Abstract
Colonization of plant roots by root knot and cyst nematodes requires a functional ethylene response pathway. However, ethylene plays many roles in root development and whether its role in nematode colonization is direct or indirect, for example lateral root initiation or root hair growth, is not known. The temporal requirement for ethylene and localized synthesis of ethylene during the life span of soybean cyst nematode (SCN) on soybean roots was further investigated. Although a significant increase in ethylene evolution was not detected from SCN-colonized roots, the concentration of the immediate precursor to ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC), was higher in SCN-colonized root pieces and root tips than in other parts of the root. Moreover, expression analysis of 17 ACC synthase (ACS) genes indicated that a select set of ACS genes is expressed in SCN-colonized root pieces that is clearly different from the set of genes expressed in non-colonized roots or root tips. Semi-quantitative real-time PCR indicated that ACS transcript accumulation correlates with the high concentration of ACC in root tips. In addition, an ACS-like sequence was found in the public SCN nucleotide database. Acquisition of a full-length sequence for this mRNA (accession GQ389647) and alignment with transcripts for other well-characterized ACS proteins indicated that the nematode sequence is missing a key element required for ACS activity and therefore probably is not a functional ACS. Moreover, no significant amount of ACC was found in any growth stage of SCN that was tested.
Keywords: 1-Aminocyclopropane-1-carboxylic acid, ACC synthase, cyst nematode, ethylene, Glycine max, Heterodera glycines, soybean
Introduction
Soybean cyst nematode (SCN), Heterodera glycines, is an important pathogen of soybean (Wrather and Koenning, 2006). Understanding the biology of SCN and its host interactions is of academic and economic interest. In addition to evading plant defences, which is common to most successful pathogens, the nematode establishes inside the host root an elaborate feeding structure that is built from existing and new plant cells induced to divide at the site of infection. In order to do this the nematode must co-opt plant gene expression to do its bidding (Williamson and Gleason, 2003; Davis et al., 2004). The cyst nematode feeding structure, the syncytium, forms from parenchyma cells within the vascular bundle of the root by degradation of plant cell walls and membranes between adjacent cells (Goverse et al., 2000). A single syncytium can incorporate as many as 200 cells into one very large cell (Jung and Wyss, 1999). Although the syncytium itself does not form by initiation of new cell division, new cell division may occur in cells surrounding the syncytium and some of these cells may then be incorporated into the established syncytium (Golinowski et al., 1996; de Almeida Engler et al., 1999). In addition, many ingrowths appear along the syncytium. The ingrowths, which consist of thickened cell wall lined with plasmalemma, are presumed to increase the transfer of nutrients from the plant into the syncytium where the nutrients then move to the growing nematode (Grundler et al., 1998; Jung and Wyss, 1999).
The processes and signals associated with the formation of the feeding structure are of great interest. Many of these signals originate from the nematode and initiate changes in gene expression, alter protein function, or modify metabolism of plant nutrients (Davis et al., 2004, 2008). One possible approach for nematodes to alter root development and formation of a feeding structure is to alter the concentration or movement of plant hormones. There is good evidence that an intact response pathway for both auxin and ethylene is important for successful colonization of roots by both root knot and cyst nematodes (Goverse et al., 2000; Wubben et al., 2001). However, both auxin and ethylene have marked effects on root growth, including root elongation, lateral root initiation, and root hair development (Clark et al., 1999; Rahman et al., 2002; Negi et al., 2008). How these hormones influence the initial infection process and establishment of a feeding structure is unclear.
It has been demonstrated that ethylene-insensitive mutants in Arabidopsis cause a reduction in the number of sugar beet cyst nematodes, Heterodera schachtii, that mature on Arabidopsis roots (Wubben et al., 2001). Moreover, treatment of Arabidopsis roots with 1 mM aminoethoxyvinylglycine (AVG), an ethylene synthesis inhibitor, greatly reduced the number of nematodes that matured on the roots (Wubben et al., 2001). Conversely, Arabidopsis mutants that overproduce ethylene or treatment of roots with 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene, increased the number of adult females on the roots (Wubben et al., 2001). Similarly, a soybean mutant that has reduced sensitivity to ethylene also showed a 50% decrease in the number of SCNs that fully matured on the mutant roots (Bent et al., 2006). Clearly, ethylene plays a role in nematode colonization of roots, but the role could be indirect and only at the early stages of infection.
In an earlier report using an Affymetrix GeneChip for soybean to compare transcription profiles in SCN-infected and non-infected root pieces, it was observed that transcripts encoding proteins involved in ethylene synthesis, ACC synthase (ACS) and ACC oxidase (ACO), decreased (Puthoff et al., 2007). This was the opposite of what was expected. Three possible explanations for this decrease in ethylene-associated gene expression were given: (i) the GeneChip might be missing the ethylene synthesis genes that increase during SCN colonization; (ii) the peak in ethylene synthesis occurred prior to sampling at 8 days post-infection (dpi); or (iii) the nematode itself synthesized ethylene and this caused a feedback inhibition of the host ethylene synthesis genes.
