Abstract
Ankyrin repeat and suppressor of cytokine signaling box-containing protein 4 (Asb-4) is specifically expressed in the energy homeostasis-related brain areas and colocalizes with proopiomelanocortin (POMC) neurons of the arcuate nucleus (ARC). Injection of insulin into the third ventricle of the rat brain increased Asb-4 mRNA expression in the paraventricular nucleus but not in the ARC of the hypothalamus, whereas injection of leptin (ip) increased Asb-4 expression in both mouse paraventricular nucleus and ARC. A transgenic mouse in which Myc-tagged Asb-4 is specifically expressed in POMC neurons of the ARC was made and used to study the effects of Asb-4 on ingestive behavior and metabolic rate. Animals with overexpression of Asb-4 in POMC neurons demonstrated an increase in food intake. However, POMC-Asb-4 transgenic animals gained significantly less weight from 6–30 wk of age. The POMC-Asb-4 mice had reduced fat mass and increased lean mass and lower levels of blood leptin. The transgenic animals were resistant to high-fat diet-induced obesity. Transgenic mice had significantly higher rates of oxygen consumption and carbon dioxide production than wild-type mice during both light and dark periods. The locomotive activity of transgenic mice was increased. The overexpression of Asb-4 in POMC neurons increased POMC mRNA expression in the ARC. The transgenic animals had no observed effect on peripheral glucose metabolism and the activity of the autonomic nervous system. These results indicate that Asb-4 is a key regulatory protein in the central nervous system, involved in the control of feeding behavior and metabolic rate.
Transgenic over-expression of Asb-4 in POMC neurons of mouse arcuate nucleus produces a hyperphagic, lean phenotype via increasing motor activity and energy expenditure.
Asb-4 is a member of the ankyrin repeat and suppressor of cytokine signaling (SOCS) box-containing protein family (Asb-1 to Asb-18). Representatives of the Asb family have two functional domains: an ankyrin repeat region and a SOCS box region (1). The ankyrin repeat domain directs specific protein-protein interactions, and the SOCS box region serves as an adapter directing the degradation of proteins targeted by the ankyrin repeat region (2,3,4). The 18 Asb proteins vary in the form and numbers of ankyrin repeats and contain other novel regions, suggesting they bind differing target proteins. Asb-4 was identified by database searches using a SOCS box consensus sequence and was originally demonstrated to be expressed in the testis. Asb-4 has nine ankyrin repeats N-terminal to its SOCS box (5). In a previous study using microarray analysis of arcuate nucleus (ARC) RNAs from fed and fasted rats, we identified 321 genes for which mRNA expression was ether up- or down-regulated by fasting. One of the down-regulated genes was Asb-4 (6). Based on its molecular characteristics, we chose Asb-4 for further study. Real-time quantitative PCR (RT-QPCR) showed that Asb-4 mRNA was also down-regulated in the ARC of the genetically obese Zucker rat, a model of long-term energy imbalance. In situ hybridization revealed that expression of Asb-4 mRNA was restricted to neuroanatomical areas in the hypothalamus and amygdala associated with energy homeostasis. Furthermore, Asb-4 mRNA was differentially expressed in two types of ARC neurons critical to feeding behavior, proopiomelanocortin (POMC) and neuropeptide Y neurons. In previous work, we also used yeast two hybridization to search for proteins that interact with Asb-4 and identified G protein pathway suppressor 1 as an Asb-4 interacting protein (7). Although these findings imply that Asb-4 might have a role in the regulation of energy homeostasis and be involved in signal transduction, the function of Asb-4 within the hypothalamus remains to be elucidated.
The ARC of the hypothalamus is a key integrative center for peripheral and central signals controlling appetite and metabolism, and ARC neurons express receptors for circulating factors including insulin and leptin. The best described anabolic neurons within the ARC use neuropeptide Y and agouti-related peptide (AgRP) as neurotransmitters. A parallel and opposing pathway is comprised of neurons expressing POMC and cocaine- and amphetamine-regulated transcript (CART) (8). Melanocortinergic neurons in the hypothalamus are regulated by circulating leptin. For example, POMC neurons in the ARC express leptin receptors through which leptin regulates POMC expression (9,10). Within the hypothalamus, the paraventricular nucleus (PVN) is also integral to melanocortinergic signaling. The PVN receives projections from several areas of the brain and acts as an integrator of signals regulating food intake and energy expenditure. The PVN receives input from POMC neurons in the ARC (11).
