Abstract
Ascorbic acid (AA) is an antioxidant fulfilling a multitude of cellular functions. Given its pivotal role in maintaining the rate of cell growth and division in the quiescent centre of the root, it was hypothesized that the AA-deficient Arabidopsis thaliana mutants vtc1-1, vtc2-1, vtc3-1, and vtc4-1 have altered root growth. To test this hypothesis, root development was studied in the wild type and vtc mutants grown on Murashige and Skoog medium. It was discovered, however, that only the vtc1-1 mutant has strongly retarded root growth, while the other vtc mutants exhibit a wild-type root phenotype. It is demonstrated that the short-root phenotype in vtc1-1 is independent of AA deficiency and oxidative stress. Instead, vtc1-1 is conditionally hypersensitive to ammonium (NH4+). To provide new insights into the mechanism of NH4+ sensitivity in vtc1-1, root development, NH4+ content, glutamine synthetase (GS) activity, glutamate dehydrogenase activity, and glutamine content were assessed in wild-type and vtc1-1 mutant plants grown in the presence and absence of high NH4+ and the GS inhibitor MSO. Since VTC1 encodes a GDP-mannose pyrophosphorylase, an enzyme generating GDP-mannose for AA biosynthesis and protein N-glycosylation, it was also tested whether protein N-glycosylation is affected in vtc1-1. Furthermore, since root development requires the action of a variety of hormones, it was investigated whether hormone homeostasis is linked to NH4+ sensitivity in vtc1-1. Our data suggest that NH4+ hypersensitivity in vtc1-1 is caused by disturbed N-glycosylation and that it is associated with auxin and ethylene homeostasis and/or nitric oxide signalling.
Keywords: Ammonium (NH4+), Arabidopsis thaliana, ascorbic acid (AA), auxin (IAA), GDP-mannose pyrophosphorylase (GMPase), glutamine synthetase (GS), N-glycosylation, root growth
Introduction
Vitamin C (L-ascorbic acid, AA) is an essential antioxidant in plants and animals. It protects against oxidative stress caused by adverse environmental conditions and functions in various aspects of plant cell growth (Cordoba and Gonzalez-Reyes, 1994). Most notably, AA plays a pivotal role in maintaining the rate of cell growth and division in the quiescent centre of the root (Kerk and Feldman, 1995), in the transition from the G1 to the S phase in the cell cycle. Actively dividing root meristem cells have high levels of AA and glutathione compared to cells in the quiescent zone. Exogenous application of AA and glutathione stimulates cell division and growth (Kerk and Feldman, 1995). Furthermore, redox activity (Sanchez-Fernandez et al., 1997) and the formation of reactive oxygen species (Shin and Schachtman, 2004) are known to influence root elongation through cell proliferation in response to nutrient alterations.
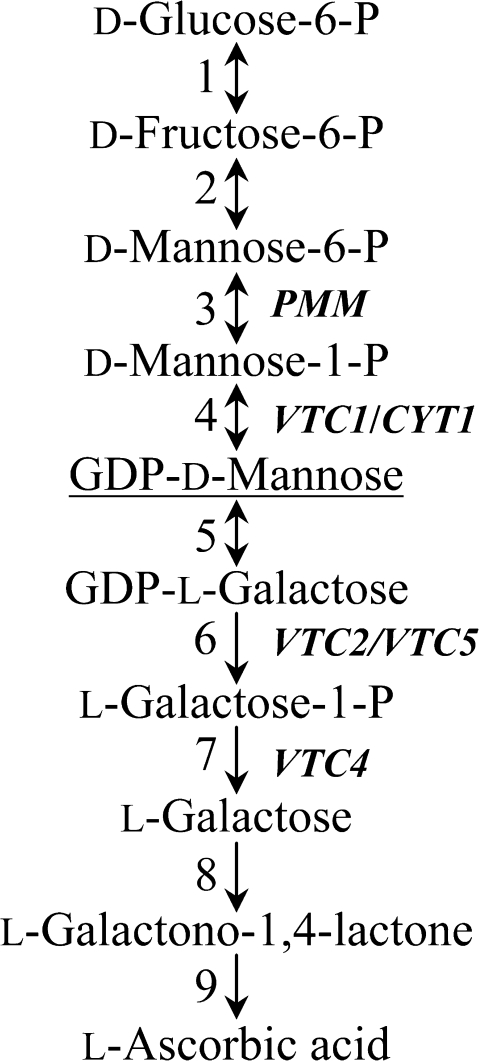
To investigate the physiological role of AA in root development, advantage was taken of the availability of AA-deficient Arabidopsis thaliana vtc mutants. Five vtc mutant lines, vtc1, vtc2, vtc3, vtc4, and vtc5 have been isolated so far (Conklin et al., 2000; Dowdle et al., 2007). Except for vtc3, whose genetic defect is still unknown, the vtc mutants were shown to contain mutations in genes encoding enzymes in the predominant L-galactose/D-mannose AA biosynthetic pathway (Fig. 1; Wheeler et al., 1998). The VTC1 gene encodes a GDP-mannose pyrophosphorylase (GMPase; Conklin et al., 1999). VTC2 and VTC5 encode a GDP-L-galactose phosphorylase (Dowdle et al., 2007), while VTC2 also has GDP-L-galactose orthophosphate guanylyltransferase activity (Linster et al., 2007, 2008; Laing et al., 2007). VTC4 encodes an L-galactose-1-phosphate phosphatase (Laing et al., 2004; Conklin et al., 2006). The vtc mutants contain between 25% and 50% of the wild-type leaf AA content (Conklin, 2001).
Fig. 1.
Simplified representation of the D-mannose/L-galactose L-ascorbic acid biosynthetic pathway in higher plants. Enzymes are (1) phosphoglucose isomerase, (2) phosphomannose isomerase (3), phosphomannose mutase (PMM), (4) GDP-mannose pyrophosphorylase (VTC1), (5) GDP-mannose-3′,5′-epimerase, (6) GDP-L-galactose phosphorylase (VTC2/VTC5), (7) L-galactose-1-phosphate phosphatase (VTC4), (8) L-galactose dehydrogenase, (9) L-galactono-1,4-lactone dehydrogenase. (Adapted from Dowdle et al., 2007.)
Given the pivotal function of AA in root development, we hypothesized that AA-deficient mutants have altered root growth. To test this hypothesis, the wild type and vtc mutants were grown on full-strength Murashige and Skoog (MS) medium and it was discovered that only the vtc1-1 mutant has strongly retarded root growth, while the other vtc mutants exhibited a root developmental phenotype similar to the wild type. Thus, the goal of this study was to elucidate the mechanism through which root growth is inhibited in vtc1-1. Interestingly, our investigations demonstrate that vtc1-1 is conditionally hypersensitive to ammonium (NH4+) and that the short-root phenotype in the mutant is independent of AA deficiency and the accumulation of H2O2. Therefore, we focused on investigating NH4+ metabolism and hormone homeostasis in vtc1-1 to better understand root growth inhibition by NH4+ in vtc1-1.
NH4+ and nitrate are the major sources of nitrogen for plants. NH4+ is the preferred nitrogen source with concentrations ranging from 20–200 μM in agricultural soils (Loque and von Wiren, 2004; Miller and Cramer, 2004). It is assimilated into essential amino acids. However, excess amounts of NH4+ may be toxic and inhibit root growth (Cao et al., 1993; Britto and Kronzucker, 2002; Cruz et al., 2006). In order to understand the mechanism of NH4+ toxicity in plants, several aspects of NH4+ uptake, transport, metabolism, and interactions with plant hormones have been studied, particularly with respect to root development.
NH4+ is directly taken up from the soil at the root surface and hairs of the rhizodermis and immediately transferred into the root symplast for NH4+ assimilation by rhizodermis-localized glutamine synthetase (Ishiyama et al., 2004). Non-assimilated NH4+ might be stored in the vacuole (Miller et al., 2001) or further transported via the symplast into the root stele for assimilation or loading into the xylem where it may accumulate in millimolar concentrations (Yuan et al., 2007). The Casparian strip forms an apoplastic barrier, which favours a local accumulation of NH4+ in the endodermal apoplast (Marschner, 1995).
Transport of NH4+ is mediated by AMT-type transporters (Gazzarrini et al., 1999; Ludewig et al., 2001; Yuan et al., 2007). When nitrogen content is high, AMT genes are transcriptionally down-regulated, whereas they are up-regulated under deficiency conditions (Rawat et al., 1999). NH4+ uptake may also occur through high- or low- affinity transport systems (Rawat et al., 1999). To minimize NH4+ toxicity, NH4+-sensitive species have been reported to actively pump out NH4+ (Kronzucker et al., 2001).
Once inside the cell, NH4+ is metabolized by glutamine synthetase (GS) into glutamine (Gln), which is further metabolized by glutamate synthase (GOGAT) that converts Gln and 2-oxoglutarate into two molecules of glutamate. NH4+ may also be assimilated by glutamate dehydrogenase (GDH) that catalyses the formation of glutamate through reductive amination using NAD(P)H. Therefore, various studies have investigated the role of GS, GOGAT, and GDH in response to NH4+ nutrition. Plants with high GS activities are more tolerant to NH4+ (Magalhaes et al., 1992; Glevarec et al., 2004). Arabidopsis GS isoforms have different kinetic properties and are differentially regulated by NH4+ (Ishiyama et al., 2004). The role of GDH in NH4+ metabolism is still a matter of debate, because GDH has low affinity to NH4+ (Harrison et al., 2000). Its main role is thought to recycle glutamate (Robinson, 2001) and to return carbon in amino acids back into reactions of carbon metabolism and the tricarboxylic acid cycle (Miflin and Habash, 2002). In fact, proper C/N balance and sufficient carbohydrates in the roots have been suggested to play an important role in preventing NH4+ toxicity (Oliveira and Coruzzi, 1999; Schjoerring et al., 2002).