Here it is confirmed that ethylene is important for SCN colonization of soybean roots and furthermore shown that ethylene probably plays a role in the growth of the nematode throughout its life cycle on the root and not only at early stages in the infection process, i.e. prior to 8 dpi. In addition, it is demonstrated that a select set of ACS genes are up-regulated at the SCN infection site. Most of these ACS genes were not represented on the GeneChip used in the earlier experiments. The focus here is on ACS gene expression and not ACO gene expression because it is generally agreed that ACC synthesis is the limiting step in ethylene synthesis and therefore changes in ACS gene expression and ACC accumulation may have a greater effect on ethylene synthesis than changes in ACO (Yamagami et al., 2003).
As an initial step to determine if it was possible that the nematode synthesized ethylene, the public H. glycines sequence databases at the National Center for Biotechnology Information (NCBI) was searched for ACS-like sequences. Surprisingly, a partial ACS-like sequence was identified in the nucleotide database. Interestingly, the source of the sequence was a nematode oesophageal gland cDNA library (Gao et al., 2001). A full-length clone for the SCN, ACS-like transcript was obtained (accession number GQ389647) and experiments conducted to determine if the nematode either secretes an ACS into the host or synthesizes ACC for internal or external synthesis of ethylene. The evidence indicates that SCN probably does not synthesize ACC or secrete an active ACS.
Materials and methods
Plant material, nematode inoculation, and RNA extraction
The nematodes and methods used have been previously described (Tucker et al., 2007). Briefly, SCNs (population NL1-RHg, HG-type 7, race 3) were reared and maintained in USDA greenhouses, Beltsville, MD, USA, and infective juveniles (stage 2) prepared according to Matthews et al. (2003). Seeds for Glycine max cv Williams82 were germinated in Perlite (Geiger, Harleysville, PA, USA) in the greenhouse and after 2 weeks seedlings were washed free of Perlite, combined into groups of six seedlings, and inoculated by pipetting 5000 J2 per seedling onto the roots. Infected and non-infected whole roots were collected at 0, 2, 4, 8, 12, and 20 dpi and frozen in liquid nitrogen. For collection of root pieces at 8, 12, and 16 dpi, the roots were examined under a stereomicroscope and regions displaying a cluster of ≥3 SCNs (SCN+) were dissected out and lateral roots trimmed and discarded. Root pieces were collected and trimmed from similar positions of non-inoculated aged roots to serve as control samples (SCN–). Similarly, under a stereomicroscope, root tips were dissected into 0–2, 2–7, and 7–12 mm sections and thereafter frozen in liquid nitrogen. Frozen roots were ground to a fine powder under liquid nitrogen, and RNA extracted using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA).
Identification of ACS genes in genomic sequence and real-time PCR
The translated peptide for the soybean ACS cDNA sequence, accession X67100 (Liu et al., 1993), was used in a BLASTx search of the soybean genomic sequence maintained at the Phytozome website (http://www.phytozome.net/soybean). Twenty-one similar sequences were discovered. If the open reading frames (ORFs) were not already identified in Phytozome, they were identified by nucleotide pairing to a well-defined ACS transcript and the presence of a conserved GT/AG splice junction at the boundary of each intron (Brown et al., 1996). The GCG Wisconsin Package (Accelrys, San Diego, CA, USA) and MacVector (MacVector Inc., Cary, NC, USA) software were used for nucleotide and protein comparisons. The nucleotide sequences for the predicted transcripts (see Supplementary Table S1 available at JXB online) were aligned and PCR primer pairs prepared to unique sequences in the 3′ ends of the ACS sequences (see Supplementary Table S2). To ensure gene-specific amplification, the 3′ end of each primer included one or more divergent nucleotides not found in the other most similar ACS sequence. Primer pair specificity was further indicated by a single dissociation peak in the melting curve of the real-time PCR end-product and the occurrence of a single band of the expected size after agarose gel electrophoresis. Primer annealing, extension, and denaturing temperatures of 60, 70, and 95 °C, respectively, were used for real-time PCR.
The semi-quantitative procedures used to standardize the real-time PCR have been described previously (Tucker et al., 2007). The cDNA synthesis was completed with 5 μg of DNase-treated RNA using the Superscript III-First Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). A single bulk cDNA synthesis reaction was performed and the cDNA diluted to a larger volume to accommodate a large number of PCRs and reduce differences that might occur between cDNA synthesis reactions. Real-time PCRs were completed using a Brilliant SYBR Green QPCR Master Mix in an Mx3000P instrument (Stratagene, La Jolla, CA, USA). The real-time PCR signal for the constitutively expressed soybean elongation factor (GmEF1b), which encodes a protein that is part of the ribosomal protein translation complex, was used to normalize all the RNA samples.
Monoxenic cultures of SCN on soybean roots
Glycine max cv Williams82 seeds were sterilized with 95% ethanol for 3 min followed by 10% household bleach treatment for 10 min and a rinse with sterile water. Sterile seeds were germinated on Noble agar plates for 3 d and two 2–3 cm root tips were transferred to a fresh Nobel agar plate containing 1× Gamborg's B5 medium with organics and sucrose at 20 g l−1. Axenic nematodes were prepared several years ago from the same line of greenhouse nematodes described above (Meyer et al., 1997). Axenic nematodes are routinely propagated by transferring and crushing five swollen females from a 30–40 dpi monoxenic root culture to a freshly prepared axenic root culture.
