Abstract
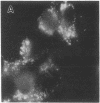
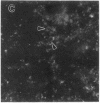
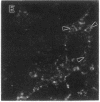
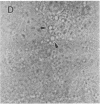
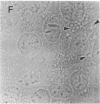
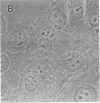
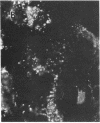
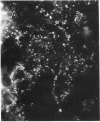
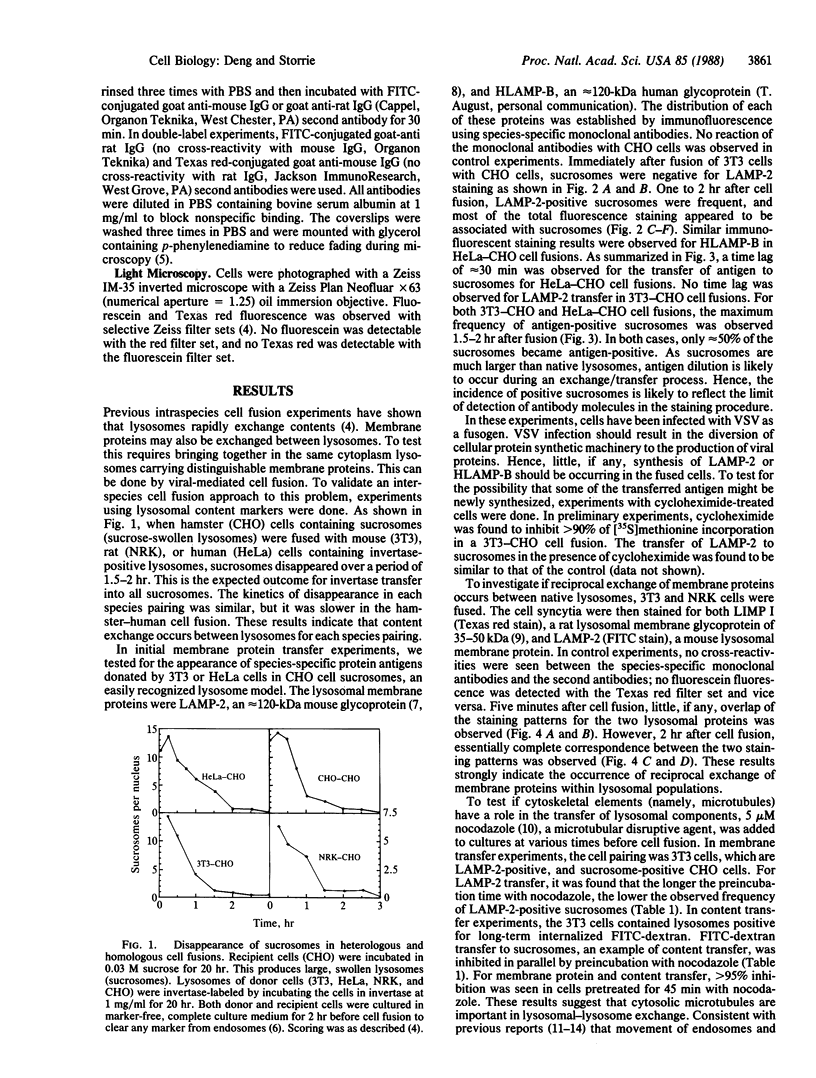
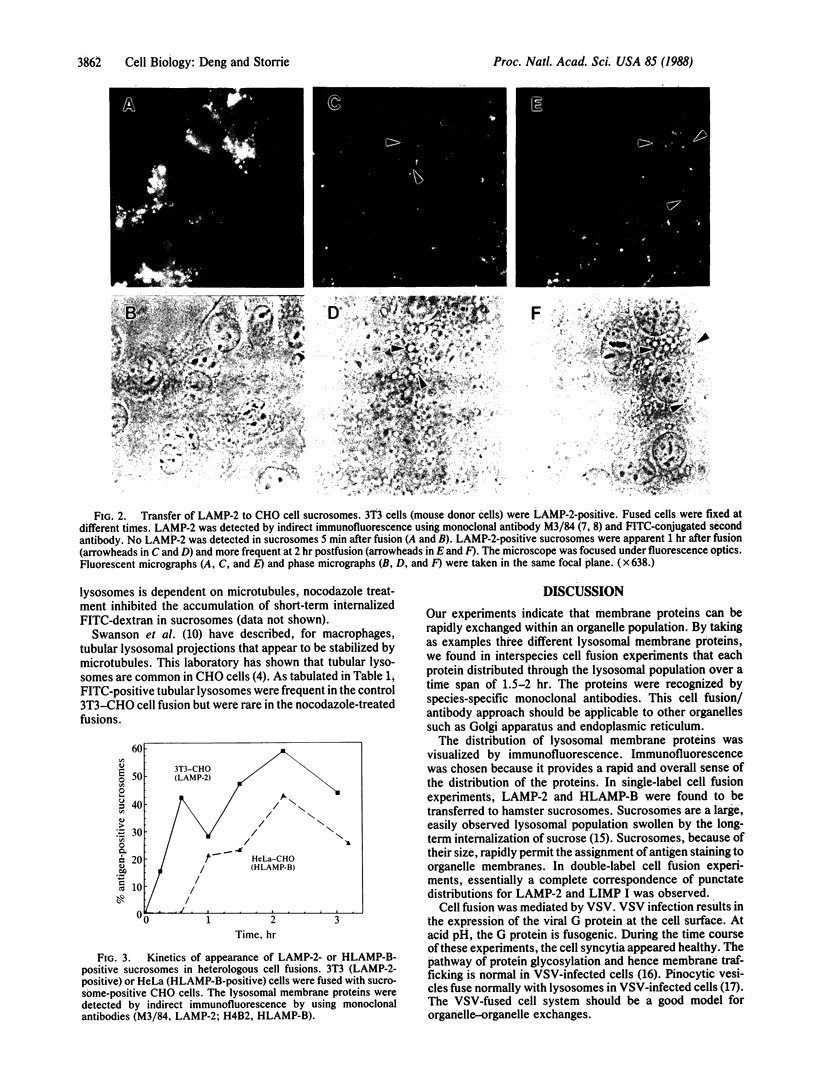
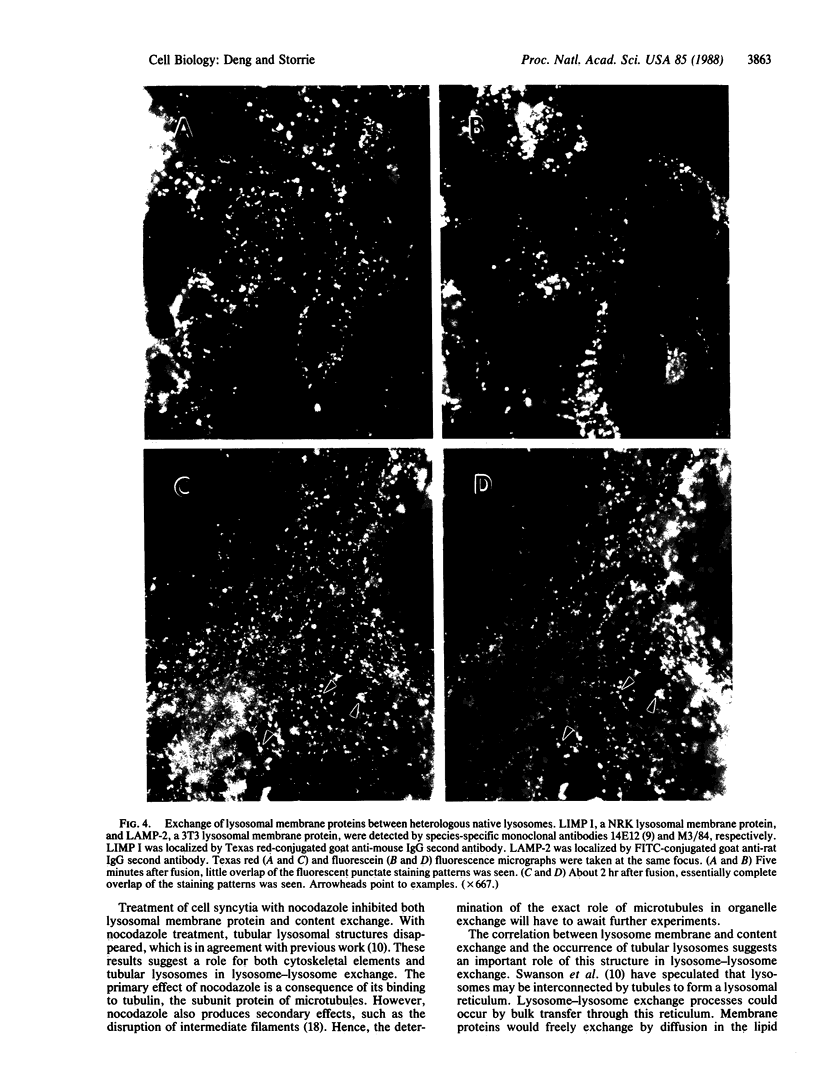
The lysosome has been chosen as a model to study the exchange of native membrane proteins within an organelle population. Heterologous lysosomes were brought together by vesicular stomatitis virus-mediated cell fusion. The distribution of lysosomal membrane protein was visualized by indirect immunofluorescence using species-specific monoclonal antibody. LAMP-2, a mouse lysosomal membrane protein, and HLAMP-B, a human lysosomal membrane protein, were found to transfer to Chinese hamster ovary cell sucrosomes (sucrose-swollen lysosomes). This transfer occurred in the presence of cycloheximide. The exchange of LAMP-2 and LIMP I, a rat lysosomal membrane protein, was observed between native lysosomes in a mouse (3T3)-rat (normal rat kidney) cell fusion. Extensive transfer/exchange was observed within 1.5-2 hr postfusion, which is consistent with the kinetics of endocytic content exchange between lysosomes. Both