Abstract
Aluminium (Al) is phytotoxic when solubilized into Al3+ in acidic soils. One of the earliest and distinct symptoms of Al3+ toxicity is inhibition of root elongation. To decipher the mechanism by which Al3+ inhibits root elongation, the role of ethylene and auxin in Al3+-induced inhibition of root elongation in Arabidopsis thaliana was investigated using the wild type and mutants defective in ethylene signalling (etr1-3 and ein2-1) and auxin polar transport (aux1-7 and pin2). Exposure of wild-type Arabidopsis to AlCl3 led to a marked inhibition of root elongation, and elicited a rapid ethylene evolution and enhanced activity of the ethylene reporter EBS:GUS in root apices. Root elongation in etr1-3 and ein2-1 mutants was less inhibited by Al3+ than that in wild-type plants. Ethylene synthesis inhibitors, Co2+ and aminoethoxyvinylglycine (AVG), and an antagonist of ethylene perception (Ag+) abolished the Al3+-induced inhibition of root elongation. There was less inhibition of root elongation by Al3+ in aux1-7 and pin2 mutants than in the wild type. The auxin polar transport inhibitor, naphthylphthalamic acid (NPA), substantially alleviated the Al3+-induced inhibition of root elongation. The Al3+ and ethylene synthesis precursor aminocyclopropane carboxylic acid (ACC) increased auxin reporter DR5:GUS activity in roots. The Al3+-induced increase in DR5:GUS activity was reduced by AVG, while the Al3+-induced increase in EBS:GUS activity was not altered by NPA. Al3+ and ACC increased transcripts of AUX1 and PIN2, and this effect was no longer observed in the presence of AVG and Co2+. These findings indicate that Al3+-induced ethylene production is likely to act as a signal to alter auxin distribution in roots by disrupting AUX1- and PIN2-mediated auxin polar transport, leading to arrest of root elongation.
Keywords: Aluminium toxicity, Arabidopsis thaliana, auxin polar transport, ethylene, root elongation
Introduction
Aluminium (Al) is the most abundant metal in the Earth's crust. Most Al occurs in soil as aluminosilicate, which is usually non-toxic to living organisms (May and Nordstrom, 1991). However, Al3+ is hydrolysed into Al3+ cations in acidic environments, and becomes a major factor limiting crop production and yield in many acid soils throughout the world (Foy, 1988). Inhibition of root elongation is one of the earliest and most distinct symptoms exhibited by plants exposed to micromolar concentrations of Al3+ in solution cultures (Zhang and Rengel, 1999; Doncheva et al., 2005). Although recent studies suggested that Al3+ can induce a rapid change in the position of cell division activity in maize (Doncheva et al., 2005), it is generally believed that the rapid inhibition of root growth induced by Al3+ is primarily caused by inhibition of cell elongation (Horst, 1995; Matsumoto, 2000). Furthermore, several studies have demonstrated that the root apex, particularly the root distal transition zone, is a critical site of perception and expression of Al toxicity (Ryan et al, 1993; Sivaguru and Horst, 1998). Although extensive research has demonstrated that Al3+ alters numerous physiological processes, including disruption of cytosolic Ca2+ homeostasis, alterations of cytoskeleton dynamics (see reviews by Matsumoto, 2000; Barcelo and Poschenrieder, 2002; Rengel and Zhang, 2003), and disturbance of endogenous nitric oxide in root tips (Illes et al., 2006; Tian et al., 2007), the primary mechanisms underlying Al toxicity in plants remain largely unknown.
Phytohormones, particularly auxin and ethylene, play critical roles in modulating root growth. For instance, it has been shown that ethylene affects root growth by inhibiting the rapid expansion of cells leaving the root meristem (Le et al., 2001; Swarup et al., 2007). This feature resembles the widely observed rapid inhibition of root elongation by Al3+. In higher plants, ethylene is produced from methionine through S-adenosyl-L-methionine and 1-aminocyclopropane-1-carboxylic acid (ACC), catalysed by ACC synthase (ACS) and ACC oxidase (ACO), respectively (Kende, 1993). ACS and ACO are encoded by multigene families and regulated by many biotic and abiotic factors (Wang et al., 2002). Our previous work has established that ethylene plays a critical role in Al-induced inhibition of root elongation in Lotus japonicus such that inhibition of Al3+-induced ethylene production from root apices by ethylene synthesis antagonists markedly alleviates the Al-induced inhibition of root elongation (Sun et al., 2007). In addition to ethylene, root growth and development are also closely related to auxin synthesis, distribution, and transport (Blilou et al., 2005; Tanaka et al., 2006). Several studies have demonstrated that Al3+ may interact with auxin signalling pathways by possibly targeting auxin polar transport systems, leading to alterations of auxin accumulation and distribution in roots (Kollmeier et al., 2000; Doncheva et al., 2005; Shen et al., 2008). Auxin, which is transported to roots by polar transport systems through the specific subcellular localization of auxin efflux and auxin influx machineries, modulates root growth and development (Benjamins et al., 2005). It has been identified that the PIN FORMED (PIN) proteins function to mediate auxin efflux (Blilou et al., 2005; Paponov et al., 2005; Teale et al., 2006). Among the PIN proteins, PIN2 is involved in the transport of auxin from the root tip into the elongation zone and back again via the cortex toward the root tip (Blilou et al., 2005). AUXIN RESISTANT 1 (AUX1) is an auxin influx carrier (Bennett et al., 1996) which facilitates polar auxin delivery to the root apex (Swarup et al., 2005). A recent study indicated that Al3+ reduces the auxin concentration in the transition zone of Arabidopsis roots by inhibiting the transport of PIN2 vesicles from plasma membranes to endosomes (Shen et al., 2008). However, there has been no detailed study to investigate the role of PIN2 and AUX1 in Al3+-induced inhibition of root elongation.