ACC measurements
Mature female nematodes were collected from greenhouse plants, and eggs were harvested and hatched as previously described (Matthews et al., 2003). Approximately 50 000 J2 were frozen in liquid nitrogen and later used for extraction of ACC. Approximately 300 swollen females were collected from monoxenic soybean roots at 16 dpi and 20 dpi, and frozen in liquid nitrogen. The root tips used for ACC measurements were collected from non-infected axenic soybean roots cultured as described above.
ACC extraction and conversion to ethylene were essentially as described by Lizada and Yang (1979). Frozen roots were weighed and 100 mg of tissue were homogenized in 400 μl of 85% ethanol. The 50 000 J2 and 300 swollen SCN females were estimated to be ∼50 mg and 20 mg, respectively. These were brought to 100 μl with sterile distilled water and homogenized in 85% ethanol in the same way as the roots. The homogenate was centrifuged at 10 000 rpm for 5 min and the supernatant collected. The pellet was suspended in 500 μl of 80% ethanol and then centrifuged again, and the supernatants were combined and dried in an Eppendorf Vacufuge overnight at room temperature. The dried pellet was suspended in 0.5 ml of chloroform, vortexed, 0.5 ml of sterile distilled water added, and the mixture vortexed again. The mixture was centrifuged at 10 000 rpm for 5 min. The aqueous phase was collected and mixed a second time with 250 μl of chloroform, centrifuged, and the aqueous phase collected and put into a 15 ml culture tube and sealed with a rubber vaccine cap. A 100 μl aliquot of 10 mM HgCl2 was injected through the vaccine cap, the solution mixed, and the tube placed in ice. A saturated NaOH solution was prepared separately and added to household bleach (5.5% NaOCl) immediately prior to use at a ratio of 1:2. Cold bleach/NaOH (100 μl) was injected and the mixture kept cold for at least 3 min with intermittent agitation. After 3 min, the 15 ml culture tube and mixture were allowed to warm to room temperature with intermittent agitation and then 2 ml of airspace was extracted with a gas syringe and injected into a gas chromatograph (GC). The GC was fitted with a 1/8 inch×3 ft long activated alumina column and a flame ionization detector (FID). GC settings were: oven 100 °C, injection port 110 °C, nitrogen carrier at 25 ml min−1, and FID at 200 °C with hydrogen at 40 ml min−1 and air at 400 ml min−1.
Inhibitor studies
All the inhibitor studies were completed using monoxenic (SCN-inoculated) or axenic roots grown on agar medium as described above. For treatment of roots with 1-methycyclopropene (1-MCP) or 2,5-norbornadiene (NBD), five plates were stacked and sealed in a 2.5 l desiccator fixed with a vaccine cap for injection of water for gasification of the 1-MCP in a 1.5 ml tube hung inside the desiccator or injection of the appropriate volume of liquid NBD onto a small wad of tissue in the desiccator. The liquid NBD quickly vaporizes after injection. To reduce accumulation of carbon dioxide in the desiccator and maintain the desired concentration of the inhibitor, the desiccators were opened in the hood for several minutes at 5 d intervals and the 1-MCP or NBD replenished, or not replenished in the case where NBD was given for only 10 d.
Ethylene measurements
When ethylene was to be measured in the airspace above the roots, the roots were grown inside 75 ml tissue culture flasks (Falcon 3013) with a vaccine cap inserted into the plastic screw cap. The roots with or without SCNs were grown for 4 d with the caps loose, which allows for diffusion of gases, and then the caps were screwed on tightly for another 3 d so that the ethylene could accumulate to measurable concentrations, i.e. >25 nl l−1. The cycle of loosening the caps for 4 d and then tightening them for 3 d was repeated every 7 d until near the end (28 dpi) of the 30 day life cycle of the SCNs.
Results
Confirmation of the importance of ethylene to SCN colonization of soybean roots
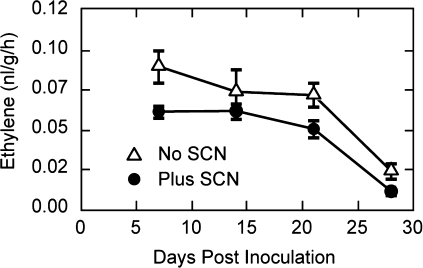
Ethylene synthesis was measured at 7 d intervals in control, non-inoculated roots (no SCNs) and SCN-inoculated roots grown on Gamborg's medium containing sucrose. Each flask averaged 114 swollen females on two root systems per flask (∼1 g of roots per flask). Ethylene production was highest on the seventh day and remained steady until 21 d in the control roots (Fig. 1, No SCN), and thereafter steeply declined. The same pattern was observed in the SCN-infected roots, however, with consistently lower values (Fig. 1, Plus SCN). SCN colonization slightly inhibited root growth and, although the ethylene synthesis was normalized to root weight, the infected roots consistently produced slightly lower levels of ethylene than non-infected roots.
Fig. 1.
