OHase, CYP24A1/24OHase, VDR, and IGF-I genes was assessed in hMSCs obtained from 27 consented subjects for whom clinical data were available (each lane for 17 examples shown for clarity). B, The relationship between serum 25OHD of 27 subjects and CYP27B1 gene expression in their hMSCs was assessed. Serum 25OHD less than 20 ng/ml was defined as vitamin D deficiency, and more than 32 ng/ml was defined as vitamin D sufficiency. C, There was an inverse correlation between serum 25OHD and CYP27A1/25OHase gene expression in hMSCs (r = −0.36; P = 0.065; n = 27; F, females; M, males). D, There was significantly lower expression of CYP27B1 in hMSCs from subjects with serum 1,25(OH)2D higher than 50 pg/ml (P = 0.0014, Mann-Whitney U test) and in subjects with serum PTH higher than 45 pg/ml (P = 0.002).
In vitro CYP27B1 gene expression and 1α-hydroxylase activity in hMSCs
hMSCs from a vitamin D-deficient subject (72-yr-old female, 10.5 ng/ml serum 25OHD) were treated with or without 25OHD3 (10 nm) with or without CYP inhibitor ketoconazole (10 μm) for 24 h. The hMSCs expressed CYP27B1, which was up-regulated by 25OHD3. Both basal and up-regulated levels of CYP27B1 were inhibited by ketoconazole (Fig. 3A). The 1α-hydroxylase activity was assessed in hMSCs from the same subject by measuring 1,25(OH)2D3 in the medium with an ELISA. Media were collected from confluent hMSCs treated for 24 h with or without 1 μm 25OHD3 as exogenous substrate with or without 10 μm ketoconazole (Fig. 3B). The 1α-hydroxylase activity was expressed as biosynthesized 1,25(OH)2D3 in medium. In the presence of ketoconazole, biosynthesis of 1,25(OH)2D3 was 34.6% of control with only exogenous substrate (P < 0.05, Mann-Whitney U test).
Figure 3.
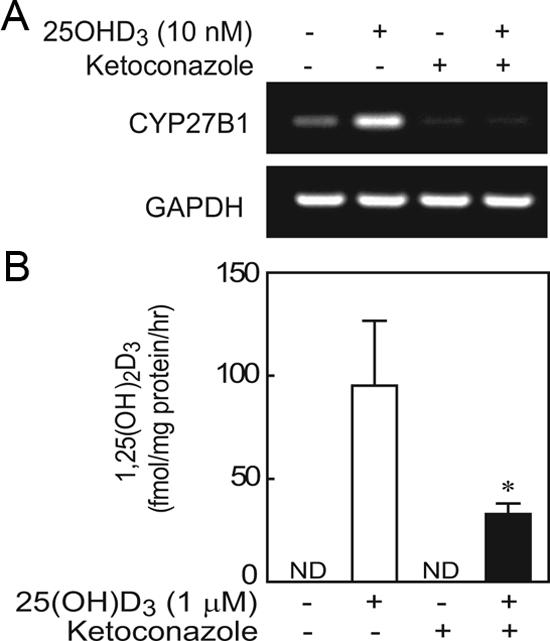
Effects of CYP inhibitor ketoconazole on CYP27B1/1αOHase gene expression and activity in hMSCs from a 72-yr-old vitamin D-deficient female subject. A, After 24 h treatment, CYP inhibitor ketoconazole (10 μm) blocked the stimulation by 25OHD3 (10 nm) on CYP27B1/1αOHase gene expression. B, Ketoconazole (10 μm) significantly inhibited synthesis of 1,25(OH)2D3 in the presence of 25OHD3 (*, P < 0.05; n = 3). There was no detectable (ND) 1,25(OH)2D3 in cultures without 1 μm 25OHD3 exogenous substrate.
Constitutive expression of IGF-I in hMSCs
Expression of the growth factors IGF-I, TGF-β1, FGF-2, and PDGF-β1 was evaluated in all hMSCs because of their potential roles in skeletal homeostasis. None of the growth factors showed correlations with serum parameters. Of those growth factors, IGF-I was the only growth factor correlated with expression of a hydroxylase. There was a significant correlation between constitutive expression levels of IGF-I and CYP27A1 (r = 0.71; P = 0.0003). These findings prompted in vitro experiments for regulation of and by IGF-I.