Synergistic effects of auxin and ethylene on root growth have been extensively studied using an array of Arabidopsis mutants defective in signalling of ethylene and auxin (Stepanova et al., 2005, 2007; Růžička et al., 2007; Swarup et al., 2007). For example, Růžička et al. (2007) demonstrated that ethylene stimulates auxin biosynthesis and basipetal auxin transport toward the elongation zone, leading to the inhibition of root cell elongation. Because ethylene (Massot et al., 2002; Sun et al., 2007) and auxin (Kollmeier et al., 2000; Doncheva et al., 2005; Shen et al., 2008) have been implicated in Al3+-dependent inhibition of root growth, it is conceivable that cross-talk between the two hormones may exist in Al3+-dependent inhibition of root growth in plants. In the present study, this issue was addressed by using pharmacological agents and several Arabidopsis mutants with impaired auxin and ethylene signalling (pin2, aux1-7, etr1-3, and ein2-1). The effect of Al3+ on auxin and ethylene production and distribution was also studied by monitoring activities of auxin (DR5:GUS) and ethylene (EBS:GUS) reporter genes.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia (Col), ethylene-insensitive mutants etr1-3 and ein2-1, and auxin polar transport mutants aux1-7 and pin2 were obtained from the Arabidopsis Biological Resource Centre, Columbus, OH, USA. The EBS–GUS reporter line, in which the GUS reporter gene is driven by a synthetic EIN3-responsive promoter, was generously provided by Dr J Alonso, and was originally generated by Dr Anna Stepanova (Stepanova et al., 2005). DR5 is a synthetic auxin-responsive promoter which has been widely used to monitor auxin responses in planta. The DR5–GUS report line used in the present study is described by Ulmasov et al. (1997) and is a kind gift of Professor Tom Guilfoyle. All seeds were surface-sterilized by incubation for 1 min in 75% ethanol, and rinsed thoroughly with sterile distilled water followed by exposure to 10% (v/v) sodium hypochlorite for 15 min, and then washed with sterile water. The sterilized seeds were sown on 1/2 MS agar plates [0.6% agar (w/v), pH 5.8]. Wild-type, etr1-3, ein2-1, aux1-7, and pin2, and DR5–GUS and EBS–GUS reporter seedlings (5 d old) grown on 1/2 MS agar plates were transferred to agar medium containing 1/2 MS nutrients, 0.8% sucrose, and 0.7% (w/v) agar, with pH adjusted to 5.8 for another 7 d. All seedlings were grown in 9 cm diameter glass dishes, oriented vertically, in a controlled environment with a temperature of 20/23 °C, 14/10 h light cycle, and photosynthetic photon flux density of 100–120 μmol m−2 s−1.
Root elongation assays
To study the inhibitory effect of AlCl3 on root elongation, Arabidopsis seedlings were incubated in 1/2 MS agar plates for 7 d and then transferred into Petri dishes with solutions containing 0.5 mM CaCl2 with and without 50 μM AlCl3 (pH 4.5) for 24 h or with agar (0.7%) containing AlCl3 (0, 50, 100, and 200 μM, pH 4.5) for 4 d. Elongation of the primary root was measured after treating the roots for varying periods under a microscope. To study the effect of AlCl3 on root elongation, seedlings of Col-0, etr1-3, ein2-1, aux1-7, and pin2 were exposed to 50 μM AlCl3 and root elongation was measured after exposure of seedlings to AlCl3 for 24 h. To study the effect of aminoethoxyvinylglycine (AVG), Co2+, AgNO3, and naphthylphthalamic acid (NPA) on root elongation in the absence and presence of 50 μM AlCl3, seedlings of Arabidopsis wild type (Col-0) were first exposed to 10 μM AVG, 10 μM CoCl2, or 10 μM NPA for 2 h and then incubated in 50 μM AlCl3 for another 24 h. For treatment with Ag+, seedlings were first incubated in 10 μM AgNO3 as control and then exposed to 50 μM Al(NO3)3 for 24 h to determine the effect of Ag+ on root elongation of Arabidopsis wild-type (Col-0). Values are given as the mean±SE of at least 10 independent measurements. All experiments were repeated at least three times.
Determination of ethylene production
After exposure of Arabidopsis seedlings to 50 μM AlCl3 for varying durations, root tips (∼1 cm in length) of ∼0.2 g were excised and put into 5 ml gas-tight vials containing 1 ml of agar medium (0.7% agar). A 1 ml volume of the headspace was taken from the vials, and then injected into a gas chromatograph (GC) equipped with an alumina column (GDX502) and a flame ionization detector (GC-7AG; Shimadzu Japan) for measurement of the ethylene concentration.
GUS staining
GUS staining was carried as described in the literature (Jefferson et al., 1987; Malamy and Benfey, 1997; Stepanova et al., 2005). Briefly, 7-d-old seedlings were pulled out of agar and exposed to control solution and to solutions supplemented with 50 μM AlCl3 (pH 4.5), 10 μM ACC, or 10 μM NPA for 2 h, fixed in an ice-cold 90% acetone, washed once with the rinse buffer, which is composed of 100 mM NaPO4 buffer (pH 7.0), 1 mM K3Fe(CN)6, and 1 mM K4Fe(CN)6, and stained for 4 h in the dark at 37 °C. Staining buffer comprises 100 mM NaPO4, pH 7.0, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6, 10 mM Na2EDTA, 0.1% (v/v) Triton X-100, 20% (v/v) methanol and 0.5 mg ml−1 X-Gluc. For observation of whole mounts, stained seedlings were transferred to small Petri dishes containing 0.24 N HCl in 20% methanol and incubated on a 57 °C heat block for 15 min. This solution was replaced with another solution containing 7% NaOH, 7% hydroxylamine-HCl in 60% ethanol for 15 min at room temperature. Roots were then rehydrated for 5 min in 40, 20, and 10% ethanol, respectively, and infiltrated for 15 min in 5% ethanol, 25% glycerol. Roots were mounted in 50% glycerol on glass microscope slides and individual seedlings were photographed as described by Malamy and Benfey (1997).
