Abstract
Pearl millet, a key staple crop of the semi-arid tropics, is mostly grown in water-limited conditions, and improving its performance depends on how genotypes manage limited water resources. This study investigates whether the control of water loss under non-limiting water conditions is involved in the terminal drought tolerance of pearl millet. Two pairs of tolerant×sensitive pearl millet genotypes, PRLT 2/89-33–H77/833-2 and 863B-P2–ICMB 841-P3, and near-isogenic lines (NILs), introgressed with a terminal drought tolerance quantitative trait locus (QTL) from the donor parent PRLT 2/89-33 into H77/833-2 (NILs-QTL), were tested. Upon exposure to water deficit, transpiration began to decline at lower fractions of transpirable soil water (FTSW) in tolerant than in sensitive genotypes, and NILs-QTL followed the pattern of the tolerant parents. The transpiration rate (Tr, in g water loss cm−2 d−1) under well-watered conditions was lower in tolerant than in sensitive parental genotypes, and the Tr of NILs-QTL followed the pattern of the tolerant parents. In addition, Tr measured in detached leaves (g water loss cm−2 h−1) from field-grown plants of the parental lines showed lower Tr values in tolerant parents. Defoliation led to an increase in Tr that was higher in sensitive than in tolerant genotypes. The differences in Tr between genotypes was not related to the stomatal density. These results demonstrate that constitutive traits controlling leaf water loss under well-watered conditions correlate with the terminal drought tolerance of pearl millet. Such traits may lead to more water being available for grain filling under terminal drought.
Keywords: Drought stress, fraction of transpirable soil water, pearl millet, stomatal density, transpiration rate
Introduction
Water deficit is one of the major abiotic factors limiting crop productivity in the semi-arid tropics, and climate change is likely to make drought stresses even more severe in the future. Therefore, sustainable and equitable global food security is, at least in part, dependent on the development of crop plants with better adaptation to water-limited environments.
Under drought, the leaf gas exchange of plants is reduced and this leads to lower biomass accumulation and grain yield. Previous work in several crops shows genotypic differences in how leaf gas exchange responds to water stress, with certain genotypes being capable of sustaining plant transpiration until the soil becomes fairly dry, whereas others react with a decline in transpiration when the soil is still relatively wet. This has been documented in maize (Ray and Sinclair, 1997), soybean (Vadez and Sinclair, 2001; Hufstetler et al., 2007), and groundnut (Bhatnagar-Mathur et al., 2007). The relevance of either type of behaviour for performance under drought conditions in the field would depend on the pattern of drought: a decline in transpiration at high soil moisture would allow some water saving and would be beneficial in the case of long drought spells, but the related decrease in light capture and carbon fixation would eventually be reflected in a yield penalty in conditions of short drought spells. Therefore, the soil moisture threshold [the fraction of transpirable soil water (FTSW)] where transpiration declines is extremely useful to understand and forecast genotypic behaviour in the face of a water deficit (Sinclair and Ludlow, 1986; Sadras and Milroy, 1996; Ray and Sinclair, 1997).
One aspect of water management that is often overlooked relates to the control of the overall water loss at the leaf level when water is available. A conservative use of water, even if soil moisture is sufficient to supply plant water demand fully, would maintain water in the soil profile for a longer period of time and might be advantageous under conditions of a long drought spell and/or terminal drought. The control of leaf area (LA) and leaf conductance are the main factors determining plant water losses. At a given LA, stomata regulation is the prime actor for the control of that water loss. A low stomata conductance, which could be in part related to a difference in the stomatal density (SD), would probably confer such a conservative pattern of water use. Recent findings on genes involved in the regulation of transpiration efficiency in Arabidopsis shows that a single gene, ERECTA, is involved in the regulation of SD and mesophyll cell proliferation (Masle et al., 2005). Differences in stomatal conductance would have a direct impact on the gas exchange rate (Henson et al., 1983; Muchow and Sinclair 1989; Masle et al., 2005). Yet, in pearl millet there are limited data on the possible variation of that trait and how it can relate to differences in tolerance.
Stomatal conductance is heavily regulated, being influenced by many factors internal and external to the leaf. Stomatal conductance is not only linked to internal biochemical processes but is influenced by a range of physical factors such as the hydraulic conductance of xylem (Sperry et al., 2005) whose short-term variation can be explained by physicochemical processes such as cavitation (Salleo et al., 2001), wall collapse (Cochard et al., 2004), changes of water viscosity with temperature (Cochard et al., 2004), changes of wall permeability with sap chemical composition (Zwieniecky et al., 2001), or leaf architecture (Tsuda and Tyree, 2000; Sack et al., 2003). Yet, no clear relationship was established between stomatal conductance under well-watered conditions and tolerance to terminal drought. Since stomatal conductance is highly variable and is difficult to compare on many genotypes, methods are needed for comparing conductance of water in a way that is independent of environmental fluctuations.
Previous data (Black and Squire, 1979) showed that stomatal conductance in pearl millet was capable of adjustment in response to LA restriction or to a change in the LA ratio (LAR) (Henson and Mahalakshmi, 1985). In the study of Black and Squire (1979), a restriction of photosynthetically active LA led to an increased stomatal conductance of the remaining LA, showing the capacity of stomata to adjust to changes in LA. So pearl millet stomatal conductance and LA appear to be closely related. Thus, while work on pearl millet has focused on understanding how the leaf canopy develops to maximize water use (Bidinger and Hash, 2003), or considers the reduction of LA under drought as an adaptation (Wallace et al., 1993), it is argued here that it is important to study how both conductance and LA interact in the control of water loss.
Lines of pearl millet contrasting in yield under terminal drought conditions are known (Bidinger et al., 1987). Quantitative trait loci (QTLs) for that trait have been identified (Yadav et al., 2002) and confirmed in another genetic background (Yadav et al., 2004). Near-isogenic lines (NILs) containing a major terminal drought tolerance QTL on linkage group 2 have been generated and these lines have confirmed the role of the QTL in achieving a higher yield under terminal drought conditions (Serraj et al., 2005). The major effect of the QTL is to improve grain filling, but the underlying mechanisms are not known. Root growth under drought varies among these contrasting lines (Vadez et al., 2007). However, differences in how plants regulate their water loss have not been investigated.