Ethylene synthesis by SCN-inoculated and non-inoculated soybean roots cultured on Gamborg's B5 medium plus organics and 20% sucrose.
Although no increase in ethylene synthesis was observed in SCN-infected whole roots, there still may be a localized increase in ethylene synthesis in the region colonized by SCNs that was masked by the greater amount of ethylene synthesized in other parts of the root. In order to determine if this might be true, the concentration of ACC, the immediate precursor of ethylene and the rate-limiting step in ethylene synthesis (Yamagami et al., 2003), was measured in SCN-colonized and non-infected root pieces at 20 dpi, and in root tips (Table 1). The ACC concentration was ∼3-fold higher in the SCN-colonized root pieces compared with similar pieces of root that were not infected with SCNs. The highest concentration, however, was in the non-infected root tips (Table 1).
Table 1.
ACC concentration in SCNs, SCN-infected and non-infected root pieces, and root tips
| Mean±SE (nmol ACC/g) | |
| J2a (∼50 000) | NDb |
| SCNs (∼300) 16 dpi | ND |
| SCNs (∼300) 20 dpi | ND |
| Internal root pieces—no SCNs 20 dpi | 1.1±0.14 |
| Internal root pieces—many SCN (∼100) 20 dpi | 3.2±0.54 |
| Root tips 0.0–0.5 cm | 9.3±3.5 |
| Proximal to root tips 0.5 2.0 cm | 3.9±0.48 |
Three stages of SCN growth were examined: infective juveniles (J2) and swollen females at 16 dpi and 20 dpi. Root pieces that were either colonized with SCNs or free of SCNs were collected from cultured roots and then trimmed free of lateral roots. Root tips (2.0 cm) were collected from non-inoculated roots and then dissected into 0–0.5 cm and 0.5–2.0 cm pieces for ACC extraction.
The number in parentheses indicates the approximate number of individual nematodes in the sample.
ND, ethylene not detected by the GC integrator.
Bent et al. (2006) reported that the number of nematodes on identically inoculated roots was inhibited by 50% in a soybean mutant that was partially resistant to ethylene action. To determine if a more complete block of ethylene action might produce a greater inhibition of SCN numbers, ethylene action was inhibited with 2 μl l−1 1-MCP or 5000 μl l−1 NBD. 1-MCP and NBD reduced nematode numbers on cultured roots by nearly 90% (Fig. 2A). 1-MCP is a non-competitive inhibitor of ethylene action and its effect on ethylene action appears to be irreversible until new receptors are synthesized in the absence of 1-MCP (Sisler, 2006). NBD, on the other hand, is a competitive inhibitor of ethylene and its effect on ethylene action can be reversed by addition of an excessive amount of ethylene (Sisler, 2006). To determine which stage of SCN development was sensitive to ethylene inhibition 5000 μl l−1 NBD was applied for 10 d intervals at 0–10, 10–20, and 20–30 dpi. Treatment of the infected roots with NBD at 10 d intervals reduced the number of mature females counted at 30 dpi by ∼20% at all three stages (Fig. 2B). This is in contrast to the continuous (0–30 dpi) treatment that inhibited SCN numbers by >95% (Fig. 2B). NBD at the high concentration of 5000 μl l−1 was chosen to maximize inhibition of the ethylene response (Sisler et al., 1986); however, NDB inhibition of SCN numbers was only partly reversed by the addition of 100 μl l−1 ethylene. A reduced level of NBD, 2000 μl l−1, was less effective on nematode numbers but the inhibition was fully reversed with 25 μl l−1 ethylene; however, root morphology looked similar between the NBD-treated and non-treated roots (result not shown), indicating that ethylene action was not fully inhibited at 2000 μl l−1. NBD at 5000 μl l−1, as did 2 μl l−1 1-MCP, caused the roots to grow long and thin with less branching and a slightly lower total root mass than the controls.
Fig. 2.
Inhibition of ethylene action reduces the numbers of SCNs that develop on soybean roots, and addition of ethylene increases the number. (A) Continuous exposure of SCN-inoculated monoxenic roots to no inhibitors (control), 2 μl l−1 1-MCP, 5000 μl l−1 NBD, or 1 μl l−1 ethylene. (B) Exposure of monoxenic roots to 5000 μl l−1 NBD for 10 d intervals preceded or followed by exposure to air with no inhibitor, or continuous exposure of roots to 5000 μl l−1 NBD for 30 d or to 5000 μl l−1 NBD plus 100 μl l−1 ethylene. (C) Continuous exposure of monoxenic roots to 2000 μl l−1 NBD or 2000 μl l−1 NBD plus 25 μl l−1 ethylene.
SCNs at 30 dpi appeared smaller in all of the 5000 μl l−1 NBD treatments compared with SCNs on the non-treated SCN-colonized roots. The small size might indicate that the inhibitors impeded development of the feeding structure or nutrient flow by their inhibition of ethylene action. The observation that 5000 μl l−1 NBD given at 10 d intervals consistently inhibited SCN numbers suggests that ethylene might be important throughout the SCN life cycle in the root.