In the present work, we studied the regulation of Asb-4 mRNA in the hypothalamic ARC and PVN by insulin and leptin. Because Asb-4 was found to colocalize with POMC neurons of the ARC, we hypothesized that it may play a role in the regulation of POMC neuronal function. The in vivo effects of hypothalamic Asb-4 were further examined using transgenic mice in which Asb-4 was specifically overexpressed in ARC POMC neurons. The POMC-Asb-4 mice exhibited significant alterations in food intake, somatic growth, and metabolic rate.
Materials and Methods
All studies were approved by the University of Michigan Committee on Use and Care of Animals.
Production of transgenic mice
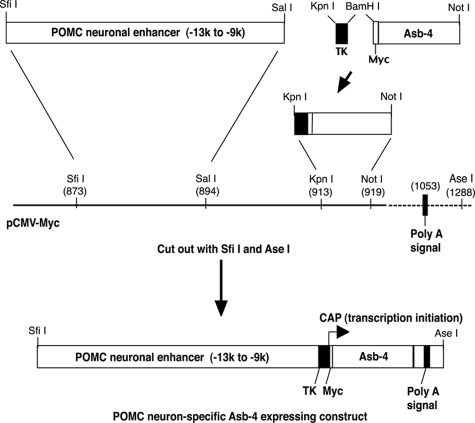
The POMC neuronal enhancer is located approximately 10–12 kb upstream of the mammalian POMC transcriptional unit and has been characterized (12). The mouse POMC neuronal enhancer (8.6–13 kb upstream of mouse POMC transcriptional unit according to the mouse genomic sequence (GenBank no. NT_039548.6)) including nPE1 and nEP2 was cloned by PCR from 129 svj mouse genomic DNA with the SfiI and SalI sites incorporated into the 5′ and 3′ ends, respectively. The PCR primers are forward (−13097 to −13066) 5′-ATGGCCATGGAGGCCTTTGGAGTCAACCGGCCTTTCCAAGTAAAAG-3′ (the underlined sequence is the SfiI site) and reverse (−8605 to −8636) 5′-ACGCGTCGACGTCACAAGGGAAGACTGCACATGGTGGGCACG −3′ (the underlined sequence is the SalI site). The sequence of the PCR product was confirmed by DNA sequencing. The POMC neuronal enhancer was subcloned into SfiI and SalI sites of a pCMV-Myc vector (Fig. 1). A minimal herpes simplex virus thymidine kinase (TK) promoter (157 bp) was connected to a Myc epitope-tagged (13 amino acids) Asb-4 cDNA with a BamHI site. The TK promoter with the Myc-Asb-4 was then subcloned into KpnI and NotI sites of the pCMV-Myc vector. The correct conjunctions and reading frame were confirmed by DNA sequencing using TK forward and reverse primers: forward, 5′-GCCGCCCCGACTGCATCTGCGTGTTC-3′; reverse, 5′-CTGCTTCATCCC-CGTGGCCCGTTGC-3′. The POMC neuron-specific Asb-4 expressing cosset with an SV40 polyadenylation signal was cut out from the vector using SfiI and AseI and then purified from gel. Transgenic mice were made by injecting the construct DNA into the pronucleus of the fertilized eggs of C57BL mice at the University of Michigan Transgenic Core.
Figure 1.
Making of POMC neuron-specific Asb-4-expressing transgenic construct. The POMC neuronal enhancer (9–13 kb upstream of mouse POMC transcriptional unit), minimal herpes simplex virus TK promoter, and Myc epitope-tagged (13 amino acids) Asb-4 cDNA were subcloned into a pCMV-Myc vector. The POMC neuron-specific Asb-4-expressing cosset with a SV40 polyadenylation signal was cut out from the vector. Transgenic mice were made by injecting the construct DNA into the pronucleus of the fertilized eggs of C57BL mice.
Transgenic pups were identified by tail genomic DNA PCR. The forward primer was in the POMC enhancer region (5′-CCCCCAAGAGAACAAGATAAGATT-3′), and the reverse primer was in the Asb-4 region (5′-TCAGTTTTCCGAAGTCATTTGTTT-3′). The primers amplified a 562-bp product. Five transgenic positive mice were identified (two males and three females). The transgenic mice were used as founders to mate with wild-type C57BL mice. The transgenic positive pups and their wild-type littermates were used to characterize Myc-Asb-4 expression and resulting phenotypes.
Immunohistochemistry
Dual immunohistochemistry was used to determine the expression of Myc-Asb-4 in POMC neurons. Anti-Myc monoclonal antibody (BD Biosciences, Palo Alto CA) and anti-POMC polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used.
Diets and measurement of food consumption
After weaning at 3 wk of age, the mice were fed either standard laboratory chow or high-fat-content chow (45% fat D12451; Research Diet, New Brunswick, NJ). For the measurement of food consumption, mice were housed individually for at least 1 wk before experiments (13). Over a 2-wk period, mouse chow was weighed and provided ad libitum. Each day, the remaining food was measured.