The overall objective of the present study was to assess whether pearl millet genotypes varying for a QTL responsible for terminal drought tolerance differed in how plants controlled leaf water loss. Specific objectives were to: (i) compare whether these pearl millet genotypes differed in their response to a progressive exposure to water deficit; (ii) assess whether they differ in the regulation of their leaf water loss when water is non-limiting; and (iii) test how the rate of water loss changes upon alteration of the LA (LAR in cm2 g−1).
Materials and methods
Genetic material
Parental lines:
Two pairs of pearl millet (Pennisetum americanum L.) genotypes contrasting in tolerance under drought stress [PRLT 2/89-33 (tolerant) versus H77/833-2 (sensitive) and 863B-P2 (tolerant) versus ICMB 841-P3 (sensitive)] were selected for the study based on previous experiments (Yadav et al., 2004; Serraj et al., 2005). Tolerance/sensitivity was assessed on test-cross hybrids of these inbred parental lines, developed by crossing the inbred parental lines to male-sterile line tester 843A for PRLT 2/89-33 and H77/833-2 and to male-sterile line tester H77/833-2 for 863B-P2 and ICMB 841-P3 (Stegmeier et al., 1998). Tolerance of these hybrids was based on yield under terminal drought stress in several years of field trials and on the panicle harvest index (PNHI), an index that assesses the success of spikelet fertilization and the degree of grain filling (Bidinger et al., 1987). Sensitive genotypes H77/833-2 and ICMB 841-P3 are of North Indian origin and are heat-resistant parental genotypes of many commercial hybrids of this area. Tolerant genotypes PRLT 2/89-33 and 863B-P2 derive from the ICRISAT Bold Seeded Early Composite, which is an elite breeding population based on Iniadi landrace germplasm from West Africa.
Near-isogenic lines:
From the two crosses between tolerant/sensitive pairs reported above, a major QTL for terminal drought tolerance was identified on linkage group 2 (Yadav et al., 2002, 2004). To develop the QTL introgression lines in the background of H77/833-2, the latter was crossed to PRLT 2/89-33 followed by four backcrosses with H77/833-2 to recover most of its genetic background. At each backcross the assessment of the presence or absence of the terminal drought tolerance QTL was made using simple sequence repeat (SSR) flanking markers on LG2 (Xpsmp2059, Xpsmp2066, and Xpsmp2237). Two steps of selfing were performed to generate inbred NILs-QTL. Test-cross hybrids of the NILs-QTL, made using the same male-sterile line 843A, were produced with five introgression lines ICMR 1029, ICMR 1031, ICMR 2041, ICMR 2042, and ICMR 2044. The test-cross hybrids involving ICMR 1029, ICMR 1031, and ICMR 2041 were previously found to be superior in terms of their yield under terminal drought tolerance, whereas ICMR 2042 and ICMR 2044 had a yield response in the field that was similar to that of the test-cross hybrid from the sensitive parent H77/833-2 (Serraj et al., 2005).
Plant growth and response to drought
Plants were grown in pots during May–June 2007 and January–February 2008 in a glasshouse under near-optimal conditions [day/night temperature 32/25 °C, relative humidity oscillating between 40% and 80% during the course of the day, and the resulting vapour pressure deficit (VPD) varying between 2.86 kPa and 0.63 kPa]. The soil used was an Alfisol collected from ICRISAT's farm and had a pH of ∼7. The soil was mixed with sand and manure (5:3:1). Pots were filled with 5 kg and 9 kg of the Alfisol:sand:manure mixture for the assessment at the vegetative and reproductive stage, respectively (details provided below). The soil was amended with N, P, and K by mixing di-ammonium phosphate and muriated potash at a rate of 300 mg kg−1 and 200 mg kg−1. In addition plants were top dressed with 1 4g urea per plant at 4 weeks after sowing.
The transpiration response of plant to progressive exposure to water deficit was assessed in parental lines [PRLT 2/89-33 (tolerant) and H77/833-2 (sensitive), 863B-P2 (tolerant) and ICMB 841-P3 (sensitive)] in May–June 2007. The assessment was initiated before the flag leaf stage in Experiment 1 (30 d after sowing) and after the panicles were fully emerged in Experiment 2 (40 d after sowing). For the sake of brevity, the former stage of assessment is hereafter called the ‘vegetative stage’ whereas the latter is referred to as the ‘reproductive stage’. These two experiments were designed to estimate whether the FTSW threshold where transpiration declines varied with genotypes and phenological stages. Experiment 3, carried out in January–February 2008, was similar to Experiments 1 and 2, and used PRLT 2/89-33, H77/833-2, and four QTL introgression lines (ICMR1029, ICMR 1031, ICMR 2042, and ICMR 2044) to assess a putative relationship between the FTSW threshold for transpiration decline and the known performance of NILs-QTL in the field and the putative presence/absence of the introgressed QTL. Based on results from Experiments 1 and 2, Experiment 3 was performed at the vegetative developmental stage only.
For each experiment there were two sets of plants with six replicates of each genotype. At the time of imposing the treatment, depending on development stage, pots were saturated with water and allowed to drain overnight. The following morning, plants were bagged in a plastic bag wrapped around the stem, and pots were subsequently weighed. Pot weight was thereafter taken every day in the morning. Two water treatments were imposed: a well-watered and a water stress treatment. The WW set of plants was maintained close to 80% field capacity by bringing the pot weight to that level (i.e. 100 g and 200 g below the saturated weight for the 5 kg and 9 kg pots, respectively) every day. The WS set of plants was exposed to a gradual water stress by partially compensating water loss from transpiration, i.e. plants were allowed to lose no more than 70 g and 100 g of water on each day for the vegetative and reproductive stage assessment, respectively. The difference in re-watering was related to the pot size and allowed the imposition of relatively similar kinetics of stress imposition in these pots varying in size and therefore in water availability. Any transpiration in excess of these maximum daily water losses allowed was added back to the pots, as previously described (Vadez and Sinclair, 2001).