Testing the possibility of ACC synthesis in the nematode
A tBLASTn search of translated H. glycines expressed sequence tag (EST) sequences at the NCBI with the protein sequence for the Arabidopsis ACS 2 (AtACS2) produced an alignment to the H. glycines accession CK350381 with a score of 72 and an expect value of 2E-13. This similarity score and expect value are not particularly high or low, respectively; nevertheless, interestingly the CK350381 clone was from an oesophageal gland cell library, where an enzymatic product, i.e. ACC, might be secreted into the host. Using 5′ and 3′ RACE (rapid amplification of cDNA ends), a full-length sequence (accession GQ389647) for the CK350381 EST was obtained. Semi-quantitative real-time PCR was used to demonstrate that the ACS-like transcript (GQ389647) was expressed in eggs, J2, and at 2, 4, 8, 12, and 20 dpi. The normalized expression of the ACS-like transcript was highest in J2 and at 20 dpi but was also present in eggs at ∼50% of that in J2 (data not shown). The ORF for the full-length sequence does not appear to encode an N-terminal signal peptide that would be expected for a protein secreted from the nematode (Davis et al., 2008). The translated ORF was used for a tBLASTn search of Arabidopsis sequences at the NCBI. Several ACSs aligned with the GQ389647 peptide, having bit scores between 150 and 174 and expect values between 2E-35 and 1E-42. However, alignment of the H. glycines ACS-like peptide with eight authentic Arabidopsis ACSs indicated that the H. glycines sequence did not contain the box2 motif (FQDYHGL) that Yamagami et al. (2003) found to be essential for ACS activity.
However, another as yet undiscovered nematode protein might have ACS activity. To test this possibility, ACC was measured in hatched infective juveniles (∼50 000 J2) and SCNs pulled off the roots (∼300 females) at 16 dpi and 20 dpi. The female SCNs can be pulled off the roots at these stages of growth with the oesophageal glands and mouth parts intact (Tucker et al., 2005). No detectable amount of ACC was found at any of these stages of development (Table 1).
ACS gene expression in infected and non-infected soybean roots
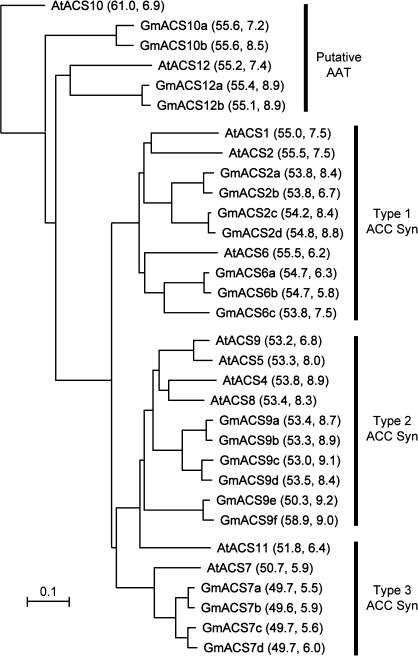
Twenty-one ACS-like sequences were found in the soybean genomic sequence database. Alignment of translated ORFs for all 21 of the soybean sequences with 11 ACS-like sequences from Arabidopsis (Fig. 3) indicated that 17 GmACS-like peptides were similar to eight AtACS peptides demonstrated to synthesize ACC (Yamagami et al., 2003). Four of the 21 GmACS-like sequences matched most closely to AtACS10 and AtACS12, which are presumed to be amino acid transferases (AATs) and not ACSs (Yamagami et al., 2003). The percentage identity between the eight different Arabidopsis ACSs and any of the 17 soybean ACS-like peptides ranged between 74% and 86% (data not shown). The soybean sequences were numbered based on the most similar Arabidopsis sequence and then a letter follows the number to distinguish among similar soybean sequences (Fig. 3).
Fig. 3.
Dendrogram of soybean and Arabidopsis ACS-like protein sequences. AtACS10 and AtACS12 do not have ACS activity and are most probably amino acid transferases (AATs) (Yamagami et al., 2003). AtACS1 also does not have ACS activity but AtACS2 does (Yamagami et al., 2003). The ACS sequences are labelled by types based on similarity in their C-terminal sequence (Yoshida et al., 2005). The numbers in parentheses are the molecular weight and isoelectric point, respectively, of the predicted proteins.
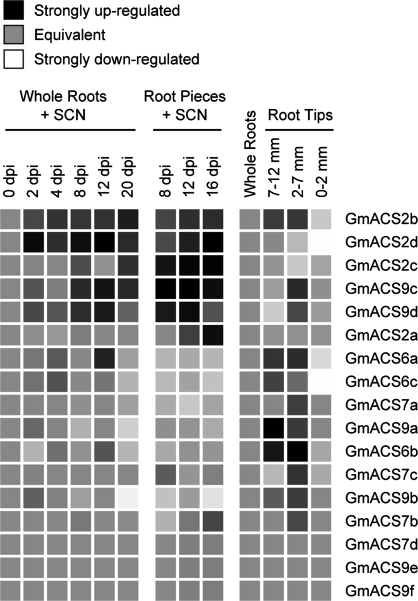
Semi-quantitative real-time PCR was used to quantify relative change in expression of each of the 17 GmACS genes. The results are plotted as a heat-map of the log base 2 ratios of the SCN-inoculated roots relative to the non-inoculated roots or, for root tips, the expression in the dissected portions relative to the expression in the whole root system (Fig. 4). The elevated expression for some of the ACS genes observed in the SCN-colonized root pieces is supported by a lower but similar expression pattern observed in the whole root collections (Figs 4 and 5). Of interest is that the expression patterns for ACS in root tips, where the highest concentration of ACC occurs (Table 1), is different from those in the SCN-colonized roots (Fig. 4).