Multiple studies suggest that nutrient signalling is intertwined with plant hormones (Feng and Barker, 1992; Cao et al., 1993; Barker, 1999b; Rubio et al., 2009). With regard to NH4+ nutrition, IAA (Cao et al., 1993; Sattelmacher and Thoms, 1995), cytokinin (Cao et al., 1993; Gerendas et al., 1997), and ethylene (Feng and Barker, 1992; Barker, 1999a) have been shown to be linked to NH4+-induced alterations in growth and development. The work by Cao et al. (1993) not only demonstrated that NH4+ inhibition of Arabidopsis root growth can be reversed by auxin resistance mutations, but can also be rescued by the addition of potassium.
In the present study, evidence is provided that the genetic defect in vtc1-1, causing defective protein N-glycosylation, results in pleiotropic alterations in NH4+ metabolism, hormone homeostasis, and nitric oxide content.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana L. Heynh wild-type ecotype Columbia (Col-0) and the previously described Arabidopsis mutants vtc1-1, vtc2-1, vtc3-1, and vtc4-1 (Conklin et al., 2000; mutant seeds kindly provided by Dr Patricia Conklin) were grown in a growth chamber (Percival, Perry, IA). Surface-sterilized seeds (see below) were grown on full-strength (1×) Murashige and Skoog (MS) or variations of the MS medium (Caisson Laboratories, Inc., North Logan, UT). All growth media contained 1% phytoblend agar (Caisson Laboratories, Inc.) and were adjusted to a pH of 5.7 with KOH before autoclaving. None of the growth media contained sucrose. Seeds were germinated in 120×80 mm omni trays (Fisher Scientific, Pittsburgh, PA), which were filled with 30 ml of medium and sealed with parafilm. Trays were placed vertically for 7 d or 14 d under long-day (16/8 h light/dark) conditions. Light intensity was 160 μmol photons m−2 s−1 (fluorescent bulbs) and the temperature in the chamber was 23 °C day and night.
To assess root growth in the soil, wild-type and vtc mutant plants were grown on Metromix 360 soil (BFG Supplies, Burton, OH) in flats containing 32 inserts. Otherwise, growth conditions were the same as above.
Seed-surface sterilization
Seeds were soaked for 1 min in 50% ethanol, followed by washing the seeds in 50% bleach plus 0.01% sodium dodecyl sulphate for 8 min. Finally, seeds were rinsed six times with sterile water and stored in 0.1% sterile phytoblend agar for 2 d at 4 °C (Weigel and Glazebrook, 2002).
Root length measurements
Seven days or 14 d after germination, primary root length was measured using a ruler.
Ascorbic acid content assay
The AA content was determined using the AA oxidase assay as described previously (Conklin et al., 1997).
Hydrogen peroxide content assay
Hydrogen peroxide production in whole seedlings of the wild type and vtc mutants (50–100 mg fresh tissue) was measured following a previously described protocol (Shin and Schachtman, 2004) using the Amplex red hydrogen peroxide/peroxidase assay kit (Invitrogen, Carlsbad, CA).
Nitric oxide content assay
Using liquid nitrogen, 20–50 mg of 7-d-old whole seedlings were ground in a Harbil paint shaker (Midwest Paint Equipment, Elwood, KS) and resuspended in 500 μl extraction buffer (0.1 mM CaCl2, 10 mM KCl, and 10 mM MES at a pH of 5.6). Samples were centrifuged at 4 °C at 10 000 rpm for 10 min. The supernatant was collected and used for the GRIESS assay according to the manufacturer's protocol (Invitrogen, Carlsbad, CA) with the following modifications: Equal volumes of Component A and Component B from the GRIESS reagent kit were mixed. To a 150 μl nitric oxide-containing sample, 130 μl of sterile water, and 20 μl of the GRIESS reagent (Component A and Component B) were added and mixed. The reaction mixture was incubated for 30 min at room temperature. Absorbance of each sample was recorded at 548 nm. The nitric oxide content was determined based on a standard curve of known sodium nitrite concentrations and normalized to the amount of fresh weight.
Ammonium and glutamine content assay
NH4+ content was determined in whole seedlings crushed in liquid nitrogen and tissue was extracted in 500 μl water or the extraction buffer used for the NO assay. Fifty microlitres of the supernatant were added to 50 μl of Nessler reagent. Absorbance was measured at 404 nm (Leleu and Vuylsteker, 2004). Concentration of NH4+ was determined using an NH4Cl standard curve and normalized to gram fresh weight of the sample. Glutamine content was determined using the L-asparagine, L-glutamine, ammonia Rapid Kit from Megazyme (Megazyme International Ireland Ltd, Co. Wicklow, Ireland).
Indole-3-acetic acid content assay
The IAA content was determined in whole seedlings grown on 1× MS and 1× MS lacking NH4NO3. Extraction of IAA from plant tissue was performed using 80% methanol as previously described (Tanaka et al., 2001). Methylated IAA was quantified using the Phytodetek IAA kit following the manufacturer's protocol (Agdia, Elkhart, IN).
Gutamine synthetase activity assay
Total glutamine synthetase activity was determined following a spectrophotometric assay (Mäck, 1995; Hirano et al., 2008). In brief, 30–60 mg tissue was extracted using 500 μl of an imidazole buffer consisting of 50 mM imidazole-HCl, 0.5 mM EDTA, 1 mM DTT, 10 mM MgSO4, and 1 mM phenylmethanesulphonyl fluoride. Fifty-five microlitres of sample were used in the enzyme reaction containing 20 μl of each of the following reagents: 80 mM imidazole-HCl, 8 mM MgSO4, 160 mM hydroxylamine-HCl, 4 mM ATP, and 8 mM glutamate. The reaction was allowed to run for 15 min at 37 °C and was terminated after 15 min by adding 45 μl of a termination buffer (670 mM HCl, 88 mM FeCl3, and 200 mM trichloracetic acid). A glutamine synthetase activity standard curve was prepared using the reaction end-product γ-glutamyl hydroxamate. Standards and samples were measured at both 498 nm and 540 nm with similar results.
Glutamate dehydrogenase activity assay
Enzyme activity was determined from the extract used for measuring GS activity. Aminating-GDH activity was assayed as previously described (Groat and Vance, 1981).
Exogenous application of pharmacological compounds
Growth media were supplemented with methione sulphoximine (MSO, a GS inhibitor), sodium nitroprusside (SNP, an NO donor), 2-(4-carboxyphenyl)4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (cPTIO, an NO inhibitor), indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylate (ACC, an ethylene precursor), tunicamycin (an inhibitor of N-glycosylation), D-mannose, GDP-D-mannose, or L-galactose. The final concentration of these compounds in the growth media is indicated in the figures.
Generation and identification of double mutants
The vtc1-1 mutant was crossed with the salicylic acid (SA)-deficient mutants pad4-1 and eds5-1, respectively (Weigel and Glazebrook, 2002). The F1 progeny of these crosses were allowed to grow and self-fertilize. The F2 progeny were screened for AA deficiency (Conklin et al., 2000). Genomic DNA was extracted from AA-deficient individuals, PCR products spanning the two respective mutations were generated, purified, and analysed by sequencing to verify the presence of the mutations. F3 and F4 plants of homozygous double mutants were used for experimental analyses.
RNA isolation, cDNA synthesis, and NOS gene expression analysis
Total RNA was extracted from whole seedlings using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Five micrograms of total RNA were subjected to reverse transcription using a first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA) and 10 pg of oligo dT primers. Two micrograms of cDNA were utilized for PCR using gene-specific primers for genes encoding nitric oxide synthase and TUBULIN, which served as an internal control, running 25 amplification cycles (linear range of amplification). PCR fragments were separated on 1% agarose gels containing ethidium bromide. Band intensities were quantified using ImageQuant 5.0 (Amersham Biosciences). Primer sequences were 5′-TCTGGGGAGGTCTCGTTAGGATTG-3′ (NOS-F), 5′-CTCGTGTTTTGCGGATTGGTTCA-3′ (NOS-R), 5′-CTCAAGAGGTTCTCAGCAGTA-3′ (TUB2-RT-F2), and 5′-TCACCTTCTTCATCCGCAGTT-3′ (TUB2-RT-R2).
Statistical analysis
Experiments were performed at least three times. Figures represent individual experiments. Data were expressed as mean values ±SE. P values were determined by Student's t test analysis.