Measurement of oxygen consumption (VO2), carbon dioxide production (VCO2), and spontaneous motor activity
VO2 and VCO2, spontaneous motor activity was measured using the Comprehensive Laboratory Monitoring System (Columbus Instruments, Columbus, OH), an open-circuit calorimeter equipped with an optical beam activity monitoring device. Animals were fed standard laboratory chow and were maintained on 12-h light, 12-h dark cycles beginning at 0600 and 1800 h. Animals were acclimated in measuring chambers for 1 d before recording. Measurements of VO2 and VCO2 were made every 10 min for each animal, and the motor activity was recorded every second over a period of 3 d.
Measurement of body composition and serum leptin
Body composition was analyzed in the conscious state with Minispec LF90 II (Bruker Optics, Billerica, MA), a nuclear magnetic resonance-based whole-body composition analyzer. Serum leptin levels were measured with RayBio mouse leptin ELISA kit (RayBiotech, Inc., Norcross, GA).
Measurement of heart rate and heart rate variability
Heart rate and heart rate variability were measured using the Anonymouse instrument (Mouse Specifics, Inc., Quincy, MA).
Glucose tolerance and insulin sensitivity tests
For glucose tolerance testing, mice were injected ip with 1.5 mg glucose/g body weight at 1000 h after a 16-h fast (14). Blood glucose was determined at the indicated times with samples of tail blood obtained using the OneTouch Ultra Glucometer (LifeScan Canada Ltd., Burnaby, British Columbia, Canada). For insulin sensitivity, mice were fasted for 4 h, and then insulin (0.5 U/kg body weight) was administered ip and blood samples were collected at the indicated times.
Intracerebroventricular (icv) cannulation
Sprague Dawley rats with a body weight of 280–300 g were used. Rats were anesthetized with ketamine/xylazine and placed on a stereotaxic device with the incisor bar 3.3 mm below the interaural line according to Paxinos and Watson (15). A stainless steel 26-gauge guide cannula was implanted into the third ventricle using the following stereotaxic coordinates: 2.2 mm posterior to the bregma, 8.2 mm ventral to the surface of the skull, and directly along the midline. The cannula was anchored to the skull with screws and dental cement. An internal cannula was placed into the guide cannula to maintain patency. Rats were allowed to recover for 1 wk. Guide cannula patency was assessed by injection of 10 ng angiotensin II in 5 μl saline. Cannulas were considered patent if rats consumed at least 5 ml water within 1 h of injection. Rats with correct third ventricle cannulation were used 5 d later.
Insulin injection
After a 24-h fast, the rats were injected with either insulin (8 mU in 5 μl saline) or saline twice (1100 and 1300 h) into the third ventricle through the guide cannula (16). The rats were killed at 1500 h. Brains were embedded in powderized dry ice and then sectioned using a coronal brain matrix. Brain sections were placed on dry ice, and under ×4 magnification, the PVN and ARC were obtained using a Stoelting brain punch set.
Leptin injection
Eight-week-old male mice were fasted for 24 h with water ad libitum. Leptin (3 μg/g body weight) or vehicle was injected twice ip (at 1100 and 1500 h) (17). The mice were killed 2 h after the second injection and the PVN and ARC were obtained.
Isolation of total RNA and RT-QPCR
To measure POMC and Asb-4 mRNA levels in wild-type and Asb-4 transgenic mice, ARC were obtained using a Stoelting brain punch set.
Total RNA was extracted from brain using TRIzol reagent (Invitrogen, Carlsbad, CA). An additional cleanup step was then performed using the QIAGEN RNeasy Mini kit (QIAGEN, Valencia, CA) per manufacturer’s instructions, and 100–200 ng RNA was converted into cDNA using Superscript II reverse transcriptase (Invitrogen) with an oligo-dT primer. RT-QPCR was performed using a Bio-Rad iCycler iQ real-time PCR detection system (Bio-Rad Laboratory, Carlsbad, CA) and FAM-labeled fluorogenic probes for rat or mouse Asb-4 and mouse POMC with Taq DNA polymerase. The RT-QPCR primers for rat Asb-4 were forward 5′-CTACGACAAACTC-CCCTAATT-3′, probe 5′-/56-FAM/ATGAGGAACACCTTGTCCGCTTGT/36-TAMTph/-3′, and reverse 5′-GTGCTAGACTGATTCATCACAG-3′. The RT-QPCR primers for mouse Asb-4 were forward 5′-CTGAGATCTGCTACCAGCT-3′, probe 5′-/56-FAM/AGTTCCACAAGGTGAT-CCAGGCT/36-TAMSp/-3′, and reverse 5′-CCATCGAATGTGTTCATAGGC-3′. The RT-QPCR primers for mouse POMC were forward 5′-TGAACAGCCCCTGACTGAAAAC-3′, probe 5′/56-FAM/CACCGCCTCTTCCTCCGCACGC/36 TAMSp/-3′, and reverse 5′-CTCTGGACTGCCATCTCCC-3′. GAPDH was used as an internal control. RT-QPCR conditions were one cycle at 95 C for 5 min followed by 40 cycles of 95 C for 30 sec, 60 C for 30 sec, and 72 C for 30 sec. Relative mRNA expression was normalized to GAPDH using the 2−ΔΔCT methodology (18).