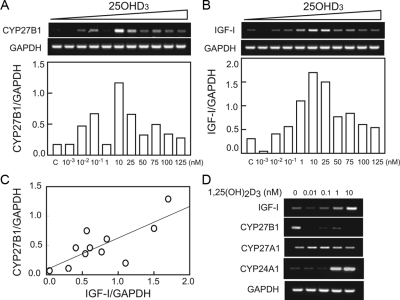
In vitro, dose-dependent effects of 25OHD3 and 1,25(OH)2D3 on CYP27B1/1αOHase and IGF-I gene expression
hMSCs obtained from a vitamin D-deficient subject (72-yr-old female) were cultured to confluence and treated with or without 25OHD3 or 1,25(OH)2D3 for 24 h. The effect of 25OHD3 on CYP27B1 (Fig. 4A) and IGF-I (Fig. 4B) gene expression depended on the concentration of 25OHD3. There was a dose-dependent increase in CYP27B1 and IGF-I gene expression with treatment from 0.001 nm (0.0004 ng/ml) to 10 nm (4 ng/ml) of 25OHD3. With more than 10 nm (4 ng/ml) 25OHD3, there was a decline in magnitude of stimulation of CYP27B1. There was a significant correlation between IGF-I and CYP27B1 gene expression in all of those samples (P = 0.0063; n = 11) (Fig. 4C). Twenty-four hours treatment with 1,25(OH)2D3 stimulated IGF-I and CYP24A1, down-regulated CYP27B1, and had no detectable effect on CYP27A1 gene expression (Fig. 4D). In addition, 25OHD3 (10 nm) up-regulated by 8-fold the expression of CYP24A1 compared with vehicle control.
Figure 4.
In vitro effects of 25OHD3 and 1,25(OH)2D3 on CYP27B1 and IGF-I gene expression in hMSCs obtained from a vitamin D-deficient subject (72-yr-old female). After 24 h treatment with 25OHD3, expression levels of CYP27B1 (A) and IGF-I (B) were modulated in dose-dependent manner. C, There was a significant correlation between IGF-I and CYP27B1 gene expression in those samples (r = 0.76; P = 0.0063; n = 11). D, IGF-I, CYP27B1, and CYP24A1 but not CYP27A1 were modulated by 1,25(OH)2D3 (0.01–10 nm).
In vitro effects of IGF-I on CYP27B1/1αOHase gene expression and activity and osteoblast differentiation in hMSCs
The effects of IGF-I on CYP27B1 gene expression in hMSCs were determined. Treatment with IGF-I for 24 h stimulated CYP27B1 expression in hMSCs in a dose-dependent manner above 5 ng/ml (Fig. 5A). MSCs from another subject were used to measure in vitro biosynthesis of 1,25(OH)sD3. Cells were incubated for 24 h with or without 1 μm 25OHD3 exogenous substrate with or without 100 ng/ml IGF-I in serum-free α-MEM (1% ITS+) with antioxidant [10 μm 1,2-dianilinoethane (N,N′-diphenylethylene-diamine)]. IGF-I significantly increased 1,25(OH)2D3 biosynthesis by 40% (P < 0.05; n = 4, Mann-Whitney U test) in the presence of exogenous substrate 25OHD3 (Fig. 5B). In addition, after 7 d treatment in osteogenic medium, IGF-I (1 ng/ml) stimulated AlkP activity in hMSCs by 73% (P < 0.05; n = 3, Mann-Whitney U test) (Fig. 5C).
Figure 5.