membrane protein and content transfer between lysosomes were inhibited by nocodazole, a disrupter of microtubules, as was endocytic delivery to sucrose-swollen lysosomes. In the presence of nocodazole, tubular lysosomes disappeared. Both tubular lysosomes and microtubules may be important for the transfer/exchange. The interspecies cell fusion/monoclonal antibody approach developed here should be readily applicable to determining if membrane protein exchange is a property of other organelles such as Golgi apparatus and mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris A. L., Brown J. C., Park R. D., Storrie B. Chinese hamster ovary cell lysosomes rapidly exchange contents. J Cell Biol. 1987 Dec;105(6 Pt 1):2703–2712. doi: 10.1083/jcb.105.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P. Synthesis and maturation of glycoproteins of enveloped animal viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):26–39. doi: 10.1093/clinids/2.1.26. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B., Albertini D. F. A time-lapse video image intensification analysis of cytoplasmic organelle movements during endosome translocation. J Cell Biol. 1984 Feb;98(2):565–576. doi: 10.1083/jcb.98.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983 Jan 10;258(1):636–642. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaire-Washington L., Silverstein S. C., Wang E. Phorbol myristate acetate stimulates microtubule and 10-nm filament extension and lysosome redistribution in mouse macrophages. J Cell Biol. 1980 Aug;86(2):641–655. doi: 10.1083/jcb.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Miller R. L., Urbani L. J. Intercompartmental transport in the Golgi complex is a dissociative process: facile transfer of membrane protein between two Golgi populations. J Cell Biol. 1984 Jul;99(1 Pt 1):260–271. doi: 10.1083/jcb.99.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Sachdeva M., Viers V. S. Chinese hamster ovary cell lysosomes retain pinocytized horseradish peroxidase and in situ-radioiodinated proteins. Mol Cell Biol. 1984 Feb;4(2):296–301. doi: 10.1128/mcb.4.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bushnell A., Silverstein S. C. Tubular lysosome morphology and distribution within macrophages depend on the integrity of cytoplasmic microtubules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1921–1925. doi: 10.1073/pnas.84.7.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox D. K., Whitaker-Dowling P. A., Youngner J. S., Widnell C. C. Rapid inhibition of pinocytosis in baby hamster kidney (BHK-21) cells following infection with vesicular stomatitis virus. J Cell Biol. 1983 Nov;97(5 Pt 1):1444–1451. doi: 10.1083/jcb.97.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]