The transpiration values were normalized to facilitate comparison. First, the transpiration ratio (TR) was calculated by dividing the transpiration of each individual plant of a given genotype by the average WW transpiration of that genotype. Secondly, the TR was normalized by dividing each TR value over time by the average of the TR value for the first 3 d of the experiment when there was still no water limitation. This second normalization gave the normalized transpiration ratio (NTR), which accounted for plant to plant variation in transpiration within each genotype. When the NTR of stressed plants fell below 0.10, i.e. when the transpiration of WS plants was <10% of that of WW plants, all the plants were harvested and LA and the dry weights of their parts were measured.
After harvest, the FTSW for each day of the experiment was calculated. The FTSW values represent the portion of remaining volumetric soil water available for transpiration on each day of the experiment and were used as the indicator of stress (Ritchie, 1981). FTSW on each day n was calculated as:
Rate of water loss per unit of leaf area
The rate of water loss per unit of LA was assessed under well-watered conditions over the course of 3 d. It was assumed that the LA changes during this period would not differ across genotypes and this index would ‘integrate’ the behaviour of stomata over the 3 d. To do so, the daily transpiration of control plants was averaged over the last 3 d before being harvested. At harvest the LA was measured and the transpiration data used for conversion of transpiration to water loss per unit of LA and per day (Tr, transpiration rate in g cm−2 d−1). The purpose of this was not to obtain an absolute value of Tr but rather a comparative estimate for different genotypes. The Tr was measured before the panicles emerged (‘vegetative phase’) and after the panicles had emerged (‘reproductive phase’).
All four parental lines (H77/833-2, PRLT 2/89-33, ICMB 841-P3, and 863B-P2) were compared for their Tr at the vegetative and reproductive stage, using plants of the WW treatment of Experiments 1 and 2. Tr was also evaluated in NILs-QTL (ICMR1029, ICMR1031, and ICMR1041) along with two parental lines (H77/833-2, PRLT 2/89-33) in Experiment 4, using six replicated plants per genotypes. Plants of Experiment 4 were grown under well-watered conditions in a glasshouse in August 2008, following the conditions described above and used for the assessment of Tr. The arrangement in the glasshouse was such that pots were widely spaced with ∼25–30 cm between each pot. Therefore, the plant density of the experimental set-up was <5 plants m−2, which limited leaf shading of the bottom leaves.
In Experiment 5, Tr was measured on detached leaves that were sampled from the well-watered block of a field experiment where the four parental lines were assessed for yield under terminal drought (data not shown). It had previously been determined with container-grown plants that the Tr measured from whole plants was consistent with the detached leaf Tr (DLT) from detached leaves from these plants (data not shown). The first fully developed leaves of plants grown in the field were used to determine the DLT. The leaves were placed in test tubes with 0.1 mM EDTA to prevent the clogging of xylem vessels and tightly covered with plastic film (Parafilm) and aluminium foil to prevent evaporation. Subsequently, leaves were acclimated for 30 min in a growth chamber (30 °C, 80% relative humidity, 600 μm light intensity at the leaf level), and the tubes were then weighed. The leaves were weighed every hour, and after 3 h they were harvested, LA was determined, and, finally, DLT was calculated as the Tr (g water cm−2 h−1).
Influence of defoliation on the Tr
In Experiment 6, tests were conducted to determine whether the Tr (g cm−2 d−1) was affected by partial defoliation of the plants. Saturated pots with plants of the parental line hybrids were bagged with foil to avoid soil evaporation and placed in a growth chamber under standard conditions (30 °C, 80% relative humidity, 600 μm light intensity at the canopy level). Previous assessment had shown that the transpiration in the chamber was relatively constant throughout the day except for ∼2 h after light set. After 1 d of acclimation to these conditions, plant transpiration was measured during 3 h by weighing every hour, and subsequently plants were partially defoliated. Every second leaf from the top was eliminated, leading to an LA reduction of 57, 50, 53, and 53% in PRLT 2/89-33, H77/833-2, 863B-P2, and ICMB 841-P3, respectively. It took about an hour for defoliation of the plants and then transpiration was estimated during another 3 h. Finally, reduced LA and remaining LA were measured and used for calculation of the Tr (g cm−2 h−1) before and after LA reduction. The percentage change in Tr was also calculated by dividing each individual Tr value after defoliation by the mean Tr for each respective genotype before defoliation.
Stomatal density
SD was estimated on the first fully developed leaf of well-watered plants of the four parental lines. Colourless nail polish was spread on the abaxial leaf side of the most fully expanded leaves sampled before the panicle emerged from WW plants of Experiment 1. The leaf print was observed under the light microscope (magnification 20×10, area 9.86 mm2). The number of stomata was counted at three randomly chosen places, avoiding large vessels in the leaf print.
Statistical analysis
The experimental design for Experiments 1–3 was a randomized complete block design with two water treatments (WW and WS) as main factors and genotypes as subfactors with six replications. The experimental design for Experiments 4–6 was a randomized complete block design with one treatment as main factor (WW in Experiments 4 and 5 and defoliation in Experiment 6) and genotypes as subfactor, with six (Experiment 4), five (Experiment 5), and four replications (Experiment 6). Analyses of variance (ANOVAs) were done with the statistical program package CoStat version 6.204 (CoHort Software, Monterey, CA, USA). One-way ANOVA was carried out to test for genotypic differences within treatment (LSD in Table 5) and to compare genotype means across treatment where the Tukey–Kramer test was then used for the analysis of differences between genotype means across treatments (letters in Table 1 and 2). For the FTSW threshold analysis, SAS (SAS Institute, Inc., 1988, Cary, NC, USA) was used. Each NTR value was plotted to a corresponding FTSW value for each day of the experiment, and the FTSW thresholds where NTR initiated its decline were determined using a plateau regression procedure as described previously (Ray and Sinclair, 1998). This analysis provided a confidence interval for each threshold value.