Statistical analysis
Data are expressed as mean ± sem. Two-way ANOVA with Bonferroni post hoc test was used for statistical analyses. Significance was accepted as P < 0.05.
Results
Effects of insulin and leptin on hypothalamic Asb-4 expression
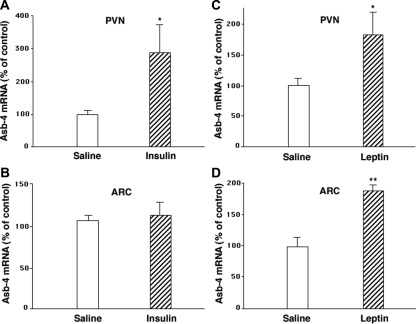
Insulin was administered icv to rats, and in a second series of experiments, leptin was administered to mice ip. Injection of insulin into the third ventricle of the rat brain increased Asb-4 mRNA expression in the PVN but not in the ARC (Fig. 2, A and B), whereas injection of leptin ip in mice increased Asb-4 expression in both the PVN and ARC of the mouse hypothalamus (Fig. 2, C and D).
Figure 2.
Effect of insulin and leptin on hypothalamic Asb-4 mRNA expression. A and B, Insulin was administered icv to rats (8 mU in 5 μl saline or 5 μl vehicle). In a second series of experiments, 8-wk-old male mice were fasted for 24 h with water ad libitum. C and D, Leptin (3 μg/g body weight) or vehicle was injected twice ip (1100 and 1500 h). Doses of insulin or leptin were used that have been demonstrated to inhibit food intake in rats or mice, respectively. Animals were killed 2 h after the second injection, and under magnification, the PVN and ARC were obtained using a brain punch. Data are expressed as mean ± sem for groups of six animals each. *, P < 0.05; **, P < 0.01 vs. control injection.
Transgenic expression of Asb-4 in the ARC
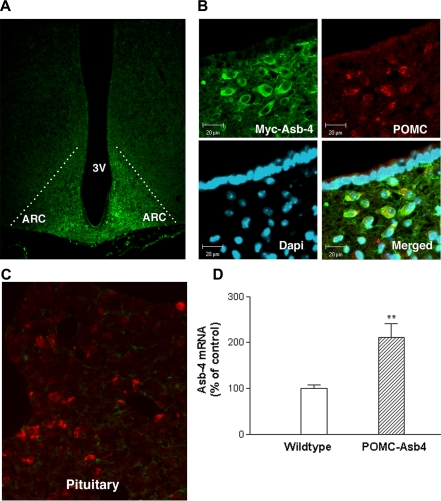
To elucidate the function of Asb-4 in POMC neurons, we made POMC neuron-specific transgenic mice. A Myc tag of 13 amino acids was incorporated into the N terminus of Asb-4 for the assessment of transgenically overexpressed Myc-Asb-4 in POMC neurons. Five lines of transgenic positive mice were identified using PCR genotyping methodology. Immunohistochemistry using anti-Myc antibody revealed that Myc-Asb-4 was specifically expressed in the ARC of all five lines, with line 493 and line 504 having stronger signals than the other lines. No Myc signal was found in other central nervous system sites or in any peripheral tissues. We then focused on these two lines and further performed dual immunohistochemistry using both anti-Myc and anti-POMC antibodies and found colocalization of Myc-Asb-4 with POMC in both lines. Figure 3 shows representative slides of double staining from line 493. Anti-Myc positivity was demonstrated in the ARC of the hypothalamus (Fig. 3A). Confocal microscopy showed that, within the ARC, virtually 100% of Myc-Asb-4-positve cells also expressed POMC. Conversely, all POMC-positive cells were also positive for Myc-Asb-4 (Fig. 3B). Myc-Asb-4 was not demonstrated in POMC-positive cells of the pituitary (Fig. 3C). RT-QPCR showed that Asb-4 mRNA more than doubled in the ARC of the transgenic animals (Fig. 3D). We focused on line 493 for further analysis.