Results
Among four different vtc mutant lines, only vtc1-1 has retarded root growth
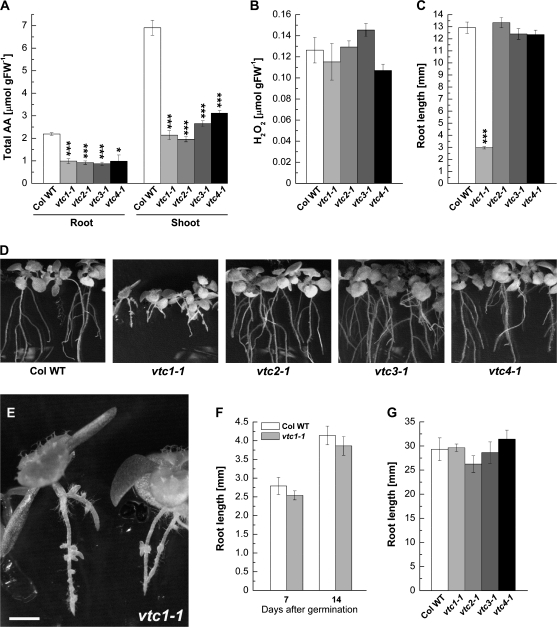
The genetic defect in the vtc mutants causes AA-deficiency to a similar degree in roots and to varying degrees in shoots. All vtc mutants contained approximately 45% of the wild-type root AA content, whereas the AA content of shoots was approximately 30–50% of that of the wild type (Fig. 2A). At this early developmental stage, H2O2 content was similar in whole seedlings of the wild type and vtc mutants (Fig. 2B). Similar results were reported previously (Kotchoni et al., 2009). Nevertheless, it was predicted that root growth would be inhibited in the vtc mutants given the function of AA in the root apical meristem (Kerk and Feldman, 1994). However, root growth on full-strength MS medium (without sucrose) was only affected in vtc1-1, while the vtc2-1, vtc3-1, and vtc4-1 mutants exhibited a root developmental phenotype similar to the wild type. The vtc1-1 mutants had four times shorter primary roots than the wild type and the other vtc mutants when plants were 7 d old (Fig. 2C). Additional developmental defects became apparent in 14-d-old seedlings. Whereas formation of lateral roots was normal in the vtc2-1, vtc3-1, and vtc4-1 mutants compared to the wild type, vtc1-1 mutants initiated lateral root primordia whose elongation was strongly retarded. Furthermore, vtc1-1 mutants initiated adventitious roots at the hypocotyl–root transition zone, which were not observed in the other genotypes (Fig. 2D, E). When wild-type and vtc1-1 mutant plants were germinated on 1× MS in darkness, root development was similar in both genotypes (Fig. 2F). Finally, root development was unaffected in vtc1-1 when plants were grown on soil (Fig. 2G).
Fig. 2.
Physiological characterization of the wild type and vtc mutants. (A) Ascorbic acid content in root and shoot tissue of 7-d-old seedlings grown on 1× MS. Mean values ±SE of three independent replicates are shown. (B) H2O2 content in whole 7-d-old seedlings of the wild type and vtc mutants. Results illustrate means ±SE of three independent replicates per genotype. (C) Primary root length in 7-d-old seedlings germinated on 1× MS. Data represent means ±SE of 53–70 replicates. (D) Phenotype of 14-d-old wild type and vtc mutant seedlings grown on 1× MS. (E) Close-up of the vtc1-1 root developmental phenotype. Bar, 1 mm. (F) Primary root length of wild-type and vtc1-1 mutant plants germinated on 1× MS medium in darkness. Data represent means ±SE of 21 replicates of the wild type and 24 replicates of vtc1-1. (G) Primary root length in 7-d-old wild-type and vtc mutant plants grown on soil. Means ±SE of 5–8 replicates are shown. Asterisks indicate significant differences between individual mutants and the wild type. *P <0.05, ***P <0.001, Student's t test.
Collectively, these data suggest that (i) inhibition of root development in vtc1-1 is not caused by AA-deficiency or oxidative stress; (ii) the root developmental defects in vtc1-1 are due to one or more nutrients present in the full-strength MS medium; and (iii) the short-root phenotype in vtc1-1 is light-dependent.
vtc1-1 is hypersensitive to NH4+
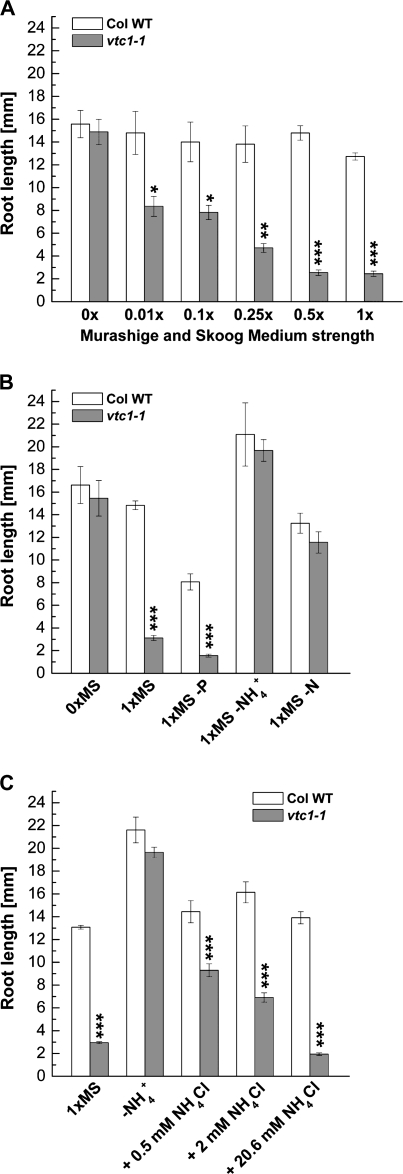
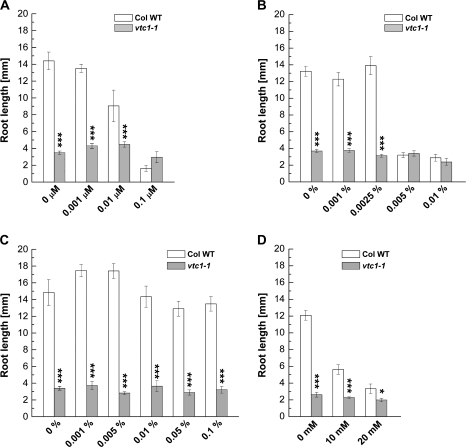
To determine whether the concentration of a nutrient in the MS medium could alter root growth in vtc1-1, wild-type and vtc1-1 mutant plants were grown on increasing strength of MS medium and primary root length was measured. In the absence of any nutrients (i.e. seedlings grown on agar), primary root length was the same in the wild type and vtc1-1. With increasing concentrations of nutrients, root elongation was strongly inhibited in vtc1-1 in a dose-dependent manner, whereas root growth was only slightly affected in the wild type and the other three vtc mutants (Fig. 3A; see Supplementary Fig. S1 at JXB online). This result confirms that one or more nutrients has or have an inhibitory effect on root development in vtc1-1 when present at high concentrations.
Fig. 3.
Effect of various MS media compositions on primary root development in 7-d-old wild type and vtc1-1. (A) Primary root length when plants were grown on increasing strength of MS medium. Results illustrate means ±SE of 9–23 individual seedlings per genotype and treatment. (B) Primary root length of plants grown in the absence of phosphorous (–P), ammonium nitrate (–NH4+, with potassium nitrate still present) and in the absence of all nitrogen (–N, i.e. no potassium nitrate and no ammonium nitrate). Data represent means ±SE of 6–18 replicates. (C) Effect of increasing concentrations of ammonium chloride (NH4Cl) on root growth in plants grown on 1× MS medium lacking ammonium nitrate (–NH4+), but potassium nitrate still present. Mean values ±SE of 44–102 individual seedlings per genotype and treatment are shown. Asterisks indicate significant differences between mutant and wild type. *P <0.05, **P <0.01, ***P <0.001, Student's t test.
To identify this/these nutrient/s, wild type and vtc1-1 mutants were germinated on MS media lacking key nutrients. In the absence of phosphorous (P), roots of vtc1-1 mutants were still approximately five times shorter than those of the wild type (Fig. 3B). A similar difference was observed in full-strength 1× MS medium (cf. Fig. 2C), suggesting that the vtc1-1 short-root phenotype is independent of P nutrition. When seedlings were germinated in the absence of NH4+ (i.e., no NH4NO3, but KNO3 still present) or when seedlings were grown in the absence of all nitrogen (–N, i.e., the medium contains neither NH4NO3 nor KNO3), root length was similar in the wild type and vtc1-1 (Fig. 3B). These data suggest that the presence of a high concentration of NH4+ in 1× MS has an inhibitory effect on root development in vtc1-1 mutants.
To further support that vtc1-1 is hypersensitive to NH4+, wild-type and vtc1-1 mutant plants were germinated on 1× MS medium lacking NH4+ (i.e., no NH4NO3, but KNO3 present), increasing concentrations of NH4Cl were added, and root length was determined. With increasing concentrations of NH4+, root elongation was strongly inhibited in vtc1-1, and to a lesser degree in the wild type (Fig. 3C). Inhibition of primary root growth was similar in the presence of 20.6 mM NH4Cl, the concentration of NH4+ that is present in the full-strength MS medium used here. These data indicate that the effect of NH4+ in vtc1-1 is dose-dependent, an effect that has been reported previously in other plant species (Lasa et al., 2002; Dominguez-Valdivia et al., 2008). To ensure that the inhibitory effect on root development in vtc1-1 was not caused by an increased concentration of Cl− ions in the medium, a control experiment was performed in which wild-type and vtc1-1 mutant seedlings were grown in the absence of NH4+ and in the presence of increasing KCl concentrations. As expected, root development was not inhibited in the presence of high amounts of KCl (see Supplementary Fig. S2 at JXB online).