Fig. 4.
A heat-map display of log2 ratios for the expression results of real-time PCR. A black box indicates a strong up-regulation, a neutral grey box equivalent expression, a white box a strong down-regulation, and shades of grey in between indicate intermediate levels of regulation. Expression profiles for each gene were grouped by hand to cluster genes with similar expression profiles. RNA was isolated from whole roots and trimmed root pieces of SCN-colonized roots or non-inoculated roots at the indicated dpi, and non-inoculated whole roots and root tips dissected at 0–2 and 2–7 mm proximal to the root apex. Where RNA was extracted from SCN-inoculated and non-inoculated whole roots or root pieces, the ratio is for the means for biological replicates of root collections taken at the same dpi. Root tip dissections are relative to the expression in whole roots collected at the same time as the root tips.
Fig. 5.
Graphic presentation of the linear gene expression profiles of normalized real-time PCR results. Results for roots inoculated with SCNs are indicated with black circles and non-inoculated roots are indicated with triangles. Note that the scale changes for some graphs in order to better illustrate differences in gene expression when they were much greater or less than the others. The means and standard error bars for whole roots are for three separate experiments and those for root pieces are for two experiments. Means without error bars are for a large number of pooled root tip and whole root collections.
Because it is difficult to assess the variability and relative expression levels in a heat-map, five of the GmACS genes that markedly change with SCN colonization and two that produced the strongest relative amplification signal are plotted as linear and bar graphs in Fig. 5. The linear graphs highlight a spike in ACS gene expression at 2 dpi that is not easily discerned in the heat-map because the spike often occurs in both the inoculated and non-inoculated roots (Fig. 5 and results not plotted). The ACS genes with the highest relative expression in the whole root RNA samples were mostly for genes that did not increase in the SCN-colonized roots (Fig. 5 and results not shown). The expression of a few genes actually decreased in the SCN-colonized root pieces compared with non-infected root pieces (Fig. 4), which is exemplified by the strongly expressed GmACS6a gene plotted in Fig. 5.
Discussion
The current study indicates that the plant hormone ethylene is important to SCN colonization of soybean roots but probably is not essential for successful colonization, which is consistent with earlier studies (Wubben et al., 2001). The possibility that SCNs produce their own ethylene or secrete an ACS or ACC into the host was considered. A partial length ACS-like sequence was found in the NCBI database and a full-length clone was obtained for this sequence. The full-length SCN ACS-like sequence does not encode an N-terminal signal peptide common to secreted proteins (Davis et al., 2008) and the predicted protein lacks an essential conserved motif found in other authentic ACSs (Yamagami et al., 2003). Moreover, no significant amount of ACC was found in infective SCNs or SCNs pulled off the root. It therefore seems unlikely that SCNs synthesize ACC itself but rely on some other means of regulating ethylene in the host.
Inhibition of ethylene action with the competitive inhibitor NBD at different stages of SCN development on cultured soybean roots suggests that ethylene impacts SCN growth throughout its 30 d life cycle on soybean roots (Fig. 2). Moreover, the sustained up-regulation of a select set of ACS genes for most of the SCN life cycle on soybean roots (Figs 4 and 5) and the higher concentration of ACC in SCN-colonized root pieces at 20 dpi (Table 1) support a prolonged role for ethylene in SCN colonization of soybean roots.
Ethylene synthesis in excised tomato roots infected with the root knot nematode Meloidogyne javanica was reported to increase several fold (Glazer and Apelbaum, 1983). However, in the current study, no increase in ethylene was observed in the ambient air surrounding the SCN-colonized roots. Nevertheless, the ACC concentration in root pieces heavily colonized by SCN was ∼3-fold higher than in control, non-infected root pieces (Table 1). The inability to detect an increase in ethylene in the infected cultured roots may be related to the observation that the ACC concentration is highest in root tips, which may have masked any increase in ethylene synthesis associated with nematode infection (Figs 4 and 5). ACC synthesis is considered to be the rate-limiting step in ethylene synthesis (Yamagami et al., 2003); however, several factors can influence the conversion of ACC to ethylene, i.e. ACC conjugation (Clarke et al., 1996), ACC deaminase (McDonnell et al., 2009), and ACC oxidase activity (Tang et al., 1994). Nevertheless, the higher concentration of ACC in the root tips correlated with the semi-quantitative real-time PCR results, indicating that the relative concentration of ACS transcripts for many ACS genes is highest in the first 12 mm of the root tips. Cell division and initiation of the vascular system all begin within the first 2 mm of the root tip (Birnbaum et al., 2003; Tucker et al., 2007). Relatively low levels of ACS transcript were found in the first 2 mm of the root tip (Figs 4 and 5). The 2–7 mm portion of the root is where most of the cell elongation occurs, the vascular system matures, and root hair initiation and growth begin (Birnbaum et al., 2003; Tucker et al., 2007). Root hair initiation and growth continue at a slightly reduced rate in the 7–12 mm portion, and radial expansion of the root cells is evident (Tucker et al., 2007). Thus, the expression patterns for ACS in the dissected root tips suggest that ACC and presumably ethylene synthesis correlate best with vascular maturation and root hair initiation and growth. The root tip is also a region where the auxin concentration is highest in roots (Ruzicka et al., 2007; Negi et al., 2008). Moreover, ethylene was demonstrated to affect the distribution of auxin in the root tip and was proposed to be involved in the movement of auxin in the root (Ruzicka et al., 2007; Negi et al., 2008).