Figure 3.
Expression of Myc-Asb-4 in the ARC. A, Myc-Asb-4 positivity within the ARC of transgenic animals. B, Colocalization of Myc-Asb-4 and POMC in mouse ARC cells; upper left, high-resolution image depicting Myc-Asb-4 cells (green); upper right, immunohistochemical staining for POMC (red); lower left, nuclear 4′,6-diamidino-2-phenylindole staining; lower right, merged images, illustrating colocalization (orange) of Myc-Asb-4 and POMC. Scale bar, 20 μm. C, Absence of Myc-Asb-4 in POMC-expressing cells (red) of the pituitary. D, Asb-4 mRNA levels in the ARC of wild-type and transgenic animals. **, P < 0.01.
Effects of POMC-Asb-4 on food intake, growth, body composition, and blood leptin
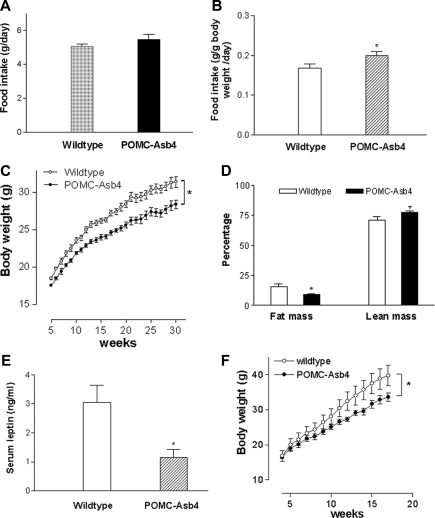
Overexpression of Asb-4 in POMC neurons of the ARC had significant effects on feeding, growth, and body composition. Although the absolute daily food intake showed no significant change (wild-type 5.07 ± 0.13 g/d, transgenic 5.48 ± 0.28 g/d; n = 8 per group; P = 0.217), when normalized for body weight, transgenic animals demonstrated an 18% increase in food intake (wild-type 0.168 ± 0.01 g/g body weight · d, transgenic 0.199 ± 0.01 g/g body weight · d; P < 0.05) (Fig. 4, A and B). Despite increased oral intake, POMC-Asb-4 transgenic animals gained significantly less weight from 6–30 wk of age (10% less at 30 wk; wild-type 31.4 ± 0.8 g, transgenic 28.4 ± 0.6 g; n = 8 per group; P < 0.01) (Fig. 4C). The body composition of POMC-Asb-4 animals was significantly different from wild-type littermates, with reduced fat mass and increased lean mass (Fig. 4D). Consistent with reduced fat mass, transgenic animals had lower levels of blood leptin (Fig. 4E). When challenged with a high-fat diet, POMC-Asb-4 animals were resistant to high-fat-induced obesity (Fig. 4F).
Figure 4.
Food intake, growth, body composition, and leptin levels of POMC-Asb-4 transgenic mice. A and B, Daily food intake for wild-type and POMC-Asb-4 mice per mouse per day (A) or per gram body weight per day (B). Error bars indicate sem. *, P < 0.05 vs. wild-type mice. Eight animals were examined for each group. C, Growth curves for POMC neuronal specific Asb-4 transgenic and wild-type mice. Weights were measured at indicated intervals. Each time point is expressed as mean ± sem for groups of eight mice. *, P < 0.01 between the two groups as determined by two-way ANOVA. D, Body composition of transgenic and wild-type mice. Error bars indicate sem. *, P < 0.05 vs. wild-type mice. E, Serum leptin levels of transgenic and wild-type mice. *, P < 0.05 vs. wild-type mice. F, High-fat-induced obesity in transgenic and wild-type mice. *, P < 0.01 between the two groups as determined by two-way ANOVA.