Finally, the effect of high amounts of NH4+ added to 1× MS devoid of all N was investigated. Increasing concentrations of NH4+ have an inhibitory effect on root growth in both the wild type and vtc1-1, although root growth was slightly more inhibited in the vtc1-1 mutant than in the wild type at higher concentrations (see Supplementary Fig. S3 at JXB online). Therefore, all experiments described in the following were carried out using media lacking NH4+, but containing KNO3. This did not alter root development in the wild type, allowing for better comparisons with vtc1-1 primary root development.
NH4+ content is not altered in vtc1-1, but glutamine synthetase activity and glutamine content are decreased
To understand the mechanism of the NH4+ hypersensitivity of vtc1-1, transcript levels of NH4+ transporters were examined, and NH4+ content and metabolism in the wild type and vtc1-1 were assessed. Wild type and vtc1-1 did not differ significantly in mRNA levels of the NH4+ transporters AMT1;1 and AMT2;1 when plants were grown in the presence or absence of NH4+ (data not shown). This was expected, because at higher concentrations (>1 mM), NH4+ transport is passive (Ullrich et al., 1984; Wang et al., 1993).
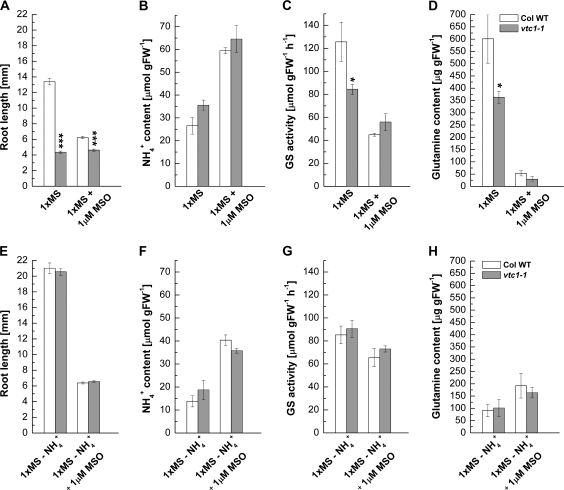
Once inside the cell, NH4+ is metabolized by GS and GDH. GS activity is inhibited by methionine sulphoximine (MSO), resulting in elevated NH4+ and reduced Gln levels (Lee et al., 1992; King et al., 1993; Hirano et al., 2008). This inhibitor was used to test whether NH4+ metabolism is altered in vtc1-1 compared to the wild type when plants were grown in the presence or absence of NH4+. As expected, in the presence of MSO, root growth was inhibited approximately 2-fold in the wild type, whereas root growth was not further impaired in vtc1-1 (Fig. 4A). In the absence of NH4+, MSO equally inhibited root growth in both genotypes (Fig. 4E). Whole seedling NH4+ content was similar in the wild type and vtc1-1 when seedlings were germinated on 1× MS. In the presence of MSO, NH4+ content doubled in both genotypes, as expected (Fig. 4B). A similar result was found when plants were grown in the absence of NH4+ (Fig. 4F). GS activity was approximately 30% lower in vtc1-1 compared to the wild type when grown on 1× MS. The GS inhibitor MSO decreased GS activity in the wild type approximately 3-fold, whereas the decrease in GS activity was less pronounced in vtc1-1 (Fig. 4C). GS activity was only slightly decreased by MSO in the absence of NH4+ (Fig. 4G). The Gln content was approximately 40% lower in vtc1-1 compared to the wild type when plants where grown on 1× MS. However, Gln content decreased dramatically in both genotypes in the presence of MSO (Fig. 4D). Gln content was significantly lower in both genotypes when grown in the absence of NH4+ and was not significantly diminished in the presence of MSO (Fig. 4H). Finally, assimilation of NH4+ by GDH was similar in the wild type and vtc1-1 (see Supplementary Fig. S4 at JXB online). Note, however, that MSO also inhibited GDH activity (see Supplementary Fig. S4A at JXB online).
Fig. 4.
Effect of methionine sulphoximine (MSO) on root development, ammonium (NH4+) content, glutamine synthetase (GS) activity, and glutamine content in whole 7-d-old seedlings of wild-type and vtc1-1 mutant plants. Plants were germinated on 1× MS in the absence and presence of ammonium nitrate (–NH4+) and MSO, respectively. (A, E) Primary root length. Mean values ±SE of 147–196 independent replicates are shown. (B, F) Ammonium content g−1 fresh weight. Data represent means ±SE of three independent replicates. (C, G) Mean glutamine synthetase activity of three independent replicates ±SE. (D, H) Glutamine content g−1 fresh weight. Means ±SE of three independent replicates are shown. Asterisks indicate significant differences between mutant and wild type. *P <0.05, ***P <0.001, Student's t test.
These data suggest that GS activity and Gln biosynthesis are negatively affected in vtc1-1 in the presence of NH4+. In the absence of NH4+, vtc1-1 behaves like the wild type and is capable of readjusting NH4+ metabolism, presumably through deamination processes. This result seems at first perplexing, given the fact that the genetic defect is of course also present when vtc1-1 is grown on media lacking NH4+. Thus, our data suggest that the mutation in GMPase in combination with high concentrations of NH4+ causes the root developmental defect in vtc1-1.
Effect of tunicamycin, mannose, GDP-mannose, and galactose on root growth in vtc1-1
To investigate whether the decreased GMPase activity in vtc1-1 (Conklin et al., 1999), resulting in lower levels of GDP-mannose and thus in disturbed protein N-glycosylation (Lukowitz et al., 2001), could be responsible for the root developmental defect in the mutant, wild-type and vtc1-1 mutant plants were treated with tunicamycin. This antibiotic inhibits N-glycosylation (Elbein, 1988). If decreased N-glycosylation causes root growth inhibition in vtc1-1, we predict that treatment of the wild type and the other vtc mutants, which are not affected in root growth in the presence of high NH4+, with tunicamycin will have an effect similar to that of the vtc1-1 mutation on root growth. Since vtc1-1 has a defect in the conversion of mannose to GDP-mannose, addition of GDP-mannose to the 1× MS growth medium should rescue the short-root phenotype in vtc1-1, while addition of mannose and galactose should not (Fig. 1).
Increasing concentrations of tunicamycin impair root elongation in the wild type, vtc2-1, vtc3-1, and vtc4-1 mutants, but not in vtc1-1. At a concentration of 0.1 μM, these four genotypes mimicked the vtc1-1 short-root phenotype (Fig. 5A; see Supplementary Fig. S5A at JXB online), suggesting that N-glycosylation is impaired in vtc1-1. Addition of mannose did not rescue the short-root phenotype in vtc1-1. Instead, high concentrations of mannose caused an inhibition in root elongation in the wild type, while root development in vtc1-1 was unchanged (Fig. 5B). An inhibitory effect of mannose on root growth has been reported previously (Lukowitz et al., 2001). Surprisingly, GDP-mannose did not rescue the vtc1-1 root developmental phenotype (Fig. 5C), even at high concentrations. It is possible that GDP-mannose was not taken up or was unstable in the medium. As expected, galactose did not rescue the short-root phenotype in vtc1-1. Instead, it also had an inhibitory effect on root development (Fig. 5D). Similar results were found when ascorbic acid was applied to the growth medium (data not shown).
Fig. 5.
Effect of the N-glycosylation inhibitor tunicamycin and ascorbic acid precursors on primary root growth in 7-d-old wild type and vtc1-1 mutants grown on 1× MS. (A) Effect of increasing concentrations of tunicamycin. Data display 7–11 replicates per genotype and treatment. (B) Effect of increasing concentrations of D-mannose. Means ±SE of 9–16 individual replicates per genotype are shown. (C) Effect of increasing concentrations of GDP-D-mannose. Results represent means ±SE of 9–14 individual replicates per genotype and treatment. (D) Effect of L-galactose. Date illustrate means ±SE of 8–11 individual seedlings per genotype. Asterisks indicate significant differences between mutant and wild type. *P <0.05, ***P <0.001, Student's t test.
Our results suggest that root growth inhibition in vtc1-1 is caused by a defect in N-glycosylation and not AA deficiency. Since defective N-glycosylation causes programmed cell death (Hauptmann and Lehle, 2008; Hoeberichts et al., 2008), it was assessed whether cell death was altered in vtc1-1. Roots of vtc1-1 grown on 1× MS exhibited enhanced cell death compared to the wild type (see Supplementary Fig. S5B at JXB online). However, only a few dead cells were detected when both genotypes were grown in the absence of NH4+ (see Supplementary Fig. S5C at JXB online). These results substantiate our findings that root growth inhibition in vtc1-1 is caused by defective N-glycosylation but only in combination with NH4+.