Yamagami et al. (2003) proposed that ACS isozymes are biochemically distinct, permitting their differential expression to have specialized tissue- or cell-specific effects. Notable regarding the ACS gene expression in the SCN-colonized roots is the observation that it is clearly different from that in the root tips (Fig. 4). A question that now comes to mind is: what environmental stimuli or developmental signal not found in the root tip might produce a pattern of ACS gene expression similar to that of SCN? There are several processes that might be considered; for example, wounding, lateral root initiation, and aerenchyma formation.
Wound-induced expression of ACS in soybean roots was not specifically addressed in this study. However, transfer of 2-week-old soybean plants from Perlite to new containers for SCN inoculation or mock inoculation may cause wounding. Moreover, the penetration of roots and migration of the nematode through the root may induce a wound response. In this regard, it is interesting that there was a spike in gene expression at 2 dpi for several of the ACS genes. Two dpi may be the tale end of a larger spike in ACS expression that occurred earlier at the root transfer stage or during SCN penetration of the root, which occurs within this time frame. Although a spike was observed in the gene expression for some other ACS genes not included in Fig. 5, the spike was generally higher in the SCN-inoculated plants, which correlates with the additional wounding associated with penetration and migration of the nematode into the root. However, although wounding may contribute to the increased expression of some of the ACSs, wounding probably is not responsible for the sustained up-regulation of ACS throughout the life cycle of SCN on the root.
The root tip collection was limited to 12 mm proximal to the root apex because lateral root initials were occasionally apparent on root sections more proximal than 12 mm. Duplication of the root tip in lateral roots would have complicated interpretation of the gene expression patterns in the root tip. Although lateral root initiation should include many developmental processes similar to the root tip, for example cell division, cell elongation, and vascular differentiation and maturation, the emergence of the lateral root through the root cortex may require enzymatic separation of cells immediately above the lateral root that would not occur in the root tips. This hypothesis is supported by studies on the expression of a polygalacturonase specific to the separating cortical cells immediately above the lateral root tip (Roberts et al., 2002). In addition to cell wall hydrolases, the process of cell separation in the cortex cells may involve localized ethylene synthesis and, therefore, up-regulation of ACS gene expression. Identification of the ACS genes, if any, which are expressed specifically in the splitting cortical cells remains to be carried out.
Roles for ethylene in SCN colonization of roots have been proposed. Wubben et al. (2004) proposed that ethylene might increase nematode susceptibility by suppressing RHD1, an Arabidopsis UDP-glucose-4-epimerase that when suppressed causes increased root hair elongation, decreased root length, and root epidermal bulging. Although root hair length might affect recognition and attachment of the nematode to the root, it is less likely to be important to sustained growth of the nematode. Goverse et al. (2000) proposed several years ago that ethylene might regulate expression of cell wall-modifying proteins that are necessary for cell wall degradation and syncytium formation. It seems likely that ethylene would play a role in this process, and many genes for cell wall-modifying proteins are up-regulated during syncytium development (Wieczorek et al., 2006; Ithal et al., 2007a, b; Tucker et al., 2007); however, ethylene cannot be the only factor that regulates their expression. Auxin very probably plays a role in regulating the expression of cell wall-modifying genes and other factors too (Goverse et al., 2000).
Considered all together, the ethylene inhibitor studies, the finding that the ACC concentration was 3-fold higher in SCN-colonized root pieces, and the unique ACS gene expression pattern in SCN-infected roots all indicate that ethylene plays an important role for much, if not all, of the SCN life cycle in roots. It remains to be determined, however, if the changes in ACS gene expression occur in syncytium or the cells surrounding the syncytium, or both. Where the changes in ACS occur will be important to identifying the precise role of ethylene and the signals that trigger the changes in ACS gene expression.
Supplementary data
Supplementary data are available at JXB online and at http://bldg6.arsusda.gov/mtucker/Public/ACSexpression.html.
Table S1. FASTA file of sequences used for primer design and protein translations. Sequences include the open reading frames plus 50 nt of 3′ untranslated regions.
Table S2. Soybean ACC synthases (GmACS) and elongation factor 1b (GmEF1b) primers used for semi-quantitative real-time PCR.
Supplementary Material
Acknowledgments
We thank Autar Mattoo and Theophanes Solomos for helpful discussions and advice on the ethylene inhibitor studies and the quantification of ACC and ethylene.