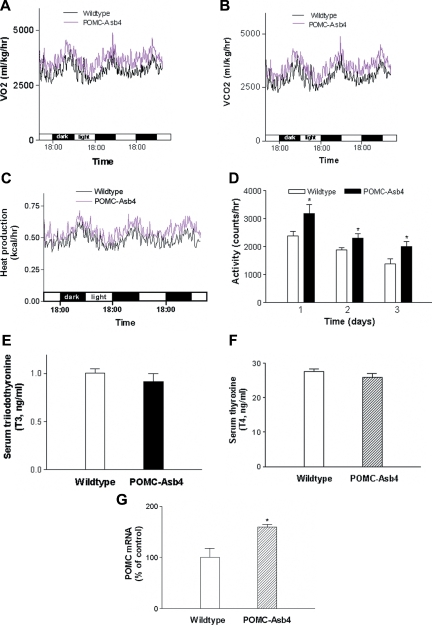
Effects of POMC-Asb-4 on energy expenditure and spontaneous motor activity
The observation that POMC-Asb-4 transgenic animals had lower weight gain and leaner body composition despite significantly increased food intake suggests that Asb-4 overexpression in POMC neurons in the hypothalamus affects rates of energy expenditure and motor activity. To examine this possibility, we next measured VO2 and VCO2 and the spontaneous motor activity in transgenic mice and wild-type littermates. Transgenic mice had significantly higher VO2 than wild-type mice during both light and dark periods (wild-type 3103 ± 24 ml/kg · h, transgenic 3527 ± 24 ml/kg · h; n = 7 per group; P < 0.01) (Fig. 5A). VCO2 was similarly increased (12%, P < 0.01) in POMC-Asb-4 animals relative to wild-type littermates (Fig. 5B). Total heat production was also increased in the transgenic animals compared with wild-type littermates (wild-type 0.49 ± 0.01 kcal/h · mouse, transgenic 0.55 ± 0.02 kcal/h · mouse, P < 0.05) (Fig. 5C) The respiratory quotient showed no significant difference (wild-type 0.91 ± 0.01, transgenic 0.92 ± 0.01). The spontaneous motor activity was increased in POMC-Asb-4 animals (5D). The increased metabolic rate was not due to disturbances in thyroid function, because circulating T3 and T4 levels were similar between groups (Fig. 5, E and F). POMC mRNA expression in the ARC of POMC-Asb-4 animals was increased compared with wild-type littermates (Fig. 5G).
Figure 5.
Energy expenditure and motor activity in wild-type and POMC-Asb4 animals. A, VO2; B, VCO2; C, total heat production. Each curve represents mean values derived from seven animals per group. P < 0.01 between the two groups for VO2, VCO2, and total heat production as determined by two-way ANOVA. D, Spontaneous motor activity. *, P < 0.05. E and F, Circulating T3 (E) and T4 (F) levels in wild-type and transgenic mice. G, POMC mRNA levels in the ARC. *, P < 0.05.
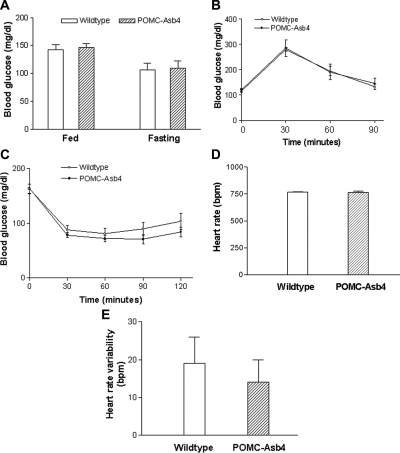
Effects of POMC-Asb-4 on glucose metabolism and heart rate
The overexpression of Asb-4 in POMC neurons of the ARC had no observed effect on peripheral glucose metabolism. Fed and fasting blood glucose levels, glucose tolerance, and insulin sensitivity were similar in the transgenic mice relative to wild-type littermates (Fig. 6, A–C). The heart rate and heart rate variability also showed no difference between the POMC-Asb-4 animals and wild-type littermates (Fig. 6, D and E), indicating that the function of the autonomic nervous system did not change in the transgenic animals.
Figure 6.
Glucose metabolism and heart rates in wild-type and POMC-Asb-4 animals. A, Fed and fasting glucose levels; B, glucose tolerance; C, insulin sensitivity; D, heart rate; E, heart rate variability in POMC-Asb-4 and wild-type animals. Error bars indicate sem. Seven animals were examined for each group.
Discussion
Our previous studies showed that Asb-4 is expressed in POMC neurons of the ARC and is down-regulated, as is POMC mRNA, by fasting (7,19). To date, the function of Asb-4 within the ARC remains unknown. To elucidate the function of Asb-4 in POMC neurons, we made POMC neuron-specific transgenic mice. Because endogenous Asb-4 is expressed in the ARC, PVN, dorsomedial nucleus, lateral hypothalamus, and amygdala, we incorporated a Myc tag of 13 amino acids at the N terminus of Asb-4 to distinguish transgenically expressed Asb-4 from endogenous Asb-4. Myc-Asb-4 was exclusively expressed in the ARC and localized in POMC neurons. Asb-4 mRNA levels in the ARC more than doubled in POMC-Asb4 transgenic animals compared with wild-type littermates. Notably, Myc-Asb-4 was not expressed in POMC endocrine cells of the pituitary, demonstrating that the neuronal enhancer of the POMC gene successfully directed Myc-Asb-4 expression to hypothalamic POMC neurons. This observation is consistent with a previous report by de Souza et al. (12) that first identified the POMC neuronal enhancer.