Table 5.
Transpiration rate (Tr) at full leaf area (LA) and at reduced LA, percentage Tr increase, and average stomatal density in genotypes H77/833-2, PRLT 2/89-33, ICMB 841-P3 and 863B-P2
| Genotype | Tr of full LA (g H2O cm−2 h−1) | Tr of reduced LA (g H2O cm−2 h−1) | % Tr increase | Stomatal density (per 9.86 mm2) |
| PRLT 2/89-33 | 0.014±0.001 | 0.021±0.003 | 44±9 | 9.51±0.70 |
| H77/2 833-2 | 0.019±0.002 | 0.031±0.005 | 77±25 | 11.50±0.58 |
| 863B-P2 | 0.011±0.001 | 0.017±0.002 | 52±10 | 10.07±0.54 |
| ICMB 841-P3 | 0.017±0.003 | 0.020±0.003 | 31±7 | 9.95±0.66 |
| LSD (P < 0.05) | 0.005 | 0.011 | 33 | 2.02 |
Stomatal density data were collected from the first fully developed leaves from greenhouse Experiment 1. Stomata were counted on set areas of 9.86 mm2. Data are means ±SE of four replicated plants for the Tr measurement and of six replicated stomatal density assessments.
Table 1.
Distribution of dry mass between plant parts in Experiment 1 (vegetative stage)
| Treatment | Genotype | RDW (g) | SDW (g) | PDW (g) | LDW (g) | LA (cm2) | SLA (cm2/g) | TDW (g) |
| Control | PRLT 2/89-33 | 5.24±0.611 a | 20.19±1.91 a | 2.74±1.04 a | 7.08±0.55 a | 1352±115 a | 191.0±4.8 a | 34.8±3.12 a |
| H77/833-2 | 14.50±0.58 a | 20.08±1.83 a | 5.42±0.79 a | 6.45±0.48 a | 1246±98 a | 193.3±2.7 a | 41.60±3.92 a | |
| 863B-P2 | 10.11±2.11 a | 22.66±1.62 a | 5.42±2.45 | 10.93±0.86 a | 2276±182 a | 208.2±6.5 a | 47.61±2.85 a | |
| ICMB 841-P3 | 3.93±0.71 a | 16.20±0.92 a | 3.38±0.34 a | 6.80±0.62 a | 1552±152 a | 228.4±8.8 a | 30.30±1.83 a | |
| Drought | PRLT 2/89-33 | 3.58±0.27 b | 18.74±1.49 a | 2.41±1.05 a | 6.78±0.26 a | 969±184 b | 124.0±1.9 b | 30.55±1.73 a |
| H77/833-2 | 5.02±0.87 b | 10.40±1.29 b | 3.24±0.36 b | 3.94±0.45 b | 599.1±41.1 b | 158.2±13.0 b | 21.75±2.94 b | |
| 863B-P2 | 4.97±0.93 a | 12.95±1.14 b | – | 10.61±1.25 a | 1462±174 b | 159.5±14.7 b | 27.16±2.02 b | |
| ICMB 841-P3 | 6.25±1.62 a | 15.29±0.93 a | 1.37±0.37 b | 6.21±0.33 a | 1087±19 b | 174.2±2.6 b | 28.65±2.75 a |
The plant parts examined were root dry weight (RDW), stem dry weight (SDW), panicle dry weight (PDW), leaf dry weight (LDW), total dry weight (TDW), and leaf parameters: leaf area (LA), specific leaf area (SLA) of four pearl millet genotypes [H77/833-2 (sensitive) and PRLT 2/89-33 (tolerant), ICMB 841-P3 (sensitive) and 863B-P2 (tolerant)]. The values are shown with ±SE; n=6. Lower case letters following means discriminate genotype means between treatments.
Table 2.
Distribution of dry mass between plant parts in Experiment 2 (reproductive stage)
| Treatment | Genotype | RDW (g) | SDW (g) | PDW (g) | LDW (g) | LA (cm2) | SLA (cm2/g) | TDW (g) |
| Control | PRLT 2/89-33 | 15.54±1.50 a | 31.52±4.06 a | 6.18±1.81 a | 11.63±1.22 a | 2132±240 a | 183.2±5.7 a | 66.00±5.70 a |
| H77/833-2 | 12.49±1.55 a | 23.05±1.88 a | 19.89±1.89 a | 7.57±0.47 a | 1328±122 a | 174.7±8.8 a | 63.32±4.72 a | |
| 863B-P2 | 24.88±2.97 a | 37.32±1.60 a | 12.17±1.81 a | 20.09±1.70 a | 3924±455 a | 200.3±19.5 a | 96.40±6.33 a | |
| ICMB 841-P3 | 23.12±3.72 a | 36.19±4.37 a | 14.63±2.61 a | 12.08±1.42 a | 2680± 351 a | 221.3±9.3 a | 86.9 ±7.56 a | |
| Drought | PRLT 2/89 33 | 17.71±3.37 a | 27.32±2.29 a | 5.33±1.54 a | 13.55±0.91 a | 738±72 b | 55.5±6.1 b | 62.13±2.74 a |
| H77/833 2 | 10.59±1.95 a | 23.95±2.25 a | 16.89±2.37 a | 7.98±0.77 a | 762±108 b | 97.8±11.3 b | 57.63±7.52 a | |
| 863B-P2 | 29.19±3.32 a | 35.86±4.31 a | 13.21±0.75 a | 20.37±3.19 a | 1902±291 b | 94.1±6.5 b | 98.63±8.10 a | |
| ICMB 841-P3 | 20.69±3.43 a | 38.01±2.81 a | 17.86±2.11 a | 13.82±1.20 a | 1267 ±199 b | 91.1±11.3 b | 87.61± 5.32 a |
The plant parts examined were root dry weight (RDW), stem dry weight (SDW), panicle dry weight (PDW), leaf dry weight (LDW), total dry weight (TDW), and leaf parameters: leaf area (LA), specific leaf area (SLA) of four pearl millet genotypes [H77/833-2 (sensitive) and PRLT 2/89-33 (tolerant), ICMB 841-P3 (sensitive) and 863B-P2 (tolerant)]. Lower case letters following means discriminate genotype means between treatments.