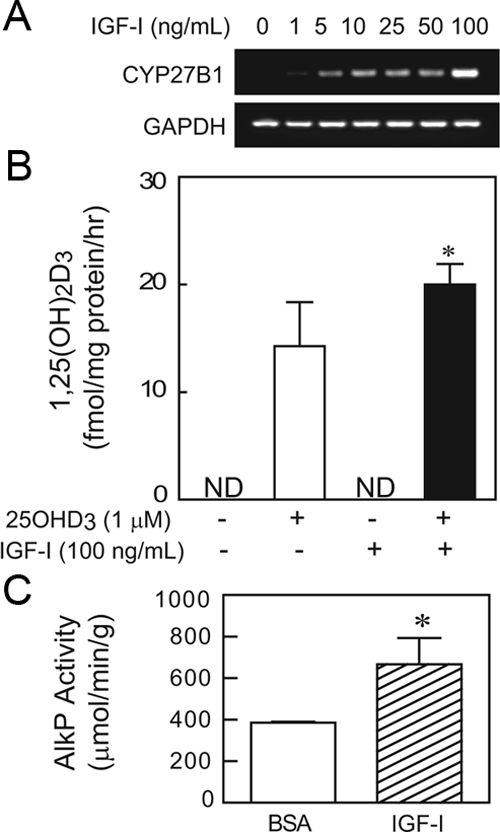
Effects of IGF-I on CYP27B1/1αOHase gene expression and 1α-hydroxylase activity and AlkP activity in hMSCs. A, After 24 h treatment, IGF-I (1–100 ng/ml) dose-dependently stimulated CYP27B1/1αOHase gene expression in hMSCs obtained from a 72-yr-old female subject (72F). B, IGF-I (100 ng/ml) significantly stimulated 1α-hydroxylase activity, shown as 1,25(OH)2D3 biosynthesis in hMSCs obtained from a 60-yr-old male subject (*, P < 0.05; n = 4). C, Osteoblast differentiation of hMSCs was assessed with AlkP activity assays in a 12-well plate after 7 d culture with or without IGF-I (1 ng/ml) in osteogenic medium (*, P < 0.05; n = 3).
Discussion
The classical actions of vitamin D concern mineral and skeletal homeostasis. Prolonged vitamin D deficiency has several skeletal consequences in humans. It can result in decreased bone formation and mineralization, known as rickets in children and as osteomalacia in adults. It can also lead to increased osteoclastic bone resorption that results in osteopenia or osteoporosis. Although these major effects relate to the actions of 1,25(OH)2D3 on intestinal calcium absorption, some information is available about direct effects on osteoblasts (33). Human trabecular bone cells responded in vitro to exogenous 1,25(OH)2D3 by increasing expression of the bone matrix genes osteocalcin and bone sialoprotein-1 (34). Whereas circulating 1,25(OH)2D originates in the kidney, local production has been shown for normal osteoblasts (35,36) and for human osteosarcoma cell lines (37). The differentiation of hMSCs to osteoblasts is enhanced by 1,25(OH)2D3 (25), but there is no information about vitamin D metabolism in hMSCs. Our finding that both 25OHD3 and 1,25(OH)2D3 stimulated osteoblastogenesis, as measured by AlkP, in the majority of preparations of hMSCs and, in some cases, to equal extents raised the possibility of 1α-hydroxylation in human marrow stromal cells.
Vitamin D is activated in the skin or absorbed from the gastrointestinal tract and is metabolized to 25OHD in the liver (providing steady-state levels of this metabolite) and then to 1, 25(OH)2D in the kidney by the CYP27B1 enzyme. The renal 1α-hydroxylase is regulated by the circulating concentrations of calcium, PTH, and phosphorus. Emerging data now suggest that other cell types have the ability to generate and inactivate vitamin D, although there are no data for human marrow stromal cells. To gain new information about vitamin D metabolism in human marrow stromal cells, we used in vivo and in vitro approaches to test whether hMSCs participate in vitamin D metabolism and whether expression of vitamin D hydroxylases in hMSCs in vitro are influenced by the vitamin D status of the individual from whom the hMSCs were obtained and to evaluate potential mechanisms of regulation of vitamin D metabolic enzymes in hMSCs.
To test whether expression of vitamin D hydroxylases in hMSCs in vitro are influenced by the vitamin D status of the individual from whom the hMSCs were obtained, we analyzed the clinical data of 27 enrolled subjects with vitamin D-related gene expression in their hMSCs. As reported in the literature (38,39,40), our clinical data also demonstrated inverse correlations between serum 25OHD and percent body fat, BMI, and serum PTH. Although it had previously been assumed that osteoarthritis may be associated with high bone density, and in fact, many of the subjects in this study did have high T-scores and high BMIs, low T-scores and osteoporosis are now recognized to be common in patients with osteoarthritis, especially in those with low BMI or advanced age (41,42,43,44).