POMC-Asb-4 transgenic mice exhibited a lean phenotype and were resistant to high-fat diet-induced obesity. The higher rates of VO2 and VCO2 in the transgenic animals compared with wild-type littermates indicate that overexpression of Asb-4 in POMC neurons increased energy expenditure. To our knowledge, this is the first demonstration of an involvement of Asb-4 in the regulation of energy homeostasis. In normal fasting animals, energy expenditure is decreased during fasting (20,21,22). The previously observed down-regulation of Asb-4 mRNA in POMC neurons during fasting is consistent with decreased energy expenditure in this state. The respiratory quotient was similar in POMC-Asb-4 transgenic animals relative to controls, implying that transgenic animals use similar fuel sources. The higher metabolic rate in the transgenic animals was accompanied by significantly lower weight and a lean phenotype. Consistent with the lean phenotype, blood leptin levels were lower in transgenic mice. Although changes in body composition could affect VO2 and VCO2, the observed changes in VO2 and VCO2 are less likely to result from the changes in body composition per se. The increased motor activity in the POMC-Asb-4 transgenic animals may explain the hyperphagic, lean phenotype with increased energy expenditure. In addition, total heat production, which is less dependent on body composition, was also increased. Peripheral glucose metabolism and the activity of the autonomic nervous system were not substantially altered.
ARC POMC mRNA expression was increased in transgenic animals. POMC neurons in ARC play a key role in the regulation of energy homeostasis. Two cleavage products of the POMC precursor, α- and β-MSH (23,24), inhibit food intake, increase energy expenditure, and thus reduce body weight by acting on melanocortin receptor subtypes 3 and 4 (MC3R and -4R) in the ARC, PVN, and lateral hypothalamus (25,26,27). Recently, Huo et al. (28) reported that POMC neurons mediate the stimulation of locomotion by leptin. The increase in POMC mRNA expression in the transgenic animals may explain the elevation in motor activity in POMC-Asb4 transgenic animals.
Asb-4 was originally identified by a database search using a SOCS box consensus sequence (5). There are five major protein families that have SOCS boxes (1). They differ in the type of domain that is upstream of the SOCS box and include 1) SH2 domain proteins with a SOCS box, 2) ankyrin repeat proteins with a SOCS box, 3) SPRY-domain proteins with a SOCS box, 4) WD40-repeat proteins with a SOCS box, and 5) a family of small rar-like GTPases with a SOCS box. Within the context of metabolic regulation, SOCS-3 is well known. SOCS-3 down-regulates leptin receptor and insulin receptor signaling. SOCS-3 belongs to the SH2 domain protein family (29,30). Asb-4 belongs to the ankyrin repeat domain family. Ankyrin repeat domains are thought to be the sites of specific protein-protein interaction. Although several Asb proteins have been identified within the setting of a physiological process, no commonality is obvious. Asb-6 was identified as an adipocyte-specific protein that modulates the adaptor molecule containing a Pleckstin homology domain and Src homology 2 domain interaction with the insulin receptor (3). The increase in POMC mRNA levels in Asb-4 transgenic animals indicates that Asb-4 is involved in the regulation of POMC gene expression or mRNA turnover. The mechanisms underlying this possibility are unclear. We hypothesize that Asb-4 might interact with transcription factors or other proteins involved in the regulation of POMC gene expression and/or mRNA turnover.
The ARC is ideally situated to sense peripherally circulating humoral factors because it is located in a part of the brain with an impaired blood-brain barrier. ARC neurons express high levels of central nervous system receptors for circulating factors including insulin and the orexigen ghrelin (31,32,33). Prominent among peripheral signals is the adipokine leptin. The long form of the leptin receptor is enriched in the ARC, and melanocortinergic neurons in the hypothalamus are regulated by circulating leptin. Exposure of POMC neurons in the ARC to leptin increases POMC expression (10). In the current study, leptin and insulin increased Asb-4 mRNA expression in the PVN. Leptin also increased Asb-4 mRNA in the ARC. The lack of effect of insulin on ARC Asb-4 expression is worth noting. It seems unlikely that icv-injected insulin did not access the ARC because the ARC is immediately adjacent to the third ventricle. One explanation is that Asb-4 mRNA in the PVN and ARC is differentially regulated by insulin and leptin. The differential regulation of Asb-4 mRNA expression in the ARC and PVN implies that Asb-4 in these two areas may perform different functions. These data imply that Asb-4 may be involved in regulation of POMC mRNA expression by leptin in the ARC.
Acknowledgments
We thank the University of Michigan Transgenic Core for the creation of transgenic animals.