Effect of auxin, the ethylene precursor ACC, and salicylic acid on root growth in vtc1-1
The vtc1-1 mutant exhibits pleiotropic phenotypes when grown on soil. These include alterations in the content of plant hormones, such as abscisic acid, salicylic acid (SA), and gibberellic acid (Pastori et al., 2003; Barth et al., 2004; Foyer et al., 2007). Although not yet investigated, it is expected that ethylene content is affected as well, because AA serves as a co-factor in ethylene biosynthesis. While it is unknown whether IAA content is altered in vtc1-1, IAA (Cao et al., 1993; Sattelmacher and Thoms, 1995) and ethylene (Feng and Barker, 1992; Barker, 1999a) are linked to NH4+-induced alterations in growth and development. Furthermore, SA is known either to inhibit (Manthe et al., 1992) or to promote (Gutierrez-Coronado et al., 1998) root growth, although a direct link between SA and NH4+ sensitivity has not yet been established. Nevertheless, investigating the role of SA in vtc1-1 root development is of relevance, because vtc1-1 contains constitutively elevated levels of SA (Barth et al., 2004). Therefore, the effect of these three hormones was investigated to obtain clues on their role in vtc1-1 root development in the presence of NH4+.
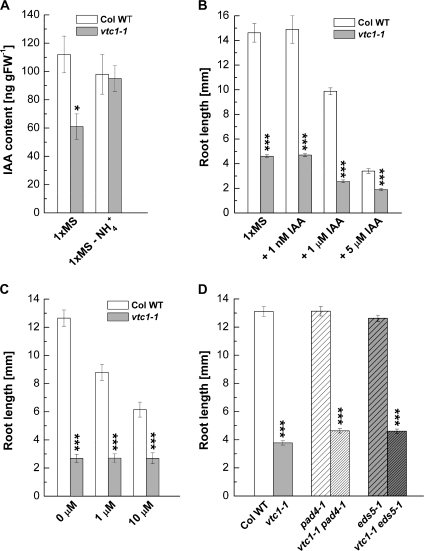
Since IAA plays a fundamental role in root development, it was predicted that IAA content is altered in vtc1-1 in the presence of NH4+, but unchanged in the absence of NH4+. In fact, IAA content was decreased by approximately 40% in vtc1-1 compared with the wild type when plants were grown on 1× MS. In the absence of NH4+, IAA content was similar in both genotypes (Fig. 6A). These data suggest that post-embryonic IAA biosynthesis and/or transport is affected in vtc1-1 in response to NH4+. To test whether exogenous IAA could complement the vtc1-1 short-root phenotype, IAA was added to the growth medium at 1 nM, at which root growth may be promoted, and at higher concentrations known to inhibit root elongation (Rahman et al., 2007). The addition of IAA at 1 nM did not promote root elongation, neither in the wild type nor in vtc1-1. By contrast, in the presence of 1 μM and 5 μM IAA, primary root growth was strongly inhibited in the wild type and to a lesser extent in vtc1-1 (Fig. 6B). However, IAA promoted the outgrowth and elongation of adventitious roots in the wild type and vtc1-1 (data not shown).
Fig. 6.
Auxin (IAA, indole-3-acetic acid) content, and effect of IAA, the ethylene precursor ACC, and salicylic acid (SA) on primary root growth in 7-d-old wild-type and vtc mutant plants grown on 1× MS. (A) Total IAA content. Means ±SE of three individual replicates per genotype are shown. (B) Primary root length in the presence of IAA. Data show means ±SE of 12–35 individual seedlings. (C) Effect of increasing concentrations of ACC. Results represent means ±SE of 8–11 individual replicates per genotype and treatment. (D) Primary root growth in SA biosynthesis mutants and double mutants deficient in AA and SA. Data illustrate means ±SE of 54–108 individual seedlings per genotype. Asterisks indicate significant differences between individual mutants and the wild type. *P <0.05, ***P <0.001, Student's t test.
Ethylene has been reported to influence primary root growth through IAA-dependent and -independent mechanisms (Ruzicka et al., 2007; Thomann et al., 2009). As illustrated in Fig. 6C, the ethylene precursor ACC had an inhibitory effect on root elongation in the wild type and the other vtc mutants (see Supplementary Fig. S6 at JXB online), but had no effect on vtc1-1. This suggests that vtc1-1 is insensitive to ACC.
If high SA contributes to root growth inhibition in vtc1-1 in the presence of high NH4+, it was predicted that double mutants of vtc1-1 and the SA-deficient pad4-1 and eds5-1 mutants would have roots like the wild type. The vtc1-1 eds5-1 and vtc1-1 pad4-1 double mutants are SA-deficient, containing SA levels similar to the pad4-1 and eds5-1 single mutants (Mukherjee et al., 2009). As shown in Fig. 6D, double mutants exhibited a root developmental phenotype similar to vtc1-1 single mutants, suggesting that the vtc1-1 short-root phenotype is independent of SA.
vtc1-1 contains elevated levels of NO when grown on high NH4+
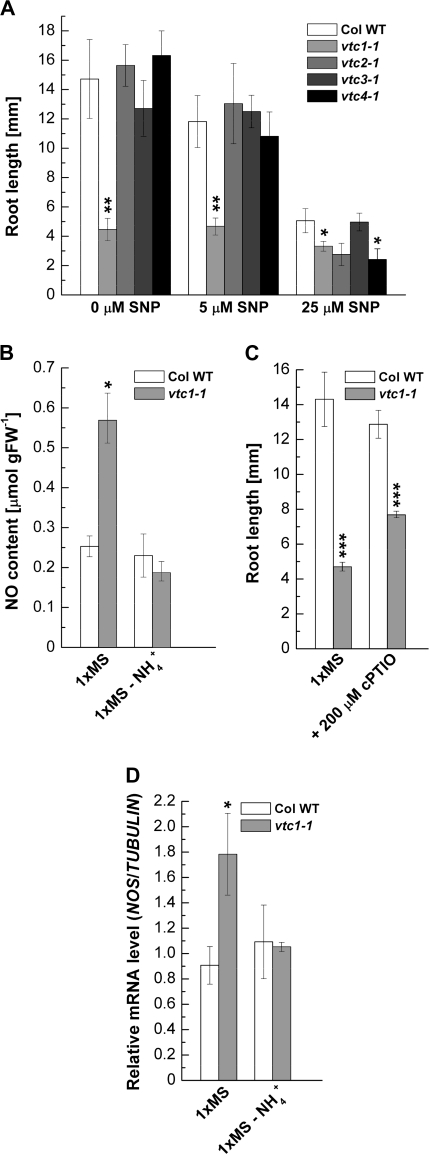
Inhibition of root elongation by high nitrate concentrations was suggested to result from a reduction of nitric oxide synthase-dependent endogenous NO levels in maize root apical cells (Zhao et al., 2007). However, a correlation between NH4+ nutrition and NO has not yet been established. Furthermore, NO is known to inhibit root elongation (He et al., 2004) and to promote adventitious rooting (Pagnussat et al., 2004), phenotypes that are present in vtc1-1 when grown on high NH4+ (Fig. 2C, D, E). Therefore, it was investigated whether NO affects root development in the NH4+-sensitive vtc1-1 mutant.
The response of the wild type and vtc mutants to exogenous NO was tested and the NO content was measured. With increasing concentrations of the NO donor, SNP, primary root growth was strongly inhibited in the wild type, vtc2-1, vtc3-1, and vtc4-1 seedlings, whereas SNP had no significant effect on the already short roots in vtc1-1 (Fig. 7A). This result suggests a high endogenous NO content in vtc1-1 compared with the wild type and the other vtc mutants. The NO content in the wild type and vtc1-1 in the presence of excess NH4+ and in the absence of NH4+ was, therefore, determined. As expected, the NO content was higher in vtc1-1 when grown on 1× MS, but decreased in the absence of NH4+ (Fig. 7B). To test whether this is an NO-specific response, it was investigated whether root growth inhibition can be rescued by the addition of the specific NO scavenger cPTIO. The short-root phenotype is partially but significantly (P <0.001) recovered in vtc1-1 in the presence of cPTIO (Fig. 7C), suggesting that NO contributes, in part, to root growth inhibition in vtc1-1 in the presence of high NH4+. Finally, it was investigated whether NO is synthesized via nitric oxide synthase (NOS) by assessing NOS transcript levels in the wild type and vtc1-1 in the presence and absence of NH4+. NOS mRNA levels were approximately 2-fold higher in vtc1-1 compared to the wild type in the presence of NH4+, whereas transcript levels were the same in both genotypes in the absence of NH4+ (Fig. 7D). These data correlate nicely with the NO content (Fig. 7C) and suggest that high concentrations of NH4+ promote the formation of NO in vtc1-1 and that NO contributes, in part, to the short-root phenotype in vtc1-1.
Fig. 7.
The role of nitric oxide (NO) in primary root development of the wild type and vtc mutants. (A) Effect of increasing concentrations of the NO donor SNP on primary root growth in 7-d-old plants grown on 1× MS medium. Data represent means ±SE of 6–12 individual seedlings per genotype and treatment. (B) NO content in the wild type and vtc1-1 in the presence of high NH4+ (1× MS) and in the absence of NH4+. Data represent means ±SE of three independent replicates. Asterisks indicate significant differences between the wild type and mutants. (C) Primary root growth in the presence of the specific NO scavenger cPTIO. Results show means ±SE of ten individual seedlings per genotype and treatment. (D) Relative NOS transcript levels in the presence and absence of NH4+. Results display means ±SE of four biological replicates of each genotype and treatment. *P <0.05, **P <0.01, ***P <0.001, Student's t test.