The expression of 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) was lower in hMSCs from subjects with elevated serum 1,25(OH)2D; this may signify feedback repression. The positive correlation between serum 25OHD (7–20 ng/ml) and CYP27B1 gene expression may signify feed-forward induction. Samples from subjects with the highest levels of serum 25OHD tended to have the lowest expression of CYP27A1; this suggests feedback repression.
Human primary osteoblasts and human osteoblastic cell lines possess the molecular machinery to both respond to and metabolize 25OHD3 (37). This study shows that osteoblast differentiation in hMSCs was stimulated by both 1,25(OH)2D3 and 25OHD3 in the majority of samples; this observation suggested that 25OHD directly acts on these osteoblast precursor cells or can be activated to 1,25(OH)2D3 in vitro. It will be valuable to determine whether clinical properties of individual subjects influence the effects of 25OHD3 on in vitro osteoblastogenesis. Among the CYP isoforms that have been shown to hydroxylate vitamin D, CYP27B1/25OHD-1α-hydroxylase hydroxylates the principal circulating vitamin metabolite, 25OHD3, into the active hormonal form, 1,25(OH)2D3 (45,46). To test whether hMSCs metabolize 25OHD3 into 1,25(OH)2D3, we analyzed gene expression and activity of 25OHD 1α-hydroxylase (CYP27B1) in hMSCs. The in vitro data show that CYP27B1 was expressed in hMSCs and was up-regulated by exogenous 25OHD3. Moreover, the 1,25(OH)2D assay showed that hMSCs have the capacity to convert 25OHD into 1,25(OH)2D. Ketoconazole, a recognized CYP inhibitor (47), inhibited both gene expression of CYP27B1 and biosynthesis of 1,25(OH)2D in hMSCs. The up-regulation of CYP27B1 by 25OHD3 and down-regulation by 1,25(OH)2D3 show a feed-forward amplification mechanism and feedback repression, respectively, at the level of gene expression. Increased substrate, 25OHD, and increased 1α-hydroxylase, CYP27B1, would result in increased synthesis of 1,25(OH)2D, which in turn, would down-regulate the enzyme, as suggested by the lower levels of CYP27B1 with the highest levels of added 25OHD3. It is notable that the range of 25OHD correlated with up-regulation of CYP27B1 in vitro is similar to the in vivo range for correlation between serum 25OHD and constitutive expression of CYP27B1 in hMSCs. Further evidence of regulation of vitamin D metabolism in marrow stroma is that the higher levels of added 25OHD3 or 1,25(OH)2D3 up-regulated 24-hydroxylase (CYP24A1), which initiates inactivation of the metabolites and prevents risk of hypercalcemia. Regulation of synthesis and inactivation of 1,25(OH)2D3 in hMSCs is similar to that described in skin and bone cells (17,47).
Skeletal IGF-I may play multiple roles in skeletal homeostasis. First, both 25OHD3 and 1,25(OH)2D3 stimulated IGF-I gene expression in hMSCs. In a number of other experimental systems, 1,25(OH)2D3 stimulates IGF-I in osteoblasts and preosteoblasts, and thus IGF-I may mediate osteoblastogenic actions of 1,25(OH)2D3 (48,49,50). Much of the information about vitamin D’s effects on bone concern in vitro actions of 1,25(OH)2D3 to decrease proliferation and increase expression of vitamin D response genes such as osteocalcin and bone sialoprotein (36).