Gene expression analysis
Real-time RT-PCR was used to study the expression patterns of ACS2, ACS6, ACS8, ACO1, ACO2, AUX1, PIN1, and PIN2 genes in Arabidopsis in response to different treatments including AlCl3, ethylene precursors, and ethylene synthesis inhibitors. Total RNAs were extracted from Arabidopsis roots with Trizol reagent (Invitrogen) and treated with RNase-free DNase I (Promega). The total RNAs were reverse-transcribed into first-strand cDNA in a 20 μl volume with M-MLV reverse transcriptase (Promega). The samples were diluted to 100 μl with water, and 5 μl of each sample (∼8 ng RNA equivalent) were PCR amplified using SYBR GreenER™ qPCR SuperMix Universal (Invitrogen) in a 25 μl reaction, containing 5 μl of diluted cDNA, 12.5 μl of SYBR GreenER™ qPCR SuperMix Universal, 0.5 μl of Rox Reference Dye, 1 μl of 10 μM forward primer, 1 μl of 10 μM reverse primer, and 5 μl of water. The Mx3000P machine was used to run quantitative RT-PCR with the following eight primer pair combinations: AtACS2, 5′-TCATGGGAAAAGCTAGAGGTGGAAG-3′ and 5′-TCAACGGTTAATTTGAAATTGTCGG-3′; AtACS6, 5′-AAACCGATGGCTGCAACAACTATGAT-3′ and 5′-TAAGTCTGTGCACGGACTAGCGGAG-3′; AtACS8, 5′-TGGGGTGATTTACTCCAACGATGATT-3′ and 5′-GACACTCGATGCCTGCAGCCTCTAG-3′; AtACO1, 5′-CCGTGTAATGACAGTGAAGCATGGAAG-3′ and 5′-TCTCAAGTCTGGGGCCTTTGTCTCC-3′; AtACO2, 5′-GGATGTCGGTTGCATCGTTTTA-3′ and 5′-TACGGCTGCTGTAGGATTCAGTTC-3′; AtAUX1, 5′-AGACGCACTTCTCGACCACTCCA-3′ and 5′-GCATCCCAATCACTTTCTCCCACA-3′; AtPIN2, 5′-CGCTCTTTTCACTATCAACACTGCCTAA-3′ and 5′-GTCTCCTATTCCGCATCGGTCTG-3′. In addition, a housekeeping gene, AtActin11, was employed as a control: 5′-CCACATGCTATTCTGCGTTTGGACC-3′ and 5′-CATCCCTTACGATTTCACGCTCTGC-3′
Primers were designed across exon–exon junctions of cDNA to avoid potential problems due to contamination of genomic DNA. The amplification efficiency for each primer pair was calculated using serial cDNA dilutions. After correcting the cycle threshold values according to the amplification efficiency, the expression values of the eight genes were normalized to the corresponding controls.
Results
Al3+ inhibited root elongation and evoked ethylene production
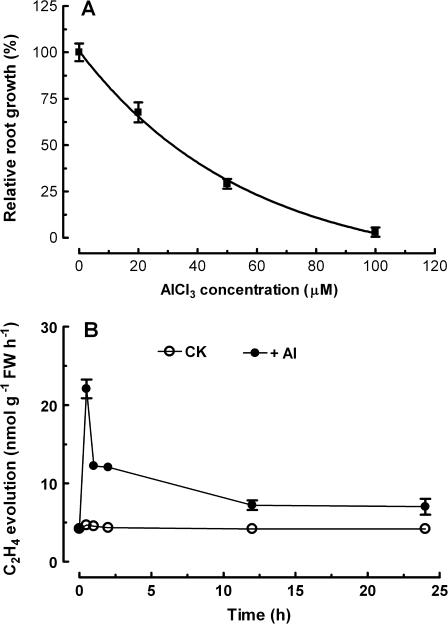
To examine the sensitivity of primary root elongation to Al3+, Arabidopsis seedlings were exposed to hydroponic solutions with varying concentrations of AlCl3 (0, 20, 50, and 100 μM, pH 4.5) for 24 h. As shown in Fig. 1A, root elongation was rapidly inhibited by exposure to Al3+, and the inhibition of root elongation was positively dependent on AlCl3 concentrations. For instance, root elongation was inhibited by 32, 71, and 97% after 24 h exposure to 20, 50, and 100 μM AlCl3, respectively. A previous study has revealed that Al3+ evokes a rapid ethylene burst from root tips of Lotus japonicas (Sun et al., 2007). To test whether a similar mechanism is operative in Arabidopsis, the effect of Al3+ on ethylene evolution from root tips of Arabidopsis was investigated. A rapid burst of ethylene evolution was observed upon exposure of Arabidopsis roots to AlCl3 (Fig. 1B). The ethylene production reached a maximum after 30 min of exposure to Al3+, and thereafter the evolution rapidly declined to a relatively steady level after exposure to Al3+ for 12 h.
Fig. 1.
(A) Response of root elongation in Arabidopsis wild-type (Col-0) seedlings to varying concentrations of AlCl3 (A). The lengths of primary roots were measured after the seedlings were exposed to solutions containing 0, 20, 50, and 100 μM AlCl3 in addition to 0.5 mM CaCl2 pH 4.5 for 24 h. Data are presented as relative root elongation compared with control values. All values are means ±SE of >8 roots. (B) Time course of ethylene evolution from root tips of wild-type Arabidopsis upon exposure to 50 μM AlCl3. Values are means ±SE of five replicates.
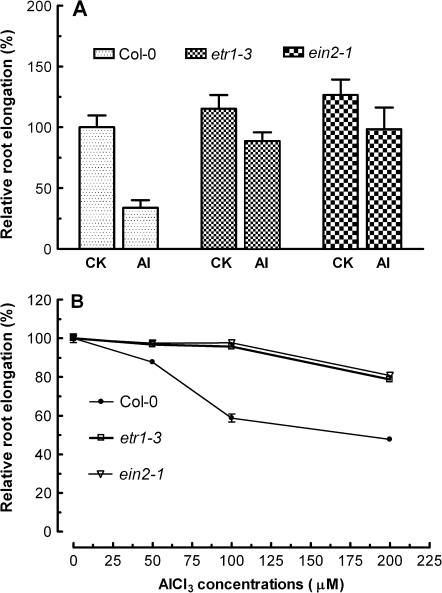
To evaluate the role of ethylene in Al3+-induced inhibition of root elongation, a genetic approach was employed by using the ethylene-insensitive mutants etr1-3 and ein2-1. Exposure of etr1-3 and ein2-1 mutants to 50 μM AlCl3 led to less inhibition of root elongation than that of the wild type (Col-0), i.e. root elongation was reduced by 71, 23, and 21% for wild-type, etr1-3 and ein2-1 plants, respectively, upon 24 h exposure to 50 μM AlCl3. Note that root elongation of etr1-3 and ein2-1 was ∼20% greater than that of wild-type plants in the absence of AlCl3 (Fig. 2A). A similar less inhibitory effect of Al3+ on root elongation in the two ethylene-insensitive mutants than in wild-type plants was also observed when these plants were grown on agar containing varying concentrations of AlCl3 (0, 50, 100, and 200 μM) for 4 d (Fig. 2B). The lower inhibitory effect of AlCl3 on root elongation when grown in agar could be ascribed to reduced Al3+ activity due to its complexing with agar.