Discussion
vtc1-1 is conditionally hypersensitive to NH4+, a response that is independent of AA deficiency and oxidative stress
Since the isolation of the AA-deficient Arabidopsis vtc mutants (Conklin et al., 2000; Dowdle et al., 2007), multiple phenotypes of these mutants have been reported (Veljovic-Jovanovic et al., 2001; Pastori et al., 2003; Barth et al., 2004; Pavet et al., 2005; Kotchoni et al., 2009). Evidence is provided here that functional GMPase, which generates GDP-mannose for AA biosynthesis and protein N-glycosylation, is essential for root growth under high NH4+ conditions in Arabidopsis. The vtc1-1 mutant, containing a point mutation in GMPase, exhibits hypersensitivity to NH4+ (Fig. 3). The root growth inhibition in vtc1-1 cannot be explained by the AA deficiency or H2O2 levels in the mutant (Fig. 2A, B). This is supported by the following facts: First, when grown on full-strength MS medium, root development is only inhibited in vtc1-1, whereas three additional AA-deficient mutants with defects in other AA biosynthetic genes have normal root growth (Fig. 2; see Supplementary Fig. S1 at JXB online). In support of these results, previous reports have demonstrated that NH4+ sensitivity is independent of antioxidant redox status and antioxidant enzymes (Dominguez-Valdivia et al., 2008). Second, vtc1-1 mutants develop roots similar to the wild type when grown in the absence of NH4+ (Figs 3B, C, 4E; see Supplementary Figs S2, S3, and S5C at JXB online). Third, increasing the concentration of NH4+ causes strong root growth inhibition in vtc1-1 mutants, whereas root growth in the wild type (Fig. 3C) and the other vtc mutants is affected to a lesser extent. Together with the results of defective N-glycosylation (Fig. 5A; see Supplementary Fig. S5A at JXB online; Conklin et al., 1999; Lukowitz et al., 2001; Qin et al., 2008) and enhanced cell death (see Supplementary Fig. S5B at JXB online), these results suggest that the defect in N-glycosylation is responsible for root growth inhibition in vtc1-1. However, the N-glycosylation defect alone does not impair root development, unless it is combined with NH4+ stress (Fig. 3B, C; see Supplementary Fig. S5C at JXB online), indicating that the NH4+ hypersensitivity phenotype of vtc1-1 is conditional. Conditional mutants with defects in N-glycosylation and N-glycan maturation have been reported in Arabidopsis (Hoeberichts et al., 2008; Kang et al., 2008) and invertebrates (Sarkar et al., 2006; Paschinger et al., 2006; Koles et al., 2007). Recently, Hoeberichts et al. (2008) reported that a mutation in phosphomannose mutase (PMM), which catalyses the interconversion of mannose 6-phospate and mannose 1-phosphate, causes conditional temperature sensitivity. PMM acts directly upstream of VTC1 and also contributes to the formation of GDP-mannose and AA (Fig. 1). The authors provide evidence that pmm mutants exhibit cell death at restrictive temperatures due to a deficiency in protein glycosylation. Finally, GDP-mannose and protein glycosylation are also necessary for proper cell wall formation, as has been demonstrated for the cyt1 mutant (Lukowitz et al., 2001) and the glycosylation-deficient cgl1 mutant, which is more sensitive to salt stress (Kang et al., 2008). However, as to how cell wall formation is affected in vtc1-1 during NH4+ stress has not yet been investigated. Taken together, these results and our data strongly suggest that mutants with defects in the synthesis of mannose or GDP-mannose are disrupted in N-glycosylation, triggering cell death. This also explains why vtc2 and vtc4 mutants are not affected in root development, because VTC2 and VTC4 genes act downstream of GDP-mannose (Fig. 1). The VTC3 gene, which has not yet been identified, is most likely not involved in the formation of mannose or GDP-mannose.
Concurrently with our investigations, Qin and co-workers reported NH4+ sensitivity in the Arabidopsis mutant hsn1, which is allelic to vtc1-1 (Qin et al., 2008). In agreement with our results, the authors report that defective protein N-glycosylation in the roots, rather than decreased AA content, correlates with the hypersensitivity of the hsn1 and vtc1-1 mutants. The authors proposed that NH4+ inhibits GMPase activity and that defective protein N-glycosylation, the initiation of unfolded protein response, and cell death are downstream responses in the regulation of NH4+ sensitivity in Arabidopsis. These results are in line with our data. However, our study provides new information, suggesting that N-glycosylation functions beyond protein folding under NH4+ stress.
NH4+ assimilation via glutamine synthetase is altered in vtc1-1
N-glycosylation constitutes a major post-translational modification. Thus, defects in N-glycosylation affect membrane and secreted proteins (Strasser et al., 2004). Therefore, it was tested whether NH4+ transport and content may be altered in vtc1-1 under high NH4+ conditions. NH4+ transport through AMT-type transporters, which are distantly related to Rhesus glycoproteins (Ludewig et al., 2007), was not altered in vtc1-1 mutants (data not shown). This was expected, because AMTs are not involved in transport at high NH4+ concentrations (used in our experiments) and because AMT genes are transcriptionally down-regulated when nitrogen content is high and up-regulated under deficiency conditions (Rawat et al., 1999). Furthermore, at NH4+ concentrations greater than 1 mM, transport is passive (Ullrich et al., 1984; Wang et al., 1993). It is not clear whether NH4+ uptake is altered in vtc1-1, because electrophysiological experiments investigating passive or active NH4+ uptake were not conducted in this study. However, NH4+ content was the same in the wild type and vtc1-1 when grown in the presence or absence of NH4+ (Fig. 4B, F). Similar results were reported by Qin et al. (2008).
Once NH4+ has entered the cell, GS catalyses the ATP-dependent condensation of NH4+ and glutamate to yield glutamine, ADP, and inorganic phosphate. Plants with high GS activity have been reported to be more tolerant to NH4+ (Magalhaes et al., 1992; Glevarec et al., 2004). Our data suggest that total GS activity is stimulated in the wild type in the presence of NH4+ when compared to GS activity in the absence of NH4+. This, however, was not the case in vtc1-1 (compare Fig. 4C and G). NH4+ may have an inhibitory effect on GS activity (Ishiyama et al., 2004). Furthermore, other metabolites, including nitrate, amino acids, and carbohydrates have been shown to influence GS activity (Oliveira and Coruzzi, 1999; Lancien et al., 2000). We surmise that GS is not inhibited by NH4+ in vtc1-1, because NH4+ content is not elevated in the mutant. Thus, one may speculate that GS activity in vtc1-1 may be affected due to altered carbon availability in this mutant when NH4+ is in excess, as GS activity and Gln content in vtc1-1 are similar to the wild type in the absence of NH4+ (Fig. 4G, H). It is important to note that chloroplastic GS is induced by light, which is mediated by phytochrome (Oliveira and Coruzzi, 1999). It has been demonstrated that the conditional short-root phenotype in vtc1-1 in the presence of high NH4+ is light-dependent (Fig. 2F). However, it appears that light exerts an indirect effect on GS expression that may depend on efficient photosynthetic activity to produce carbohydrates (Melo-Oliveira et al., 1996). Thus, a more likely explanation for the low GS activity is that GS is not fully active due to the N-glycosylation deficiency in vtc1-1, which is enhanced by NH4+.
The role of hormones and NO in mediating NH4+ sensitivity in vtc1-1
Nutrient signalling involving different metabolites and hormones has been largely characterized in response to nutrient deficiencies or in response to nitrate but not NH4+ supply (Rubio et al., 2009). Investigating the role of hormones in the inhibition of primary root growth in vtc1-1 was important considering the fact that the mutant has altered levels of various hormones (Pastori et al., 2003; Barth et al., 2004; Foyer et al., 2007).
While no evidence was found for the involvement of SA in root growth inhibition (Fig. 6D), lower IAA levels were observed in vtc1-1 in the presence of NH4+ (Fig. 6A). To inhibit root growth, NH4+ must be blocking cell division and/or expansion. It is unlikely that NH4+ directly affects these processes. It is possible that the deficiency in N-glycosylation triggering the unfolded-protein response (Qin et al., 2008) inhibits protein synthesis, causing cell-cycle arrest, as has been reported in mammals (Harding et al., 1999; Brewer et al., 1999). This response may be mediated by IAA, of which a high concentration in the root tip is required for correct cell division, cell elongation, and final cell size (Blilou et al., 2005). In fact, NH4+ feeding led to a suppression of root IAA content (Kudoyarova et al., 1997). Exogenous application of IAA did not rescue the short-root phenotype in vtc1-1 (Fig. 6B). It is known that low concentrations of IAA may promote root growth, whereas higher concentrations inhibit root growth (Rahman et al., 2007). Thus, our result is not surprising. The mutant responds to exogenous IAA, but not as dramatically as the wild type does. The vtc1-1 mutant may have a defect in IAA distribution and/or conjugation. Note that only the amount of free IAA in seedlings has been measured. Since the vtc1-1 phenotype can be mimicked in the wild type with high exogenous IAA, it is concluded that IAA synthesis, transport, and/or signalling are disrupted during NH4+ treatment. This is supported by the fact that the IAA-resistant mutants aux1, axr1, and axr2 developed roots in the presence of 6 mM NH4+ (Cao et al., 1993). Thus, the mutants are resistant to NH4+ toxicity. Perhaps high levels of IAA have an inhibitory effect on root growth, as demonstrated by our IAA application experiments. Note, however, that the NH4+ sensitivity phenotype reported by Cao et al. (1993) may be caused by a different mechanism, as the authors report almost complete root growth inhibition in the wild type, which can be rescued by the addition of potassium. Root IAA predominantly derives from shoot tissues before 10 d after germination (Ljung et al., 2001; Bhalerao et al., 2002), with IAA accumulating in the root tip between 1 d and 3 d after germination (Bhalerao et al., 2002). Therefore, NH4+ may inhibit IAA distribution or promote IAA conjugation in vtc1-1, resulting in inhibited primary root growth and lateral root elongation (Fig. 2C, D, E; Blakely et al., 1988; Celenza et al., 1995; Casimiro et al., 2001; Fukaki and Tasaka, 2009). Furthermore, outgrowth of lateral root primordia may be inhibited by the high ABA content in vtc1-1 (Pastori et al. 2003; SO Kotchoni and C Barth, unpublished results), as ABA negatively regulates the emergence of lateral root primordia (De Smet et al., 2006).