Second, our data show that exogenous IGF-I regulated synthesis of 1,25(OH)2D3 by hMSCs. IGF-I stimulated CPY27B1/1αOHase gene expression and synthesis of 1,25(OH)2D3, evidence that IGF-I may be involved in vitamin D metabolism in hMSCs. Other in vivo and in vitro studies have suggested that IGF-I regulates the renal production of 1,25(OH)2D3 (10,11,12,13,14). Finally, IGF-I stimulated osteoblast differentiation of hMSCs in osteogenic medium. Although it has been suggested that 1,25(OH)2D effects on osteoblast (49) and chondrocyte (51) differentiation may be mediated through the IGF-I, studies have shown confounding effects of duration of treatment (52), influence of dexamethasone (53), and stage of development (50). Clearly, other components of the skeletal IGF system would also be involved (50). For example, in a pilot study of marrow samples, we reported that IGF-I, its binding proteins, and IGF-binding protein-3 protease were secreted by human marrow stromal cells and that there was an age-related increase in constitutive secretion of IGF-binding protein-3 with a notable exception for marrow from a woman receiving estrogen replacement therapy at the time of surgery (54). More detailed analysis of the relationship between clinical parameters and marrow regulation of the IGF system would advance understanding of the physiological roles of factors in the bone microenvironment. We propose that circulating 25OHD, by virtue of its local conversion to 1,25(OH)2D catalyzed by CYP27B1 in hMSCs, amplifies vitamin D signaling through IGF-I up-regulation, which in turn induces CYP27B1 in a feed-forward mechanism to potentiate osteoblast differentiation initiated by IGF-I.
In summary, to our knowledge, this is the first evidence of enzymes involved in vitamin D metabolism being present in and regulated in hMSCs, cells shown to differentiate to osteoblasts in response to 1,25(OH)2D (25). In the studies using marrow obtained from subjects for whom we obtained clinical data, there were correlations between circulating serum 25OHD, 1,25(OH)2D, and PTH levels with the expression of vitamin D metabolic enzymes in their hMSCs. The in vitro data show regulation of the hydroxylases in marrow stromal cells by classic substrate induction and feedback suppression at the level of gene expression. Moreover, IGF-I appears to play an important amplification role in regulation of vitamin D metabolic enzymes and osteoblast differentiation in hMSCs. There is great interest in the significance of extrarenal hydroxylases and synthesis of 1,25(OH)2D3 (55). This study provides new information about local production of 1,25(OH)2D3 and expression of VDR and vitamin D hydroxylases in human bone marrow and suggests how paracrine/autocrine networks in the bone microenvironment may be regulated systemically and locally.
Acknowledgments
We greatly appreciate help from S. Anderson, K. Johnson, N. Glass, M. Tuteja, C. Yu, J. Hahne, L. Shen, and S.-W. Kim for aspects of these experiments. We thank Dr. J. Greenberger (University of Pittsburgh, Pittsburgh, PA) and Dr. M. Holick (Boston University Medical Center, Boston, MA) for helpful discussions about this project.
Footnotes
This work was supported by National Institutes of Health Grants R01 AG 025015 and R01 AG 028114. The discarded marrow was obtained and studied with approval and annual review from the Partners Human Research Committee and the research laboratory tests were supported by the General Clinical Research Center, National Institutes of Health Grant RR-02635.
This study was presented in part at the 30th American Society for Bone and Mineral Research Annual Meeting, 2008, in Montréal, Québec, Canada.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2009
Abbreviations: AlkP, Alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; CYP, cytochrome P450; FBS-HI, fetal bovine serum-heat inactivated; hMSC, human bone marrow stromal cell; NTX, cross-linked N-telopeptides of type I collagen; 25OHD, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; VDR, vitamin D receptor.