Footnotes
This work was supported by National Institutes of Health Grants 5R01 DK054032 and 5R01 DK043225.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 24, 2009
Abbreviations: ARC, Arcuate nucleus; Asb, ankyrin repeat and SOCS box-containing protein family; icv, intracerebroventricular; POMC, proopiomelanocortin; PVN, paraventricular nucleus; RT-QPCR, real-time quantitative PCR; SOCS, suppressor of cytokine signaling; TK, thymidine kinase; VCO2, carbon dioxide production; VO2, oxygen consumption.
References
- Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ 2002 The SOCS box: a tale of destruction and degradation. Trends Biochem Sci 27:235–241 [DOI] [PubMed] [Google Scholar]
- Debrincat MA, Zhang JG, Willson TA, Silke J, Connolly LM, Simpson RJ, Alexander WS, Nicola NA, Kile BT, Hilton DJ 2007 Ankyrin repeat and suppressors of cytokine signaling box protein Asb-9 targets creatine kinase B for degradation. J Biol Chem 282:4728–4737 [DOI] [PubMed] [Google Scholar]
- Wilcox A, Katsanakis KD, Bheda F, Pillay TS 2004 Asb6, an adipocyte-specific ankyrin and SOCS box protein, interacts with APS to enable recruitment of elongins B and C to the insulin receptor signaling complex. J Biol Chem 279:38881–38888 [DOI] [PubMed] [Google Scholar]
- Kohroki J, Nishiyama T, Nakamura T, Masuho Y 2005 ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes. FEBS Lett 579:6796–6802 [DOI] [PubMed] [Google Scholar]
- . Kile BT, Viney EM, Willson TA, Brodnicki TC, Cancilla MR, Herlihy AS, Croker BA, Baca M, Nicola NA, Hilton DJ, Alexander WS 2000 Cloning and characterization of the genes encoding the ankyrin repeat and SOCS box-containing proteins Asb-1, Asb-2, Asb-3 and Asb-4. Gene 258:31–41 [DOI] [PubMed] [Google Scholar]
- Li JY, Kuick R, Thompson RC, Misek DE, Lai YM, Liu YQ, Chai BX, Hanash SM, Gantz I 2005 Arcuate nucleus transcriptome profiling identifies ankyrin repeat and SOCS box-containing protein 4 as a gene regulated by fasting in central nervous system feeding circuits. J Neuroendocrinol 17:394–404 [DOI] [PubMed] [Google Scholar]
- Li JY, Chai BX, Zhang W, Liu YQ, Ammori JB, Mulholland MW 2007 Ankyrin repeat and SOCS box containing protein 4 (Asb-4) interacts with GPS1 (CSN1) and inhibits c-Jun NH2-terminal kinase activity. Cell Signal 19:1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL 2008 Neuronal control of energy homeostasis. FEBS Lett 582:132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA 1997 Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138:4489–4492 [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA 1997 Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066 [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS 1999 Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20:68–100 [DOI] [PubMed] [Google Scholar]
- de Souza FS, Santanelo AM, Bumaschny V, Avale ME, Smart JL, Low MJ, Rubinstein M 2005 Identification of neuronal enhancer of the proopiomelanorcortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol Cell Biol 25:3076–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F 1997 Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- Zhang W, Chai B, Li JY, Wang H, Mulholland MW 2008 Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 149:4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. 4th ed. Bowen Hills, Australia: Academic Press [Google Scholar]
- Chavez M, Riedy CA, Van Dijk G, Woods SC 1996 Central insulin and macronutrient intake in the rat. Am J Physiol 271:R727–R731 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV 1998 Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes [Erratum (1998) 47:696] 47:294–297 [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ 1982 Effect of chronic food restriction on energy balance, thermogenic capacity, and brown-adiposetissue activity in the rat. Biosci Rep 2:543–549 [DOI] [PubMed] [Google Scholar]
- Munch IC, Markussen NH, Oritsland NA 1993 Resting oxygen consumption in rats during food restriction, starvation and refeeding. Acta Physiol Scand 148:335–340 [DOI] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME 2001 Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280:R1007–R1015 [DOI] [PubMed] [Google Scholar]
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD 2004 The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Rec Prog Horm Res 59:395–408 [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD 1997 Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H 2006 A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab 3:141–146 [DOI] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O'Rahilly S, Farooqi IS 2006 A POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 3:135–140 [DOI] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbaek C 2009 Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS 1998 Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1:619–625 [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E 2000 SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275:15985–15991 [DOI] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J 1986 Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17:1127–1138 [DOI] [PubMed] [Google Scholar]
- Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA 1987 Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121:1562–1570 [DOI] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M 2005 Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept 126:55–59 [DOI] [PubMed] [Google Scholar]