Fig. 2.
Effect of AlCl3 on root elongation of Arabidopsis wild-type (Col-0) and ethylene-insensitive mutants, etr1-3 and ein2-1. (A) Seedlings of Col-0, etr1-3, and ein2-1 were exposed to 50 μM AlCl3 and root elongation was measured under a stereomicroscope after exposure of seedlings for 24 h. (B) Effect of varying concentrations of AlCl3 on root growth of seedlings grown on agar. Primary root length was measured before and after transferring wild-type seedlings to agar medium with varying concentrations of AlCl3 (0, 50, 100, and 200 μM, pH 4.5) for 4 d. Data are expressed as root elongation relative to controls, and given as means ±SE of >8 roots. The root elongation rate in the absence of AlCl3 for Col-0, etr1-3, and ein2-1 was 5.41±0.13 mm d−1 (n=17), 6.06±0.59 mm d−1 (n=6), and 6.66±0.66 mm d−1 (n=6), respectively.
Al3+ had less effect on root elongation in auxin-insensitive mutants
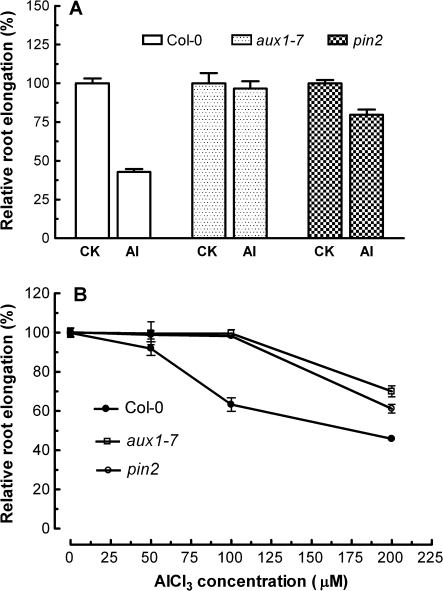
In addition to ethylene, the role of auxin in Al-induced inhibition of root elongation was also examined using the auxin polar transport mutants aux1-7 and pin2. In contrast to wild-type plants, root elongation in both aux1-7 and pin2 was relatively insensitive to Al3+ when treated with 50 μM Al3+ hydroponically for 24 h (Fig. 3A). When both wild-type and mutant seedlings were grown in agar containing varying concentrations of AlCl3 for 4 d, root elongation of aux1-7 and pin2 was also less inhibited than that of the wild type (Fig. 3B). For instance, root elongation in wild-type plants was reduced by 38% when grown in agar containing 100 μM AlCl3 (pH 4.5), while root elongation in aux1-7 and pin2 was not affected when grown under the identical AlCl3 conditions (Fig. 3B). These results are indicative that AUX1 and PIN2 are involved in Al-induced inhibition of root elongation in Arabidopsis.
Fig. 3.
Effect of AlCl3 on root elongation of Arabidopsis wild-type (Col-0) and auxin polar transport mutants, aux1-7 and pin2. (A) Seedlings of Col-0, aux1-7, and pin2 were exposed to 50 μM AlCl3 (pH 4.5) and root length was measured under a stereomicroscope after 24 h. (B) Effect of AlCl3 on root elongation of seedlings grown on agar medium containing varying concentrations of AlCl3 (pH 4.5) for 4 d. Data are expressed as relative root elongation relative to controls, and are presented as means ±SE of >8 roots. The root elongation rate in the absence of AlCl3 for Col-0, aux1-7, and pin2 was 5.41±0.13 mm d−1 (n=17), 7.14±0.47 mm d−1 (n=13), and 5.26±0.11 mm d−1 (n=10), respectively.
AVG, Co2+, and NPA alleviated Al-induced inhibition of root elongation
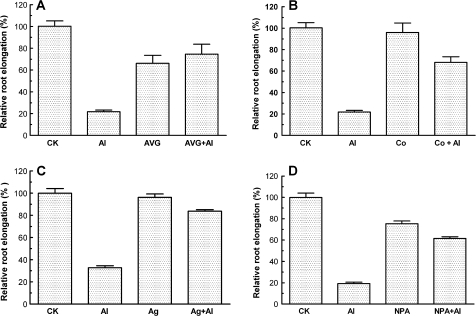
The less inhibitory effect of Al3+ on root elongation in the Arabidopsis mutants insensitive to ethylene and auxin than that in wild-type plants suggests that both ethylene and auxin may be involved in Al-induced inhibition of root elongation. To verify this hypothesis, the effects of Al3+ on root elongation in the presence of antagonists of ethylene biosynthesis (AVG and Co2+) and ethylene perception (Ag+) were examined. The Al3+-induced inhibition of root elongation was markedly recovered when Arabidopsis seedlings were exposed to 10 μM AVG and CoCl2 for 12 h prior to treatment with Al3+ (Fig. 4A, B). In the absence of Al3+, AVG reduced root elongation by ∼35% (Fig. 4A), while CoCl2 had no effect on root elongation in the absence of Al3+ (Fig. 4B). A similar ameliorative effect on the inhibition of root elongation caused by Al3+ was also observed by treatment with the ethylene perception inhibitor, AgNO3 (Fig. 4C). As root elongation in aux1-7 and pin2 mutants was also less sensitive to Al3+ than in wild-type plants (Fig. 3), the effect of an antagonist of auxin polar transport, NPA, on root elongation in the absence and presence of Al3+ was studied. As shown in Fig. 4D, NPA marginally inhibited root growth in the absence of Al3+, while NPA substantially alleviated the Al-induced inhibition of root elongation.
Fig. 4.