References
- Holick MF 2006 Resurrection of vitamin D deficiency and rickets. J Clin Invest 116:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Gallagher JC, Heaney RP, Johnston CC, Neer R, Whedon GD 1982 Vitamin D and bone health in the elderly. Am J Clin Nutr 36:1014–1031 [DOI] [PubMed] [Google Scholar]
- Henschkowski J, Dawson-Hughes B, Staehelin HB, Stuck AE, Orav JE, Egli A, Bischoff-Ferrari HA 2008 Anti-fall efficacy of oral supplemental vitamin D and active vitamin D. J Bone Miner Res 23:S350–S350 [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B 2005 Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264 [DOI] [PubMed] [Google Scholar]
- LeBoff MS, Hawkes WG, Glowacki J, Yu-Yahiro J, Hurwitz S, Magaziner J 2008 Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int 19:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J 1999 Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 281:1505–1511 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, DeLuca HF 2006 CYP27B1 null mice with LacZ reporter gene display no 25-hydroxyvitamin D3-1α-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA 103:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Armbrecht HJ, Christakos S 2009 Calcitonin, a regulator of the 25-hydroxyvitamin D3 1α-hydroxylase gene. J Biol Chem 284:11059–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T, Drezner MK 1993 Insulin-like growth factor-I regulation of renal 25-hydroxyvitamin D-1α-hydroxylase activity. Endocrinology 132:133–138 [DOI] [PubMed] [Google Scholar]
- Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabédian M 1995 Insulin-like growth factor I, a unique calcium-dependent stimulator of 1,25-dihydroxyvitamin D3 production: studies in cultured mouse kidney cells. J Biol Chem 270:25461–25467 [DOI] [PubMed] [Google Scholar]
- Wong MS, Sriussadaporn S, Tembe VA, Favus MJ 1997 Insulin-like growth factor I increases renal 1,25(OH)2D3 biosynthesis during low-P diet in adult rats. Am J Physiol 272:F698–F703 [DOI] [PubMed] [Google Scholar]
- Wong MS, Tembe VA, Favus MJ 2000 Insulin-like growth factor-I stimulates renal 1,25-dihydroxycholecalciferol synthesis in old rats fed a low calcium diet. J Nutr 130:1147–1152 [DOI] [PubMed] [Google Scholar]
- Gómez JM 2006 The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol 7:125–132 [DOI] [PubMed] [Google Scholar]
- Howard GA, Turner RT, Sherrard DJ, Baylink DJ 1981 Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem 256:7738–7740 [PubMed] [Google Scholar]
- Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P, Holick MF 1985 Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab 60:960–966 [DOI] [PubMed] [Google Scholar]
- Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I 2001 Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D3: CYP27 in epidermis completes the set of essential vitamin D3-hydroxylases. Steroids 66:399–408 [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M 2002 Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13:621–629 [DOI] [PubMed] [Google Scholar]
- Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M 2005 Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res 11:3579–3586 [DOI] [PubMed] [Google Scholar]
- Prockop DJ 1997 Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG 1999 Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med 10:165–181 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR 1999 Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- Mueller SM, Glowacki J 2001 Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 82:583–590 [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J 2008 Age-related intrinsic changes in human marrow stromal cells and their differentiation to osteoblasts. Aging Cell 7:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Oyajobi BO, Russell RG, Scutt A 1999 Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-β and 1,25(OH)2 vitamin D3in vitro. Calcif Tissue Int 65:173–180 [DOI] [PubMed] [Google Scholar]
- Glowacki J, LeBoff MS, Kolatkar NS, Thornhill TS, Harris MB 2008 Importance of vitamin D in hospital-based fracture care pathways. J Nutr Health Aging 12:291–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne TA, Morrissey TB, Gatzen C, Benfell K, Nattakom TV, Scheltinga MR, LeBoff MS, Ziegler TR, Wilmore DW 1993 Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg 218:400–416; discussion 416–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger JS, Glowacki J 2005 Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. J Biol Chem 280:22688–22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, Akerström G, Westin G 2002 25-Hydroxyvitamin D3-1α-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 87:2967–2972 [DOI] [PubMed] [Google Scholar]
- Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J 2004 Differential biological effects of 1,25-dihydroxyvitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol 89–90:375–379 [DOI] [PubMed] [Google Scholar]