Results
Effect of drought exposure on growth parameters
Water deficit reduced biomass in Experiments 1 and 2 (Tables 1, 2). The most drought-affected parts of the plants were the stem and the panicle at both growth stages assessed, in agreement with previous work (Winkel et al., 2001), compared with root and leaf biomass, which were the least affected by drought. Although leaf dry weight (LDW) changed little under drought, LA decreased 50–75% compared with control conditions, at both stages of assessment. This was related to leaf thickening in part indicated by a 30–50% decrease of the specific leaf area (SLA) in all the genotypes under drought conditions at the vegetative stage and reproductive stage. 863B-P2 had the highest LA and LDW values, followed by ICMB 841-P3 along with PRLT 2/89-33, while H77/833-2 had the lowest LA and LDW values.
It was also found at the vegetative stage that, while the SLA decreased (leaves thickened) in all genotypes, this trend was more marked in the tolerant parents (PRLT 2/89-33 and 863B-P2) than in their respective sensitive partner. In addition, it was found that as SLA increased Tr (g water cm−2d−1, see below) decreased.
Transpiration response to soil drying
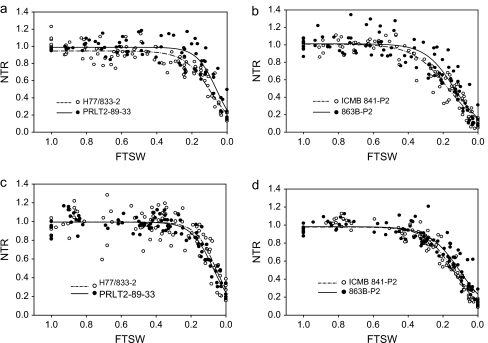
At the vegetative stage, the transpiration started declining at FTSW values ranging between 0.49 and 0.30 (Fig. 1a, b). In fact, threshold values were higher for sensitive H77/833-2 and ICMB 841B-P2 than for PRLT 2/89-33 and 863B-P2 at the vegetative stage. In contrast, at the reproductive stage the transpiration dropped at similar FTSW values ranging between 0.26 and 0.35 (Fig. 1c, d) in all genotypes (Table 3).
Fig. 1.
Relationship between the normalized transpiration rate (NTR) and the fraction of transpirable soil water (FTSW) of two pearl millet genotype pairs: H77/833-2 and PRLT 2/89-33, and ICMB 841-P3 and 863B-P2 (H77/833-2, ICMB 841-P3—sensitive; PRLT 2/89-33, 863B-P2—tolerant) during the vegetative (a, b) and reproductive (c, d) stage in 2007. The FTSW thresholds where transpiration initiated its decline were calculated with a plateau regression procedure from SAS. Then the regression lines of the relationships between NTR and FTSW were drawn by fitting NTR to FTSW data above and below the respective threshold for transpiration decline in each genotype and assessment stage.
Table 3.
Statistical analysis of data from Figs 1a, b, 2 showing the FTSW threshold where transpiration declines upon exposure to progressive water deficit in vegetative and reproductive stage (Experiments 1 and 2) in several pearl millet genotypes
| Genotype | FTSW threshold | Approximate SE | 95% CI |
| Vegetative stage | |||
| PRLT 2/89-33 | 0.3036 | 0.0353 | 0.2333–0.3738 |
| H77/833-2 | 0.4923 | 0.0476 | 0.3977–0.5869 |
| ICMB 841-P2 | 0.4146 | 0.0158 | 0.3849–0.4478 |
| 863B-P2 | 0.3267 | 0.0176 | 0.2918–0.3616 |
| Reproductive stage | |||
| PRLT 2/89-33 | 0.2645 | 0.0227 | 0.2194–0.3096 |
| H77/833-2 | 0.2489 | 0.0258 | 0.1977–0.3001 |
| ICMB 841-P2 | 0.3553 | 0.00836 | 0.3387–0.3791 |
| 863B-P2 | 0.3565 | 0.0141 | 0.3285–0.3846 |
Data are the means of six replicated plants per genotype.
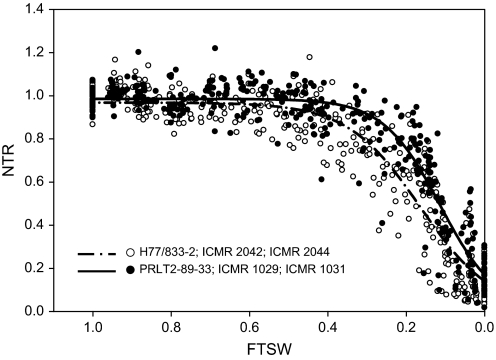
Based on the above results, this dry down at the vegetative stage only was reproduced with the first parental pair PRLT-2/89-33 and H77/833-2 along with their NILs-QTL (Fig. 2). Again parental genotypes differed and the sensitive parent H77/833-2 had a higher FTSW threshold than tolerant PRLT-2/89-33. Moreover, the FTSW for superior NILs-QTL ICMR 1029 and ICMR 1031 was similar to that of the tolerant parent (PRLT-2/89-33), whereas the FTSW threshold of sensitive NILs-QTL ICMR 2042 and ICMR 2044 was similar to that of the sensitive parent (H77/833-2) (Table 4).
Fig. 2.
Relationship between the normalized transpiration ratio (NTR) and the fraction of transpirable soil water (FTSW) of two pearl millet genotype pairs: H77/833-2 and PRLT 2/89-33 and their four NILs-QTL [ICMR 1029 and ICMR 1031 that yielded similarly to tolerant PRLT 2/89-33 under terminal drought and ICMR2042 and ICMR 2044 that yielded similarly to sensitive H77/833-2 (Serraj et al., 2005)] during the vegetative developmental stage in 2008 (Experiment 3).
Table 4.