Effect of (A) AVG, (B) Co2+, (C) AgNO3, and (D) NPA on root elongation in the absence and presence of 50 μM AlCl3 (pH 4.5). To minimize the effect of these chemical agents on Al3+ activity, seedlings were first exposed to 10 μM AVG (A), 10 μM CoCl2 (B), or 10 μM NPA (D) for 2 h followed by incubation in 50 μM AlCl3 for another 24 h. For treatment with Ag+, seedlings were first incubated in 10 μM AgNO3 and then exposed to 50 μM Al(NO3)3 for 24 h to determine the effect of Ag+ on root elongation of Arabidopsis wild-type (Col-0) (C). Root elongation was expressed relative to root elongation in the control solution (0.5 mM CaCl2, pH 4.5). Data are means ±SE of >8 roots.
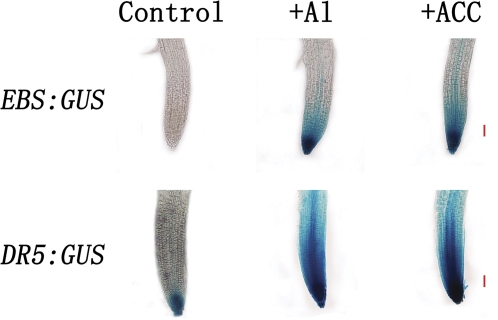
Al3+ stimulated the activity of EBS:GUS and DR5:GUS
To investigate the mechanism by which Al3+ affects the synthesis and distribution of ethylene and auxin, the expression levels of the ethylene reporter construct, EBS:GUS, in which the GUS reporter gene is driven by a synthetic EIN3-responsive promoter, were first tested. In the absence of Al3+ or ACC, no visible expression of EBS:GUS in root tips was observed (Fig. 5). Upon exposure to Al3+, there was a marked increase in the activity of EBS:GUS in the root apices (Fig. 5). A comparable increase in EBS:GUS activity was also observed when roots were treated with the ethylene synthesis precursor ACC (Fig. 5). In addition to EBS:GUS activity, the response of the auxin reporter DR5:GUS to Al3+ and ACC was also investigated. In control roots, DR5:GUS was mainly expressed in the quiescent zones and surrounding columella cells in root apices (Fig. 5). Treatment with Al3+ and ACC enhanced the levels of DR5:GUS in these areas as well as in the transition zone (Fig. 5). The similarity in response of DR5:GUS expression to Al3+ and ACC highlights cross-talk between ethylene and auxin in the Al-induced arrest of root elongation.
Fig. 5.
Effect of Al3+ and ACC on expression of the ethylene reporter EBS:GUS and the auxin reporter DR5:GUS after 2 h incubation in the incubating solutions with and without 50 μM AlCl3 (pH 4.5) and 10 μM ACC. The bar is 50 μm. The images are representatives of at least three independent experiments with >8 seedlings examined for each experiment.
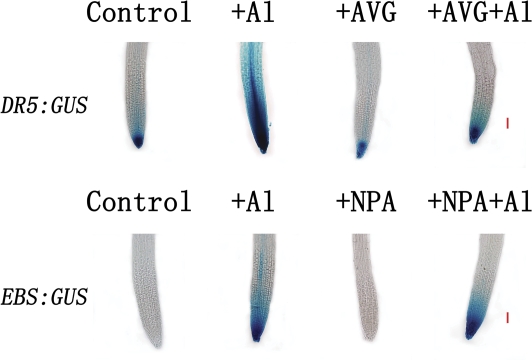
To unravel the relationship between the Al-induced increases in the activities of EBS:GUS and DR5:GUS, the effect of an ethylene synthesis inhibitor (AVG) and an auxin polar transporter inhibitor (NPA) on Al-dependent DR5:GUS and EBS:GUS expression, respectively, was investigated. As shown in Fig. 6, the Al-induced increase in activity of DR5:GUS was reduced by AVG. In contrast, NPA appeared to have a limited effect on the Al-induced increase in EBS:GUS activity (Fig. 6). These results are indicative that disruption of auxin distribution may result from elevated ethylene production evoked by Al3+. In addition, AVG may also alter auxin distribution in the absence of Al3+, as evidenced by AVG reducing DR5:GUS activity in roots without exposure to Al3+ (Fig. 6).
Fig. 6.
Effect of AVG and NPA on Al-induced activity of DR5:GUS and EBS:GUS. Arabidopsis seedlings were incubated in solution containing 10 μM AVG or 10 μM NPA for 12 h and thereafter the seedlings were incubated in 50 μM AlCl3 solution (pH 4.5) for 2 h. The bar is 50 μm. The images are representatives of at least three independent experiments with >8 seedlings examined for each experiment.
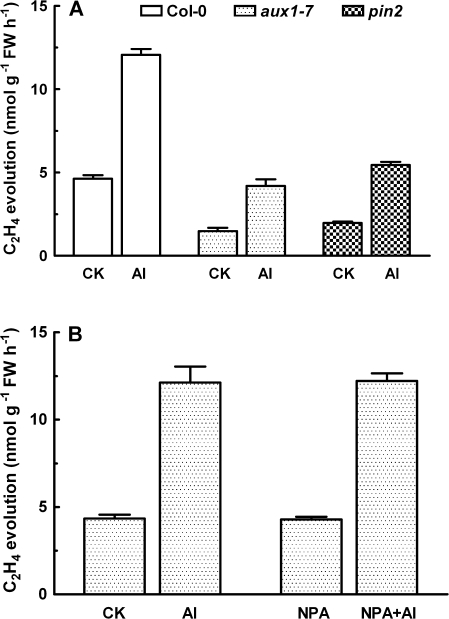
To test whether Al-induced disruption of auxin distribution in root apices occurs through Al-elicited ethylene production, the effect of Al3+ on ethylene production in aux1-7 and pin2 was studied. Ethylene evolution from the two auxin-insensitive mutants was lower than that in their wild-type counterpart in the absence Al3+ (Fig. 7A). Despite the lower basal levels of ethylene in the two mutants, both mutants exhibited increases in ethylene production when exposed to Al3+ (Fig. 7A). For instance, ethylene evolution was increased by 221, 290, and 262% in response to a 2 h exposure to Al3+ in the wild-type, aux1-7 and pin2 mutants, respectively. Moreover, it was found that NPA had no effect on Al-dependent ethylene evolution from root tips of wild-type seedlings (Fig. 7B).