While the outgrowth of lateral roots is suppressed, vtc1-1 mutants form adventitious roots (Fig. 2D, E), a process that requires IAA (Hausman et al., 1995; Guerrero et al., 1999) and light (Sorin et al., 2005). Since a lower content of free IAA was measured in vtc1-1 (Fig. 6A), it is not clear whether IAA is involved in the formation of adventitious roots in the mutant. It is possible that adventitious root growth in vtc1-1 is independent of IAA and instead mediated by NO, because NO content is high in vtc1-1 when grown on excess NH4+ (Fig. 7B). This hypothesis is supported by data demonstrating that NO is involved in the adventitious rooting process in cucumber with NO acting downstream of IAA (Pagnussat et al., 2004).
Finally, instead of NO or in addition to NO, ethylene may affect root development in vtc1-1, which is insensitive to ACC. The ethylene content in vtc1-1 has not yet been measured. A high endogenous ethylene content may inhibit expansion of cells leaving the root meristem (Le et al., 2001). It may also negatively impact lateral root formation by altering IAA transport (Negi et al., 2008) and stimulate adventitious root development (Clark et al., 1999). This would be in good agreement with our data.
In conclusion, we and a parallel study by Qin et al. (2008) identified GMPase as a genetic factor conferring conditional NH4+ hypersensitivity, resulting in root growth inhibition. This growth defect is not caused by AA deficiency or oxidative stress, but by a defect in N-glycosylation. Our data suggest that NH4+ sensitivity in vtc1-1 is linked to altered NH4+ metabolism, IAA, ethylene, and/or NO signalling. Future experiments evaluating NH4+ uptake, hormonal and cell cycle responses will aid in elucidating the mechanism of NH4+ sensitivity.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Root developmental phenotype in the wild type and vtc mutants grown on increasing strength of MS medium.
Supplementary Fig. S2. Root developmental phenotype in 7-d-old wild-type and vtc1-1 mutant plants grown on 1× MS in the absence of ammonium and increasing concentrations of potassium chloride.
Supplementary Fig. S3. Root developmental phenotype of 7-d-old wild-type and vtc1-1 mutant plants grown on 1× MS in the absence of all nitrogen and increasing concentrations of ammonium chloride.
Supplementary Fig. S4. Glutamate dehydrogenase activity in whole 7-d-old seedlings of the wild type and vtc1-1 grown on 1× MS in the presence and absence of ammonium and in the presence or absence of MSO, respectively.
Supplementary Fig. S5. Root developmental phenotype of 7-d-old wild-type and vtc mutant plants grown on 1× MS in the presence of increasing concentrations of tunicamycin and root cell death evaluation using Evan's blue staining.
Supplementary Fig. S6. Root developmental phenotype of 7-d-old wild-type and vtc mutant plants grown on 1× MS in the presence of increasing concentrations of the ethylene precursor ACC.
Supplementary Material
Acknowledgments
We thank Dr Patricia Conklin for providing vtc mutant seeds, Dr Rosana Schafer for providing a plate reader, and Dr Simeon Kotchoni for technical assistance during the initial phase of the project. This work was supported by a startup award by West Virginia University to CB.
Glossary
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylate
- cPTIO
2-(4-carboxyphenyl)4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- GDH
glutamate dehydrogenase
- Gln
glutamine
- MSO
methionine sulphoximine (MSO)
- MS
Murashige and Skoog
- NO
nitric oxide
- SNP
sodium nitroprusside
References
- Barker AV. Ammonium accumulation and ethylene evolution by tomato infected with root-knot nematode and grown under different regimes of plant nutrition. Communications in Soil Science and Plant Analysis. 1999a;30:175–182. [Google Scholar]
- Barker AV. Foliar ammonium accumulation as an index of stress in plants. Communications in Soil Science and Plant Analysis. 1999b;30:167–174. [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiology. 2004;134:1784–1792. doi: 10.1104/pp.103.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- Blakely LM, Blakely RM, Colowit PM, Elliott DS. Experimental studies on lateral root formation in radish seedling roots. II. Analysis of the dose–response to exogenous auxin. Plant Physiology. 1988;87:414–419. doi: 10.1104/pp.87.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proceedings of the National Academy of Sciences, USA. 1999;96:8505–8510. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology. 2002;159:567–584. [Google Scholar]
- Cao Y, Glass AD, Crawford NM. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiology. 1993;102:983–989. doi: 10.1104/pp.102.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes and Development. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiology. 1999;121:53–60. doi: 10.1104/pp.121.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, Cell and Environment. 2001;24:383–394. [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. Journal of Biological Chemistry. 2006;281:15662–15670. doi: 10.1074/jbc.M601409200. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences, USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Pallanca JE, Last RL, Smirnoff N. L-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant. vtc1. Plant Physiology. 1997;115:1277–1285. doi: 10.1104/pp.115.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba F, Gonzalez-Reyes JA. Ascorbate and plant cell growth. Journal of Bioenergetics and Biomembranes. 1994;26:399–405. doi: 10.1007/BF00762781. [DOI] [PubMed] [Google Scholar]
- Cruz C, Bio AFM, Dominguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Martins-Loucao MA. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta. 2006;223:1068–1080. doi: 10.1007/s00425-005-0155-2. [DOI] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inzé D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends in Plant Science. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dominguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Cruz C, Martins-Loucao MA, Moran JF. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and -sensitive plants. Physiologia Plantarum. 2008;132:359–369. doi: 10.1111/j.1399-3054.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Elbein AD. Glycoprotein processing and glycoprotein processing inhibitors. Plant Physiology. 1988;87:291–295. doi: 10.1104/pp.87.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Barker AV. Ethylene evolution and ammonium accumulation by tomato plants with various nitrogen forms and regimes of acidity. Journal of Plant Nutrition. 1992;15:2457–2469. [Google Scholar]
- Foyer CH, Kiddle G, Verrier P. Transcriptional profiling approaches to understanding how plants regulate growth and defence: a case study illustrated by analysis of the role of vitamin C. EXS. 2007;97:55–86. doi: 10.1007/978-3-7643-7439-6_3. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Molecular Biology. 2009;69:437–449. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wiren N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. The Plant Cell. 1999;11:937–948. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendas J, Zhu ZJ, Bendixen R, Ratcliffe RG, Sattelmacher B. Physiological and biochemical processes related to ammonium toxicity in higher plants. Zeitschrift für Pflanzenernährung und Bodenkunde. 1997;160:239–251. [Google Scholar]
- Glevarec G, Bouton S, Jaspard E, Riou MT, Cliquet JB, Suzuki A, Limami AM. Respective roles of the glutamine synthetase/glutamate synthase cycle and glutamate dehydrogenase in ammonium and amino acid metabolism during germination and post-germinative growth in the model legume. Medicago truncatula. Planta. 2004;219:286–297. doi: 10.1007/s00425-004-1214-9. [DOI] [PubMed] [Google Scholar]
- Groat RG, Vance CP. Root nodule enzymes of ammonia assimilation in alfalfa (Medicago sativa L.): developmental patterns and response to applied nitrogen. Plant Physiology. 1981;67:1198–1203. doi: 10.1104/pp.67.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero JR, Garrido G, Acosta M, Sanchez-Bravo J. Influence of 2,3,5-triiodobenzoic acid and 1- N-naphthylphthalamic acid on indoleacetic acid transport in carnation cuttings: relationship with rooting. Journal of Plant Growth Regulation. 1999;18:183–190. doi: 10.1007/pl00007068. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Coronado MA, Trejo-Lopez C, Larque-Saavedra A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiology and Biochemistry. 1998;36:563–565. [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harrison J, Brugiere N, Phillipson B, Ferrario-Mery S, Becker T, Limami A, Hirel B. Manipulating the pathway of ammonia assimilation through genetic engineering and breeding: consequences to plant physiology and plant development. Plant and Soil. 2000;221:81–93. [Google Scholar]
- Hauptmann P, Lehle L. Kex1 protease is involved in yeast cell death induced by defective N-glycosylation, acetic acid, and chronological aging. Journal of Biological Chemistry. 2008;283:19151–19163. doi: 10.1074/jbc.M801303200. [DOI] [PubMed] [Google Scholar]
- Hausman JF, Kevers C, Gaspar T. Auxin–polyamine interaction in the control of the rooting inductive phase of poplar shoots in vitro. Plant Science. 1995;110:63–71. [Google Scholar]
- He Y, Tang RH, Hao Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- Hirano T, Satoh Y, Ohki A, Takada R, Arai T, Michiyama H. Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiologia Plantarum. 2008;134:183–190. doi: 10.1111/j.1399-3054.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- Hoeberichts FA, Vaeck E, Kiddle G, et al. A temperature-sensitive mutation in the Arabidopsis thaliana phosphomannomutase gene disrupts protein glycosylation and triggers cell death. Journal of Biological Chemistry. 2008;283:5708–5718. doi: 10.1074/jbc.M704991200. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H. Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. Journal of Biological Chemistry. 2004;279:16598–16605. doi: 10.1074/jbc.M313710200. [DOI] [PubMed] [Google Scholar]