Statistical analysis of data from Figs 2 showing the FTSW threshold where transpiration declines upon exposure to progressive water deficit in several pearl millet genotypes, including NIL-QTL materials
| Genotype | FTSW threshold | SE | Approximate95% CI |
| H77/833-2 | 0.3772 | 0.017 | 0.3436 –0.4108 |
| ICMR 1029 | 0.2965 | 0.0153 | 0.2662 – 0.3269 |
| ICMR 1031 | 0.2982 | 0.0137 | 0.2711–0.3253 |
| ICMR 2042 | 0.4699 | 0.0181 | 0.4340–0.5058 |
| ICMR 2044 | 0.411 | 0.0148 | 0.3817–0.4403 |
| PRLT 2/89-33 | 0.304 | 0.00954 | 0.2851–0.3228 |
Data are the means of six replicated plants per genotype.
Rate of water loss per unit leaf area and time–transpiration rate
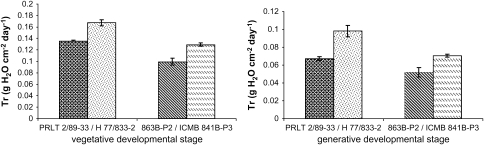
The Tr (g cm−2 d−1) was lower in tolerant PRLT 2/89033 than in H77/83302 at both the vegetative and reproductive stage (P <0.01). Similar results were found with the other pair of parental lines, with the Tr being also lower in tolerant 863B-P2 than in sensitive ICMB 841-P3 (Fig. 3a, b). These results fully confirmed data that were obtained in previous experiments (data not shown).
Fig. 3.
Transpiration rate (Tr) of pearl millet parental genotype pairs: H77/833-2 and PRLT 2/89-33, ICMB 841-P3 and 863B-P2; (H77/833-2, ICMB 841-P3 - sensitive, PRLT 2/89-33, 863B-P2 - tolerant). Tr was compared among vegetative (3a) and reproductive stage (3b) of development in 2007. Bars indicate the SE (n=6).
This measurement of Tr was repeated at the vegetative stage with parental lines PRLT 2/89-33 and H77/833-2 and three NILs-QTL (ICMR 1029, ICMR 1031, and ICMR 2041). Again the Tr was lower in tolerant PRLT 2/89-33. In addition, the Tr in the NILs-QTL was lower than in the sensitive parent H77/833-2 and similar to the tolerant PRLT2/89-33 (Fig. 4) (P <0.01).
Fig. 4.
Transpiration rate (Tr) of parental genotype H77/833-2 (sensitive) and PRLT 2/89-33 (tolerant) and their NILs-QTL (ICMR 1029, ICMR 1031, and ICMR 2041) in the vegetative stage of development in 2008. Bars indicate the SE (n=6).
Prior to assessing Tr in detached leaves from plants grown in the field, tests were carried out to determine whether the Trs of detached leaves and whole plants were similar. To do so, the Tr of detached leaves and the Tr of whole plants were simultaneously followed in two genotypes contrasting in this trait (PRLT2/89-33 and H77/833-2) during 3 h of the daylight cycle under glasshouse conditions. The Tr of detached leaves (0.0033 g cm−2 h−1) was similar to that from whole plants (0.0030 g cm−2 h−1), averaged across genotypes for both Tr measurements. In addition, Tr was lower in PRLT2/89-33 than in H77/833-2 in both cases (P <0.001).
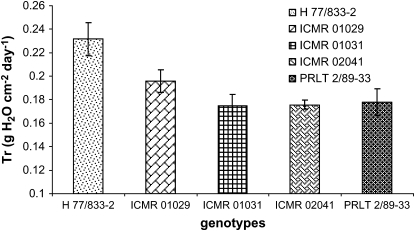
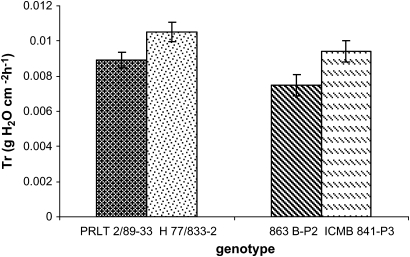
The detached leaf protocol was used to assess the transpiration rate in detached leaves sampled from the field, and a lower Tr was found in PRLT2/89-33 than in H77/833-2 and a lower Tr in 863B-P2 than in ICMB 841-P3 (Fig. 5) (P <0.01).
Fig. 5.
Average of the transpiration rate (Tr, g cm−2 h−1) of detached leaves of four genotypes (H77/833-2 and ICMB 841-P3—sensitive; PRLT 2/89-33 and 863B-P2—tolerant) grown under well-watered conditions in the field. The transpiration rate was assessed over a 3 h period in a glasshouse. Bars indicate the SE (n=6).
Effect of defoliation on transpiration rate
The reduction of LA and thus the disruption of the LAR resulted in an increase in Tr in all the genotypes, except ICMB 841-P3 (Table 5). The relationship between LAR and Tr was fitted with an exponential equation (P <0.001, data not shown). After defoliation, neither the Tr nor the percentage increase in Tr were any different between genotypes at P <0.05 (Table 5).
Stomatal density
The numbers of stomata in an area of 9.86 mm2 on the first fully developed leaf from plants of Experiment 1 were counted, and no differences in SD were found between genotypes (Table 5).
Discussion
Differences in transpiration rate
Crucial results for understanding the drought tolerance strategy of millet emerged from the comparison of Tr under well-watered conditions. Both tolerant genotypes (PRLT 2/89-33 and 863B-P2) showed lower Tr compared with their sensitive partners (H77/833-2 and ICMB 841-P3) in both developmental stages. In addition, Tr variability was also found on detached leaves sampled from the field. Evidence for the role of Tr in the terminal drought tolerance QTL was confirmed, with all tolerant NILs exhibiting a Tr similar to the tolerant parent and lower than the sensitive parent. These results show a relationship between the terminal drought tolerance of PRLT 289/33 and NILs and their lower rate of water loss per unit of LA under well-watered conditions. This trait would conserve soil moisture for later stages, in particular during the grain filling period. It would have great value under terminal drought conditions and in environments where soil evaporation is limited. This interpretation would fit well the fact that the terminal drought tolerance QTL is responsible for a better PNHI, i.e. a proxy for grain filling. Such data have not been reported so far despite the fact that the importance of plant water management in well-watered conditions has previously been discussed a number of times (Mortlock et al., 2001; Condon et al., 2002; Sinclair et al., 2005, 2007). More work is needed to test the hypothesis that Tr differences would lead to more water availability during grain filling.