Fig. 7.
Effect of Al3+ on ethylene evolution from root tips of Arabidopsis wild-type (Col-0) and auxin polar transport mutants, aux1-7 and pin2. (A) Both wild-type and mutants seedlings were treated with 50 μM AlCl3 (pH 4.5) for 2 h and the ethylene evolution from excised root tips (∼5 mm) was determined. (B) Effect of 10 μM NPA on Al-induced increase of ethylene evolution from root tips ofthe Arabidopsis wild type (Col-0). Seedlings of Col-0 were treated with 10 μM NPA solution for 12 h and then incubated in 50 μM AlCl3 (pH 4.5) for another 2 h. Values are means ±SE of five replicates.
Al3+ up-regulated expression of ASC, ACO, AUX1, PIN1, and PIN2
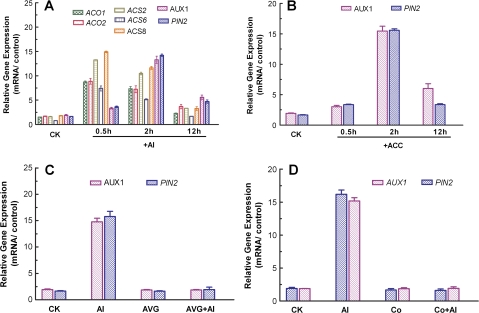
It was previously demonstrated that Al-induced ethylene evolution in L. japonicas roots is due to up-regulation of genes encoding ACS and ACO (Sun et al., 2007). To confirm whether a similar mechanism accounts for ethylene evolution in Arabidopsis roots in response to Al3+, the effect of Al3+ on expression of AtACS and AtACO was investigated by quantitative RT-PCR. In Arabidopsis, there are 12 ACS genes that encode eight functional ACS proteins (Tsuchiasaka and Theologis, 2004). It has been shown that AtACS2, AtACS6, and AtACS8 are highly expressed in roots and responsive to environmental stress and auxin (Tsuchiasaka and Theologis, 2004). Similar to AtACS, expression of both AtACO1 and AtACO2 was rapidly up-regulated in response to Al3+ treatment (Fig. 8A). The Al-dependent up-regulation of these genes exhibited transient characteristics such that the expression peaked after exposure to Al3+ for 30 min and thereafter declined with exposure time (Fig. 8A). In addition to ACS and ACO, the effect of Al3+ on expression patterns of AtAUX1 and AtPIN2 was also studied. Unlike ACS and ACO, expression of AtAUX1 and AtPIN2 was increased marginally after exposure to Al3+ for 30 min, and the expression of these genes reached a maximum after 2 h exposure to Al3+ and was reduced markedly after 12 h exposure to Al3+ (Fig. 8A). A comparable up-regulation of AtAUX1 and AtPIN2 was found in response to the ethylene synthesis precursor ACC (Fig. 8B), implying that Al-induced expression of AtAUX1and AtPIN2 may result from Al-induced ethylene evolution. To test this possibility, the responses of expression of AtAUX1 and AtPIN2 to AVG and Co2+ were further investigated. The transcriptional levels of AtAUX1 and AtPIN2 were not affected by either AVG or Co2+ in the absence of Al3+ (Fig. 8C, D). In contrast, the Al-induced increases in expression of AtAUX1and AtPIN2 genes were abolished by AVG and Co2+ (Fig. 8C, D).
Fig. 8.
Effect of Al3+ on expression of ACS, ACO, AUX1, and PIN2 of wild-type (Col-0) Arabidopsis seedlings (A). Expression of AtACS2, AtACS6, AtACS8, AtACO1, AtACO2, AtAUX1, and AtPIN2 was determined after exposure of Col-0 seedlings to 50 μM AlCl3 for varying durations (0.5, 2, and 12 h). Effect of ACC on expression of AtAUX1 and AtPIN2 of wild-type (Col-0) seedlings by exposing the seedlings to 10 μM ACC solutions for 0.5, 2, and 12 h (B). Effect of AVG and Co2+ on Al-induced up-regulation of AtAUX1 and AtPIN2 from roots of Arabidopsis wild-type (Col-0) by incubating the roots in solutions supplemented with 50 μM AlCl3 for 2 h followed by another 12 h incubation in 10 μM AVG or 10 μM CoCl2 (C, D). The relative mRNA level was normalized based on the mRNA in roots grown in 0.5 mM CaCl2 solutions. Data are means ±SE of three replicates.
Discussion
In a previous study, it was found that exposure of L. japonicus to Al3+ led to a rapid inhibition of root elongation and that the inhibition of root elongation was closely associated with the ethylene burst (Sun et al., 2007). In the present study, it was confirmed that a similar mechanism exists in Arabidopsis as evidenced by the following observations. Al3+ enhanced the expression of AtACS2, AtACS6, AtACS8, AtACO1, and AtACO2 genes (Fig. 8A). The enhanced expression of these genes would account for the observed Al-induced rapid ethylene production from root tips (Fig. 1B) and stimulation of EBS:GUS activity in the root apex (Fig. 5). In addition, the Al3+-induced inhibition of root elongation was remarkably alleviated in the presence of inhibitors of ethylene biosynthesis (AVG and Co2+) and an antagonist of ethylene perception (Ag+) (Fig. 4A–C). The responses of ethylene-insensitive mutants to Al3+ were further analysed and it was found that root elongation was less inhibited by Al3+ in the ethylene-insensitive mutants (etr1-3 and ein2-1) than in the wild-type plants (Fig. 3). The etr1-3 mutant has a reduced ethylene response due to the dominant-negative versions of the membrane ethylene receptor (O'Malley et al., 2005). The ein2-1 mutant is also insensitive to ethylene, but the biochemical function of EIN2 remains to be characterized (Alonso and Stepanova, 2004). The lower sensitivity of etr1-3 and ein2-1 to Al3+ than the wild type could be explained by the Al3+-induced ethylene signal in these plants being unable to activate downstream targets that underpin root elongation. Thus these findings highlight the important role of ETR1 and EIN2 in Al-induced inhibition of root elongation in Arabidopsis. Taken together, these findings corroborate that induction of ethylene production is a critical event in Al-induced inhibition of root elongation in Arabidopsis.
