Abstract
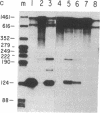
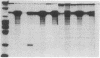
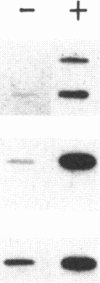
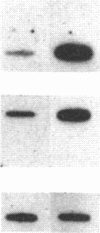
We have examined the effect of the site of integration on the expression of cloned genes introduced into cultured erythroid cells. Smithies et al. [Smithies, O., Gregg, R.G., Boggs, S.S., Koralewski, M.A. & Kucherlapati, R.S. (1985) Nature (London) 317, 230-234] reported the targeted integration of DNA into the human beta-globin locus on chromosome 11 in a mouse erythroleukemia-human cell hybrid. These hybrid cells can undergo erythroid differentiation leading to greatly increased mouse and human beta-globin synthesis. By transfection of these hybrid cells with a plasmid carrying a modified human beta-globin gene and a foreign gene composed of the coding sequence of the bacterial neomycin-resistance gene linked to simian virus 40 transcription signals (SVneo), cells were obtained in which the two genes are integrated at the beta-globin locus on human chromosome 11 or at random sites. When we examined the response of the integrated genes to cell differentiation, we found that the genes inserted at the beta-globin locus were induced during differentiation, whereas randomly positioned copies were not induced. Even the foreign SVneo gene was inducible when it had been integrated at the beta-globin locus. The results show that genes introduced at the beta-globin locus acquire some of the regulatory properties of globin genes during erythroid differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman R. B., Sprecher C., Graves R., Marzluff W. F., Skoultchi A. I. Regulated expression of a chimeric histone gene introduced into mouse fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2316–2324. doi: 10.1128/mcb.5.9.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Theoretical analysis of a model for globin messenger RNA accumulation during erythropoiesis. J Mol Biol. 1977 Feb 25;110(2):205–218. doi: 10.1016/s0022-2836(77)80069-7. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Chao M. V., Mellon P., Charnay P., Maniatis T., Axel R. The regulated expression of beta-globin genes introduced into mouse erythroleukemia cells. Cell. 1983 Feb;32(2):483–493. doi: 10.1016/0092-8674(83)90468-3. [DOI] [PubMed] [Google Scholar]
- Charnay P., Treisman R., Mellon P., Chao M., Axel R., Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984 Aug;38(1):251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Activation of phenotypic expression of human globin genes from nonerythroid cells by chromosome-dependent transfer to tetraploid mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 May;76(5):2185–2189. doi: 10.1073/pnas.76.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Skoultchi A. I. Absolute rates of globin gene transcription and mRNA formation during differentiation of cultured mouse erythroleukemia cells. J Biol Chem. 1985 Oct 5;260(22):12167–12173. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B., Skoultchi A. I., Lachman H. M. Contributions of transcriptional and post-transcriptional mechanisms to the regulation of c-myc expression in mouse erythroleukemia cells. Genes Dev. 1987 Nov;1(9):938–945. doi: 10.1101/gad.1.9.938. [DOI] [PubMed] [Google Scholar]
- Pyati J., Kucherlapati R. S., Skoultchi A. I. Activation of human beta-globin genes from nonerythroid cells by fusion with murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3435–3439. doi: 10.1073/pnas.77.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of alpha and beta mouse globin gene sequences synthesised in vitro. Gene. 1977 May;1(3-4):229–239. doi: 10.1016/0378-1119(77)90047-6. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Costantini F. A 3' enhancer contributes to the stage-specific expression of the human beta-globin gene. Genes Dev. 1987 Nov;1(9):954–961. doi: 10.1101/gad.1.9.954. [DOI] [PubMed] [Google Scholar]
- Trudel M., Magram J., Chada K., Wilson R., Costantini F. Expression of normal, mutant and hybrid human globin genes in transgenic mice. Prog Clin Biol Res. 1987;251:305–321. [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Nienhuis A. W., Anderson W. F. Selective activation of human beta-but not gamma-globin gene in human fibroblast x mouse erythroleukaemia cell hybrids. Nature. 1979 Feb 15;277(5697):534–538. doi: 10.1038/277534a0. [DOI] [PubMed] [Google Scholar]
- Wright S., Rosenthal A., Flavell R., Grosveld F. DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells. Cell. 1984 Aug;38(1):265–273. doi: 10.1016/0092-8674(84)90548-8. [DOI] [PubMed] [Google Scholar]
- Wright S., deBoer E., Grosveld F. G., Flavell R. A. Regulated expression of the human beta-globin gene family in murine erythroleukaemia cells. Nature. 1983 Sep 22;305(5932):333–336. doi: 10.1038/305333a0. [DOI] [PubMed] [Google Scholar]
- Zavodny P. J., Roginski R. S., Skoultchi A. I. Regulated expression of human globin genes and flanking DNA in mouse erythroleukemia--human cell hybrids. Prog Clin Biol Res. 1983;134:53–62. [PubMed] [Google Scholar]