The aim of this study to at least partially explain differences in plant water management by examining the number of stomata did not confirm the initial hypothesis. It was expected that variability in SD might provide an explanation for previously observed differences in Tr, as was shown in Masle et al. (2005) who provided evidence that the gene ERECTA had an impact on SD. This seems not to be the case for pearl millet. Thus, if stomata number might play a role in drought tolerance (Muchow and Sinclair. 1989), the present results agree with the previous assertions that stomatal regulation rather than SD is more important for regulating water loss in pearl millet (Henson al., 1981; Liu et al., 2003; Zhang et al., 2005). There, the use of Tr as ‘an integrated proxy’ for stomatal conductance appeared to be a simple and successful screen to discriminate tolerant and sensitive genotypes.
Effect of defoliation on the transpiration rate
The Tr may not only be dependent upon internal biochemistry of plants but may also be influenced by physical characteristics of plants’ internal architecture (Salleo et al., 2001; Zwieniecky et al., 2001; Cochard et al., 2004; Sperry et al, 2005). When the LAR was experimentally altered to test the possibility of short-term adjustment, the Tr of the remaining LA adjusted very quickly to the exponential function. This would suggest that a hydraulic control of the change in Tr is involved in such a rapid change of the stomatal opening.
Differences in FTSW threshold
A previous study has shown that pot size had no influence on the FTSW threshold (Ray and Sinclair, 1998) and the present protocol appeared adequate for comparing genotypic response to soil water deficit. It was found that the FTSW threshold of tolerant parental genotypes was lower compared with sensitive genotypes in the vegetative developmental stage. This meant that the transpiration dropped upon progressive soil drying in relatively dryer soil in the tolerant lines than in the sensitive lines. This genotypic variability in transpiration response to soil drying was in agreement with data obtained in groundnut (Bhatnagar-Mathur et al., 2007). In addition, the response measured in one contrasting parental pair and their NILs-QTL provided evidence that the FTSW threshold obtained for superior NILs-QTL was similar to that of tolerant PRLT 2/89-33 and the QTL donor parent. In contrast, the FTSW threshold obtained for NILs-QTL that did not yield better than H77/833-2 in the field was indeed similar to that of sensitive H77/833-2. These data provide evidence for a role for the QTL in explaining the differences in these thresholds and hence the role of these threshold differences in understanding the variability between lines in their terminal drought tolerance. The reasons for these differences are intriguing, given that: (i) tolerant genotypes have a lower Tr under well-watered conditions, which would denote a more ‘conservative’ water use; and (ii) tolerant genotypes have a lower FTSW threshold for transpiration decline under drought which indicates that they attempt to maximize water use. Both could in fact be related. Indeed a lower Tr in tolerant lines under well-watered conditions would lead to lower daily transpiration, which would logically drive the TR of drought-exposed plants upwards, and consequently the NTR. Therefore, the maintenance of an NTR under drought conditions at a level close to that of well-watered plants, which leads to having a lower FTSW threshold for the beginning of the transpiration drop, might simply be a consequence of the lower rate of water loss per unit of LA (Tr) in the well-watered plants of tolerant genotypes. In fact, this agrees well with the fact that the presence/absence of the QTL appeared to discriminate well for both a lower/higher Tr and lower/higher FTSW threshold. The same interpretation could be obtained from similar data in transgenic groundnut (Bhatnagar-Mathur et al., 2007). The only unexplained issue is the fact that the FTSW thresholds were not different at the reproductive stage whereas Tr was still different.
Conclusion
This study showed that genotypes contrasting in terms of terminal drought tolerance, based on seed yield in field conditions, also contrasted in the control of leaf water loss under well-watered conditions. This trend was directly related to the presence or absence of a terminal drought tolerance QTL. The tolerant/QTL holder genotypes had a lower rate of water loss per unit LA (Tr, g water cm−2 d−1). It is hypothesized that this characteristic would contribute to a more conservative water use in field conditions, making more water available for the grain filling stage, which would be very important for terminal drought conditions. This hypothesis remains to be tested. A lower Tr would also lead to having a lower FTSW threshold where transpiration declines upon progressive exposure to water deficit, making drought-stressed plant behave like well-watered plants until the soil has become dryer than for sensitive lines. Since Tr was measurable on whole plants but also on single detached leaves that could be collected from the field, Tr may be a very convenient trait to phenotype across a range of experimental conditions. Although more work is needed to understand better how Tr is regulated, Tr may be further considered as an insightful tool for selection screening in pearl millet breeding programmes.
Acknowledgments
The authors thank Mr M Anjaiah for expert technical support for the experiments and Ms Rekha Badham for support with the statistical analysis. Support for the senior author came from a grant from DFID-BBSRC, Research Contract BB/F004133/1 and from Grant No. MSM0021620858 from the Ministry of Education, Youth and Sports of the Czech Republic.