In addition to ethylene, the results revealed that Al-induced inhibition of root elongation may also be associated with disruption of auxin distribution and/or signalling. For instance, it was found that Al3+ up-regulated expression of AtAUX1 and AtPIN2 (Fig. 8A). PIN2, which is localized predominantly in cortical cells, is a key component for mediating basipetal auxin transport, and plays a pivotal role in control of cell division and growth (Blilou et al., 2005). Shen et al. (2008) recently reported that Al3+ up-regulated PIN2 expression and inhibited transport of PIN2 vesicles from plasma membranes to endosomes in Arabidopsis, leading to reductions in auxin concentration in root apical cells. The AtAUX1 gene encodes a transmembrane protein and was believed to be associated with the influx of auxin across the plasma membrane (Swarup et al., 2001; Tanaka et al., 2006). Like PIN2, the transcriptional levels of AtAUX1 were also enhanced when exposed to Al3+ (Fig. 8A). This result indicates that, in addition to PIN2 (Shen et al., 2008), Al3+ may also target the AUX1-mediated auxin transport system, leading to disruption of auxin distribution in roots. Because PIN2 and AUX1 play critical roles in mobilizing auxin [indoleacetic acid (IAA)] between root apical cells and cells in the elongation zone (Swarup et al., 2007), the enhanced expression of AtPIN2 and AtAUX1 by Al3+ may account for the changes in DR5:GUS activity in both root apical and elongation zones (cf. Fig. 6). The changes in auxin distribution would in turn contribute to the observed Al-induced inhibition of root elongation. Future studies focusing on the spatial and temporal changes in auxin distribution in response to Al3+ will shed light on the role of auxin in Al phytotoxicity.
The aux1-7 mutant that has a single lesion in the auxin influx carrier AUX1 gene is insensitive to auxin and ethylene in terms of root growth (Pickett et al., 1990). PIN2 encodes an auxin efflux carrier protein, exhibiting asymmetric PIN2 distribution in the pin2 mutant and more protein degraded at the upper side of the gravistimulated root (Roman et al., 1995). The observation that root elongation in aux1-7 and pin2 mutants was less sensitive to Al3+ in terms of inhibition of root elongation than in wild-type plants (Figs 3, 4) is consistent with the involvement of AUX1 and PIN2 in Al-induced inhibition of root elongation. It has been shown that root elongation in aux1 and pin2 mutants is less sensitive to exogenous ACC than that of wild-type plants (Růžička et al., 2007). Moreover, both Al3+ and ACC induced a similar increase in expression of AtAUX1 and AtPIN2 (Fig. 8) and of DR5:GUS activity in roots (Fig. 6). These findings imply that the same mechanism may underlie the inhibitory effect of Al3+ and ethylene on root elongation. In maize, it has been shown that accumulation of IAA in the root elongation zone is reduced by Al3+, while the IAA content in root apical cells is enhanced by Al3+ (Kollmeier et al., 2000). The present finding that expression of AtAUX1 and AtPIN2 was enhanced by Al3+ may account for the Al-induced changes in IAA distribution in maize roots because these two proteins play important roles in mobilizing auxin distribution between root apical and elongating cells (Tanaka et al., 2006; Růžička et al., 2007). In the present study, it was observed that Al3+ enhanced DR5:GUS activity in both root apical and elongating cells (Fig. 5), suggesting that Al3+ alters patterns of auxin accumulation and distribution in roots. This may in turn contribute to the observed inhibition of root elongation. The auxin polar transport inhibitor, NPA, inhibited root elongation (Fig. 4D), whereas NPA was also effective in alleviating the Al-induced inhibition of root elongation (Fig. 4D). These findings may suggest that Al3+ and NPA have opposite effects on auxin distribution such that Al3+ stimulates auxin polar transport by up-regulating AtAUX1 and AtPIN2 expression. In this context, Doncheva et al. (2005) reported that treatment with Al3+ and NPA led to a rapid inhibition of cell division in maize roots probably by changing cell patterning. Unfortunately, the authors did not investigate the interactive effects of Al3+ and NPA on cell division and root elongation.
There is ample evidence demonstrating the synergistic effects of auxin and ethylene on root growth and development (Stepanova et al., 2005, 2007; Růžička et al., 2007; Swarup et al., 2007). In the present study, it was found that both ethylene and auxin were involved in Al-induced inhibition of root elongation. Attempts were therefore made to unravel the network associated with interactive effects of auxin and ethylene on root growth in the presence of toxic Al3+ using mutants defective in ethylene signalling and auxin polar transport, and inhibitors of ethylene synthesis and perception and of auxin polar transport. It was found that treatments with the ethylene synthesis precursor ACC and Al3+ induced comparable increases in DR5:GUS activity in Arabidopsis roots (Fig. 5), while the Al-dependent increases in DR5:GUS activity were substantially reduced by the ethylene synthesis inhibitor AVG (Fig. 6). On the other hand, the auxin polar transport inhibitor NPA had a marginal effect on Al-induced increases in EBS:GUS activity (Fig. 6) and Al-induced ethylene production (Fig. 7B). These results prompted the hypothesis that Al-induced ethylene production may act as a trigger to evoke changes in auxin distribution by affecting auxin polar transport systems such as AUX1 and PIN2. In the present study, it was found that the Al-induced up-regulation of ethylene synthesis genes (ACS and ACO) preceded the Al-induced up-regulation of the AUX1 and PIN2 genes (Fig. 8). In addition, both ACC and Al3+ induced comparable expression of AUX1 and PIN2 (Fig. 8A, B). The inhibitor of ethylene synthesis AVG abolished the Al3+-evoked up-regulation of AUX1 and PIN2 (Fig, 8D). Taken together, these observations indicate that disruption of auxin distribution by Al3+ is a downstream event of Al-induced ethylene production. This claim is also in line with the consensus that auxin synthesis, transport, and signalling are required for the ethylene-induced inhibition of root elongation in Arabidopsis (Růžička et al., 2007; Stepanova et al., 2007; Swarup et al., 2007).