- Mocharla H, Butch AW, Pappas AA, Flick JT, Weinstein RS, De Togni P, Jilka RL, Roberson PK, Parfitt AM, Manolagas SC 1997 Quantification of vitamin D receptor mRNA by competitive polymerase chain reaction in PBMC: lack of correspondence with common allelic variants. J Bone Miner Res 12:726–733 [DOI] [PubMed] [Google Scholar]
- Gordeladze JO, Drevon CA, Syversen U, Reseland JE 2002 Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85:825–836 [DOI] [PubMed] [Google Scholar]
- St-Arnaud R 2008 The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 473:225–230 [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC 2003 RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 18:1088–1098 [DOI] [PubMed] [Google Scholar]
- Turner RT, Howard GA, Puzas JE, Baylink DJ, Knapp DR 1983 Calvarial cells synthesize 1 α,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Biochemistry 22:1073–1076 [DOI] [PubMed] [Google Scholar]
- Anderson PH, Atkins GJ 2008 The skeleton as an intracrine organ for vitamin D metabolism. Mol Aspects Med 29:397–406 [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O'Loughlin PD, Morris HA 2007 Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 40:1517–1528 [DOI] [PubMed] [Google Scholar]
- Looker AC 2007 Do body fat and exercise modulate vitamin D status? Nutr Rev 65:S124–126 [DOI] [PubMed] [Google Scholar]
- Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA 2004 The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 89:1196–1199 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO 2005 Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 90:5576–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki J, Hurwitz S, Thornhill TS, Kelly M, LeBoff ML 2003 Osteoporosis and vitamin D deficiency among postmenopausal osteoarthritic women undergoing total hip arthroplasty. J Bone Joint Surg 85A:2371–2377 [DOI] [PubMed] [Google Scholar]
- Sah AP, Thornhill TS, Leboff MS, Glowacki J 2007 Correlation of plain radiographic indices of the hip with quantitative bone mineral density in osteoarthritic women. Osteoporos Int 18:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki J, Tuteja M, Hurwitz S, Thornhill TS, LeBoff MS, Discordance in femoral neck bone density in subjects with unilateral hip osteoarthritis. J Clin Densitom 10.1016/j.jocd.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Mäkinen TJ, Alm JJ, Laine H, Svedström E, Aro HT 2007 The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone 40:1041–1047 [DOI] [PubMed] [Google Scholar]
- Prosser DE, Jones G 2004 Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29:664–673 [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K 2005 Metabolism of vitamin D3 by cytochromes P450. Front Biosci 10:119–134 [DOI] [PubMed] [Google Scholar]
- van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP 2006 Evidence for auto/paracrine actions of vitamin D in bone: 1α-hydroxylase expression and activity in human bone cells. FASEB J 20:2417–2419 [DOI] [PubMed] [Google Scholar]
- Linkhart TA, Keffer MJ 1991 Differential regulation of insulin-like growth factor-I (IGF-I) and IGF-II release from cultured neonatal mouse calvaria by parathyroid hormone, transforming growth factor-β, and 1,25-dihydroxyvitamin D3. Endocrinology 128:1511–1518 [DOI] [PubMed] [Google Scholar]
- Chenu C, Valentin-Opran A, Chavassieux P, Saez S, Meunier PJ, Delmas PD 1990 Insulin like growth factor I hormonal regulation by growth hormone and by 1,25(OH)2D3 and activity on human osteoblast-like cells in short-term cultures. Bone 11:81–86 [DOI] [PubMed] [Google Scholar]
- Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M 2001 1,25-Dihydroxyvitamin D3 stimulates the production of insulin-like growth factor-binding proteins-2, -3 and -4 in human bone marrow stromal cells. Eur J Endocrinol 144:549–557 [DOI] [PubMed] [Google Scholar]
- Fernández-Cancio M, Audi L, Carrascosa A, Toran N, Andaluz P, Esteban C, Granada ML 2009 Vitamin D and growth hormone regulate growth hormone/insulin-like growth factor (GH-IGF) axis gene expression in human fetal epiphyseal chondrocytes. Growth Horm IGF Res 19:232–237 [DOI] [PubMed] [Google Scholar]
- Scharla SH, Strong DD, Mohan S, Baylink DJ, Linkhart TA 1991 1,25-Dihydroxyvitamin D3 differentially regulates the production of insulin-like growth factor I (IGF-I) and IGF-binding protein-4 in mouse osteoblasts. Endocrinology 129:3139–3146 [DOI] [PubMed] [Google Scholar]
- Chen TL, Mallory JB, Hintz RL 1991 Dexamethasone and 1,25(OH)2 vitamin D3 modulate the synthesis of insulin-like growth factor-I in osteoblast-like cells. Calcif Tissue Int 48:278–282 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Verault D, Steffens C, Cheleuitte D, Glowacki J 1997 Effects of age and estrogen status on the skeletal IGF regulatory system: studies with human marrow. Endocrine 7:77–80 [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS 2007 Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103:316–321 [DOI] [PubMed] [Google Scholar]