Glossary
Abbreviations
- FTSW
fraction of transpirable soil water
- NIL
near-isogenic line
- SD
stomatal density
- Tr
transpiration rate
References
- Bhatnagar-Mathur P, Devi MJ, Reddy SD, Lavanya M, Vadez V, Serraj R, Yamaguchi-Shinozaki K, Sharma KK. Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Reports. 2007;26:2071–2082. doi: 10.1007/s00299-007-0406-8. [DOI] [PubMed] [Google Scholar]
- Bidinger FR, Hash CT. Pearl millet. In: Nguyen HT, Blum A, editors. Physiology and biotechnology integration for plant breeding. New York: Marcel Dekker; 2003. pp. 225–270. [Google Scholar]
- Bidinger FR, Mahalakshmi V, Rao GDP. Assessment of drought resistance in pearl millet [Pennisetum americanm (L.) Leeke]. II. Estimation of genotype response to stress. Australian Journal of Agricultural Research. 1987;38:49–59. [Google Scholar]
- Black CR, Squire GR. Effects of atmospheric saturation deficit on the stomatal conductance of pearl millet (Pennisetum typhoides S. and H.) and groundnut (Arachis hypogaea L.) Journal of Experimental Botany. 1979;30:935–945. [Google Scholar]
- Cochard H, Froux F, Mayr S, Coutand C. Xylem wall collapse in water-stressed pine needles. Plant Physiology. 2004;134:401–408. doi: 10.1104/pp.103.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquar GD. Improving intristic water-use efficiency and crop yield. Crop Science. 2002;42:122–131. doi: 10.2135/cropsci2002.1220. [DOI] [PubMed] [Google Scholar]
- Henson LE, Mahalakshmi V. Evidence for panicle control of stomatal behavior in water-stressed plants of pearl millet. Field Crops Research. 1985;11:281–290. [Google Scholar]
- Henson IE, Mahalakshmi V, Alagarswami G, Bidinger FR. An association between flowering and reduced stomatal sensitivity to water stress in pearl millet (Pennisetum americanum (L.) Leeke) Annals of Botany. 1983;52:641–648. [Google Scholar]
- Henson IE, Mahalakshmi V, Bidinger FR, Alagarswami G. Stomatal responses of pearl millet (Pennisetum americanum (L.) Leeke) genotypes, in relation to abscisic acid and water stress. Journal of Experimental Botany. 1981;32:1211–1221. [Google Scholar]
- Hufstetler EV, Boerma HR, Carter TE, Earl HJ. Genotypic variation for three physiological traits affecting drought tolerance in soybean. Crop Science. 2007;47:25–35. [Google Scholar]
- Liu F, Andersen MN, Jensen CR. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Functional Plant Biology. 2003;30:271–280. doi: 10.1071/FP02185. [DOI] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436:860–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- Mortlock MY, Hammer GL. Genotype and water limitation effects on transpiration efficiency in sorghum. Journal of Crop Production. 2001;2:265–286. [Google Scholar]
- Muchow CR, Sinclair TR. Epidermal conductance, stomatal. density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant, Cell and Environment. 1989;4:425–431. [Google Scholar]
- Ray JD, Sinclair TR. The effect of pot size on growth and transpiration of maize and soybean during water deficit stress. Journal of Experimental Botany. 1998;49:1381–1386. [Google Scholar]
- Ray JD, Sinclair TR. Stomatal closure of maize hybrids in response to soil drying. Crop Science. 1997;37:803–807. [Google Scholar]
- Ritchie JT. Water dynamics in the soil–plant–atmosphere system. Plant and Soil. 1981;58:81–96. [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sadras VO, Milroy SP. Soil water thresholds for the response of leaf expansion and gas exchange: a review. Field Crops Research. 1996;47:253–266. [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf miner veins: any impact on leaf gas exchange? Plant, Cell and Environment. 2001;24:851–859. [Google Scholar]
- Serraj R, Hash CT, Rizvi SMH, Sharma A, Yadav RS, Bidinger FR. Recent advances in marker-assisted selection for drought tolerance in pearl millet. Plant Production Science. 2005;8:334–337. [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology. 2005;32:945–952. doi: 10.1071/FP05047. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Ludlow MM. Influence of soil water supply on the plant water balance of four tropical grain legumes. Australian Journal of Plant Physiology. 1986;13:329–341. [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook MN. Drought tolerance in soybean associated with limiting leaf hydraulic conductance. Physiologia Plantarum. 2007;132:446–451. doi: 10.1111/j.1399-3054.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Wheeler JK. Comparative analysis of end wall resistance in xylem conduits. Plant, Cell and Environment. 2005;28:456–465. [Google Scholar]
- Stegmeier WD, Andrews DJ, Rai KN, Hash CT. Pearl millet parental lines 843A and 843B. International Sorghum and Millet Newsletters. 1998;39:129–130. [Google Scholar]
- Tsuda M, Tyree MT. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany. 2000;51:823–828. [PubMed] [Google Scholar]
- Vadez V, Krishnamurthy L, Kashiwagi J, et al. Exploiting the functionality of root systems for dry, saline, and nutrient deficient environments in a changing climate. ICRISAT and CGIAR 35th Anniversary Symposium ‘Climate-Proofing Innovation for Poverty Reduction and Food Security’. Journal of SAT Agriculture Research. 2007;4:1–61. [Google Scholar]
- Vadez V, Sinclair TR. Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. Journal of Experimental Botany. 2001;52:153–159. [PubMed] [Google Scholar]
- Wallace JS, Lloyd CR, Sivakumar MVK. Measurements of soil, plant and total evaporation from millet in Niger. Agricultural and Forest Meteorology. 1993;63:149–169. [Google Scholar]
- Winkel T, Payne W, Renno JF. Ontogeny modifies the effects of water stress on stomatal control, leaf area duration and biomass partitioning of Pennisetum glaucum. New Phytologist. 2001;179:71–82. doi: 10.1046/j.1469-8137.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- Yadav RS, Hash CT, Bidinger FR, Cavan GP, Howart CJ. Quantitative trait loci associated with traits determining grain and stover yield in pearl millet under terminal drought-stress conditions. Theoretical and Applied Genetics. 2002;104:67–83. doi: 10.1007/s001220200008. [DOI] [PubMed] [Google Scholar]
- Yadav RS, Hash CT, Bidinger FR, Devos KM, Howarth CJ. Genomic regions associated with grain yield and aspects of post-flowering drought tolerance in pearl millet across stress environments and testers background. Euphytica. 2004;136:265–277. [Google Scholar]
- Zhang X, Wang T, Li C. Different responses of two contrasting wheat genotypes to abscisic acid application. Biologia Plantarum. 2005;49:613–616. [Google Scholar]
- Zwieniecky MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]