In the present study, the effect of relatively low concentrations of Al3+ on root elongation was investigated using the wild type and mutants defective in ethylene signalling and auxin polar transport. It has been well documented that Al3+ can alter a myriad of biochemical and physiological processes (Matsumoto, 2000; Rengel and Zhang, 2003). Therefore, the inhibitory effect of Al3+ on root elongation, especially at relatively high Al3+ concentrations, may not be exclusively accounted for by the interaction of Al3+ with ethylene and auxin signalling cascades. The overall findings indicate that Al3+-induced ethylene may act as a trigger to inhibit root elongation by disrupting auxin distribution in roots. However, the possibility cannot be ruled out that Al3+ may interact with auxin in an ethylene-independent manner. The disruption of auxin may in turn affect ethylene-dependent root growth (Stepanova et al., 2007). This possibility may also account for the observation that Al3+ elicited less ethylene evolution from root apices of aux1-7 and pin2 than wild-type plants (Fig. 7).
In summary, we demonstrated that Al3+-induced inhibition of root elongation was positively correlated with ethylene production in Arabidopsis root tips, and that etr1-3 and ein2-1 mutants were insensitive to Al3+ when compared with wild-type plants. These results highlight the critical roles played by ETR1 and EIN2, two key proteins in ethylene signalling (Alonso and Stepanova, 2004), in Al toxicity. In addition to ethylene, it was found, by monitoring changes in DR5:GUS activity in Arabidopsis roots, that Al3+ disrupted auxin distribution in roots. The up-regulation of AtAUX1 and AtPIN2 expression by Al3+ and the greater tolerance of aux1-7 and pin2 mutants to Al3+ than wild-type plants suggest that AUX1 and PIN2 proteins are likely to be involved in Al3+-induced inhibition of root elongation. More importantly, we found that Al3+-induced ethylene evolution occurred very rapidly and that Al3+-induced up-regulation of ACS and ACO preceded Al3+-induced expression of AUX1 and PIN2. The up-regulation of AtAUX1 and AtPIN2 expression by Al3+ was mimicked and abolished by ACC and AVG, respectively. These findings indicate that Al-induced ethylene evolution may serve as a signal to elicit downstream changes in auxin distribution in roots by interacting with AUX1 and PIN2 proteins, leading to inhibition of root elongation in the presence of toxic Al3+.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30800706, 30788003, and 90817011). We thank Dr J Alonso at North Carolina State University for providing seeds of the EBS–GUS reporter lines which were generated by Dr Anna Stepanova in Dr Joe Ecker's lab.
References
- Alonso JM, Stepanova AN. The ethylene signaling pathway. Science. 2004;306:1513–1515. doi: 10.1126/science.1104812. [DOI] [PubMed] [Google Scholar]
- Barcelo J, Poschenrieder C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Envrionmental and Experimental Botany. 2002;48:75–92. [Google Scholar]
- Benjamins R, Malenica N, Luschnig C. Regulating the regulator: the control of auxin transport. BioEssays. 2005;27:1246–1255. doi: 10.1002/bies.20322. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Gren HG, May ST, Ward SP, Milner PA, Walker AR, Schultz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aid M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Doncheva S, Amenos M, Poschenrieder C, Barcelo J. Root cell patterning: a primary target for aluminium toxicity in maize. Journal of Experimental Botany. 2005;56:1213–1220. doi: 10.1093/jxb/eri115. [DOI] [PubMed] [Google Scholar]
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Communications in Soil Science and Plant Analysis. 1988;19:959–987. [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Zeitschrift für Pflanzenernährung und Bodenkunde. 1995;158:419–428. [Google Scholar]
- Illes P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluska F, Ovecka M. Aluminium toxicity in plants: internalization of aluminum into cells of the transition zone in Arabidopsis root apices relates to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. Journal of Experimental Botany. 2006;57:4201–4213. doi: 10.1093/jxb/erl197. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:283–307. [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiology. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen J-P. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down regulated and uncoupled from differentiation. Plant Physiology. 2001;125:519–522. doi: 10.1104/pp.125.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Massot N, Nicander B, Barcelo J, Poschenrieder CH, Tillberg E. A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+-induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.) Plant Growth Regulation. 2002;37:105–112. [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- May HM, Nordstrom DK. Assessing the solubilities and reaction kinetics of aluminous minerals in soils. In: Ulrich B, Sumner ME, editors. Soil acidity. Berlin: Springer Verlag; 1991. pp. 125–148. [Google Scholar]
- O'Malley RC, Rodriguez FL, Esch JJ, Binder BM, O'Donnell P. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. The Plant Journal. 2005;41:651–659. doi: 10.1111/j.1365-313X.2004.02331.x. [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends in Plant Science. 2005;10:170–177. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The AUX1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiology. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z, Zhang WH. Role of dynamics of intracellular calcium in aluminum toxicity syndrome. New Phytologist. 2003;159:295–314. doi: 10.1046/j.1469-8137.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell. 2007;19:2197–212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany. 1993;44:437–446. [Google Scholar]
- Shen H, Hou NY, Schlicht M, Wan YL, Baluska F. Aluminum toxicity targets PIN2 in Arabidopsis root apices: effects on PIN2 endocytosis vesicular recycling, and polar auxin transport. Chinese Science Bulletin. 2008;53:2480–2487. [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiology. 1998;116:155–163. [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. The Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis root. The Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Tian QY, Zhao MG, Dai XY, Huang JH, Li LH, Zhang WH. Aluminum-induced ethylene production is associated with inhibition of root elongation in Lotus japonicus L. Plant and Cell Physiology. 2007;48:1229–1335. doi: 10.1093/pcp/pcm077. [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes and Development. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HMO, Haseloff J, Beemster GTS, Bhalerao R, Bennett MJ. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature Cell Biology. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene regulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cellular and Molecular Life Sciences. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun DH, Zhao MG, Zhang WH. Inhibition of nitric oxide synthase (NOS) underlies aluminium-induced inhibition of root elongation in Hibiscus moscheutos L. New Phytologist. 2007;174:322–331. doi: 10.1111/j.1469-8137.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A. Unique and overlapping expression pattern among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiology. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/lAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. The Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Rengel Z. Aluminum induces an increase in cytoplasmic calcium in intact wheat root apical cells. Australian Journal of Plant Physiology. 1999;26:401–409. [Google Scholar]