- Kang JS, Frank J, Kang CH, et al. Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proceedings of the National Academy of Sciences, USA. 2008;105:5933–5938. doi: 10.1073/pnas.0800237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk N, Feldman L. The quiescent center in the roots of maize: initiation, maintenance and role in organization of the root apical meristem. Protoplasma. 1994;183:100–106. [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- King BJ, Siddiqi MY, Ruth TJ, Warner RL, Glass A. Feedback regulation of nitrate influx in barley roots by nitrate, nitrite, and ammonium. Plant Physiology. 1993;102:1279–1286. doi: 10.1104/pp.102.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles K, Lim JM, Aoki K, Porterfield M, Tiemeyer M, Wells L, Panin V. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology. 2007;17:1388–1403. doi: 10.1093/glycob/cwm097. [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiology. 2009;149:803–815. doi: 10.1104/pp.108.132324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT, Davenport RJ, Tester M. Ammonium toxicity and the real cost of transport. Trends in Plant Science. 2001;6:335–337. doi: 10.1016/s1360-1385(01)02022-2. [DOI] [PubMed] [Google Scholar]
- Kudoyarova GR, Farkhutdinov RG, Veselov SY. Comparison of the effects of nitrate and ammonium forms of nitrogen on auxin content in roots and the growth of plants under different temperature conditions. Plant Growth Regulation. 1997;23:207–208. [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E. A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proceedings of the National Academy of Sciences, USA. 2004;101:16976–16981. doi: 10.1073/pnas.0407453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proceedings of the National Academy of Sciences, USA. 2007;104:9534–9539. doi: 10.1073/pnas.0701625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancien M, Gadal P, Hodges M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiology. 2000;123:817–824. doi: 10.1104/pp.123.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa B, Frechilla S, Aparicio-Tejo PM, Lamsfus C. Role of glutamate dehydrogenase and phosphoenolpyruvate carboxylase activity in ammonium nutrition tolerance in roots. Plant Physiology and Biochemistry. 2002;40:969–976. [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiology. 2001;125:519–522. doi: 10.1104/pp.125.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RB, Purves JV, Ratcliffe RG, Saker LR. Nitrogen assimilation and the control of ammonium and nitrate absorption by maize roots. Journal of Experimental Botany. 1992;43:1385–1396. [Google Scholar]
- Leleu O, Vuylsteker C. Unusual regulatory nitrate reductase activity in cotyledons of Brassica napus seedlings: enhancement of nitrate reductase activity by ammonium supply. Journal of Experimental Botany. 2004;55:815–823. doi: 10.1093/jxb/erh088. [DOI] [PubMed] [Google Scholar]
- Linster CL, Adler LN, Webb K, Christensen KC, Brenner C, Clarke SG. A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C. Covalent intermediate and substrate requirements for the conserved reaction. Journal of Biological Chemistry. 2008;283:18483–18492. doi: 10.1074/jbc.M802594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff–Wheeler pathway to ascorbic acid in plants. Journal of Biological Chemistry. 2007;282:18879–18885. doi: 10.1074/jbc.M702094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal. 2001;28:465–474. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- Loque D, von Wiren N. Regulatory levels for the transport of ammonium in plant roots. Journal of Experimental Botany. 2004;55:1293–1305. doi: 10.1093/jxb/erh147. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Neuhauser B, Dynowski M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Letters. 2007;581:2301–2308. doi: 10.1016/j.febslet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Ludewig U, von Wiren N, Rentsch D, Frommer WB. Rhesus factors and ammonium: a function in efflux? Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-3-reviews1010. REVIEWS1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences, USA. 2001;98:2262–2267. doi: 10.1073/pnas.051625798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäck G. Organ-specific changes in the activity and subunit composition of glutamine-synthetase isoforms of barley (Hordeum vulgare L.) after growth on different levels of NH4+ Planta. 1995;196:231–238. [Google Scholar]
- Magalhaes JR, Huber DM, Tsai CY. Evidence of increased 15N-ammonium assimilation in tomato plants with exogenous α-ketoglutarate. Plant Science. 1992;85:135–141. [Google Scholar]
- Manthe B, Schulz M, Schnabl H. Effects of salicylic acid on growth and stomatal movements of Vicia faba L.: evidence for salicylic acid metabolization. Journal of Chemical Ecology. 1992;18:1525–1539. doi: 10.1007/BF00993226. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1995. [Google Scholar]
- Melo-Oliveira R, Oliveira IC, Coruzzi GM. Arabidopsis mutant analysis and gene regulation define a non-redundant role for glutamate dehydrogenase in nitrogen assimilation. Proceedings of the National Academy of Sciences, USA. 1996;93:4718–4723. doi: 10.1073/pnas.93.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ, Habash DZ. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. Journal of Experimental Botany. 2002;53:979–987. doi: 10.1093/jexbot/53.370.979. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Cookson SJ, Smith SJ, Wells DM. The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. Journal of Experimental Botany. 2001;52:541–549. [PubMed] [Google Scholar]
- Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw B, Barth C. Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid and the NPR1 gene. Molecular Plant-Microbe Interactions. 2009 doi: 10.1094/MPMI-23-3-0340. in press. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Cramer MD. Root nitrogen acquisition and assimilation. Plant and Soil. 2004;274:1–36. [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira IC, Coruzzi GM. Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiology. 1999;121:301–310. doi: 10.1104/pp.121.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiology. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Hackl M, Gutternigg M, Kretschmer-Lubich D, Stemmer U, Jantsch V, Lochnit G, Wilson IB. A deletion in the golgi alpha-mannosidase II gene of Caenorhabditis elegans results in unexpected non-wild-type N-glycan structures. Journal of Biological Chemistry. 2006;281:28265–28277. doi: 10.1074/jbc.M602878200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. The Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiology. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, Wang D, Wu P. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2008;105:18308–18313. doi: 10.1073/pnas.0806168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. Auxin, actin and growth of the Arabidopsis thaliana primary root. The Plant Journal. 2007;50:514–528. doi: 10.1111/j.1365-313X.2007.03068.x. [DOI] [PubMed] [Google Scholar]
- Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD. AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. The Plant Journal. 1999;19:143–152. doi: 10.1046/j.1365-313x.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- Robinson D. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant and Soil. 2001;232:41–50. [Google Scholar]
- Rubio V, Bustos R, Irigoyen ML, Cardona-Lopez X, Rojas-Triana M, Paz-Ares J. Plant hormones and nutrient signaling. Plant Molecular Biology. 2009;69:361–373. doi: 10.1007/s11103-008-9380-y. [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proceedings of the National Academy of Sciences, USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M, Leventis PA, Silvescu CI, Reinhold VN, Schachter H, Boulianne GL. Null mutations in Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced life span. Journal of Biological Chemistry. 2006;281:12776–12785. doi: 10.1074/jbc.M512769200. [DOI] [PubMed] [Google Scholar]
- Sattelmacher B, Thoms K. Morphology and physiology of the seminal root system of young maize (Zea mays L.) plants as influenced by a locally restricted nitrate supply. Zeitschrift für Pflanzenernährung und Bodenkunde. 1995;158:493–497. [Google Scholar]
- Schjoerring JK, Husted S, Mack G, Mattsson M. The regulation of ammonium translocation in plants. Journal of Experimental Botany. 2002;53:883–890. doi: 10.1093/jexbot/53.370.883. [DOI] [PubMed] [Google Scholar]
- Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proceedings of the National Academy of Sciences, USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin C, Bussell JD, Camus I, et al. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. The Plant Cell. 2005;17:1343–1359. doi: 10.1105/tpc.105.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Altmann F, Mach L, Glossl J, Steinkellner H. Generation of Arabidopsis thaliana plants with complex N-glycans lacking beta1,2-linked xylose and core alpha1,3-linked fucose. FEBS Letters. 2004;561:132–136. doi: 10.1016/S0014-5793(04)00150-4. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Fujikawa Y, Aly MAM, Saneoka H, Fujita K, Yamashita I. Proliferation and rol gene expression in hairy root lines of Egyptian clover. Plant Cell Tissue and Organ Culture. 2001;66:175–182. [Google Scholar]
- Thomann A, Lechner E, Hansen M, Dumbliauskas E, Parmentier Y, Kieber J, Scheres B, Genschik P. Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms. PLoS Genetics. 2009;5:e1000328. doi: 10.1371/journal.pgen.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A. Ammonium uptake in Lemna gibba G-1, related membrane-potential changes, and inhibition of anion uptake. Physiologia Plantarum. 1984;61:369–376. [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiology. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass A. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiology. 1993;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Yuan L, Loque D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wiren N. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. The Plant Cell. 2007;19:2636–2652. doi: 10.1105/tpc.107.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DY, Tian QY, Li LH, Zhang WH. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Annals of Botany. 2007;100:497–503. doi: 10.1093/aob/mcm142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.