References
- Bent AF, Hoffman TK, Schmidt JS, Hartman GL, Hoffman DD, Xue P, Tucker ML. Disease- and performance-related traits of ethylene-insensitive soybean. Crop Science. 2006;46:893–901. [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- Brown JWS, Smith P, Simpson CG. Arabidopsis consensus intron sequences. Plant Molecular Biology. 1996;32:531–535. doi: 10.1007/BF00019105. [DOI] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiology. 1999;121:53–60. doi: 10.1104/pp.121.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Kalantari KM, Smith AR. The quantification of 1-(malonylamino)cyclopropane-1-carboxylic acid in plant tissues by quantitative mass spectrometry. Plant Growth Regulation. 1996;19:133–137. [Google Scholar]
- Davis EL, Hussey RS, Baum TJ. Getting to the roots of parasitism by nematodes. Trends in Parasitology. 2004;20:134–141. doi: 10.1016/j.pt.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Mitchum MG, Baum TJ. Parasitism proteins in nematode–plant interactions. Current Opinion in Plant Biology. 2008;11:360–366. doi: 10.1016/j.pbi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Jr., Inze D, Van Montagu M, Engler G, Gheysen G. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. The Plant Cell. 1999;11:793–808. doi: 10.1105/tpc.11.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS. Identification of putative parasitism genes expressed in the esophageal gland cells of the soybean cyst nematode Heterodera glycines. Molecular Plant-Microbe Interactions. 2001;14:1247–1254. doi: 10.1094/MPMI.2001.14.10.1247. [DOI] [PubMed] [Google Scholar]
- Glazer I, Apelbaum A. Interrelationships between ethylene production, gall formation, and root-knot nematode development in tomato plants infected with Meloidogyne javanica. Journal of Nematology. 1983;15:539–554. [PMC free article] [PubMed] [Google Scholar]
- Golinowski W, Sobczak M, Grundler FMW. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma. 1996;194:103. [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J. Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Molecular Plant-Microbe Interactions. 2000;13:1121–1129. doi: 10.1094/MPMI.2000.13.10.1121. [DOI] [PubMed] [Google Scholar]
- Grundler FMW, Sobczak M, Golinowski W. Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii. European Journal of Plant Pathology. 1998;104:545. [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Hearne L, Maier T, Baum TJ, Mitchum MG. Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Molecular Plant-Microbe Interactions. 2007a;20:293–305. doi: 10.1094/MPMI-20-3-0293. [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Molecular Plant-Microbe Interactions. 2007b;20:510–525. doi: 10.1094/MPMI-20-5-0510. [DOI] [PubMed] [Google Scholar]
- Jung C, Wyss U. New approaches to control plant parasitic nematodes. Applied Microbiology and Biotechnology. 1999;51:439–446. [Google Scholar]
- Liu D, Li N, Dube S, Kalinski A, Herman E, Mattoo AK. Molecular characterization of a rapidly and transiently wound-induced soybean (Glycine max L.) gene encoding 1-aminocyclopropane-1-carboxylate synthase. Plant and Cell Physiology. 1993;34:1151–1157. [Google Scholar]
- Lizada Ma CC, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Analytical Biochemistry. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Matthews BF, MacDonald MH, Thai VK, Tucker ML. Molecular characterization of arginine kinase in the soybean cyst nematode (Heterodera glycines) Journal of Nematology. 2003;35:252–258. [PMC free article] [PubMed] [Google Scholar]
- McDonnell L, Plett JM, Andersson-Gunneras S, Kozela C, Dugardeyn J, Van Der Straeten D, Glick BR, Sundberg B, Regan S. Ethylene levels are regulated by a plant encoded 1-aminocyclopropane-1-carboxylic acid deaminase. Physiologia Plantarum. 2009;136:94–109. doi: 10.1111/j.1399-3054.2009.01208.x. [DOI] [PubMed] [Google Scholar]
- Meyer SLF, Chitwood DJ, Crowley P. Influence of soybean cultivar on reproduction of Heterodera glycines in monoxenic culture. Journal of Nematology. 1997;29:389–394. [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthoff DP, Ehrenfried ML, Vinyard BT, Tucker ML. GeneChip profiling of transcriptional responses to soybean cyst nematode, Heterodera glycines, colonization of soybean roots. Journal of Experimental Botany. 2007;58:3407–3418. doi: 10.1093/jxb/erm211. [DOI] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiology. 2002;130:1908–1917. doi: 10.1104/pp.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Elliott KA, Gonzalez-Carranza ZH. Abscission, dehiscence, and other cell separation processes. Annual Review of Plant Biology. 2002;53:131–158. doi: 10.1146/annurev.arplant.53.092701.180236. [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnology Advances. 2006;24:357–367. doi: 10.1016/j.biotechadv.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Sisler EC, Reid MS, Yang SF. Effect of antagonists of ethylene action on binding of ethylene in cut carnations. Plant Growth Regulation. 1986;4:213–218. [Google Scholar]
- Tang X, Gomes A, Bhatia A, Woodson WR. Pistil-specific and ethylene-regulated expression of 1-aminocyclopropane-1-carboxylate oxidase genes in petunia flowers. The Plant Cell. 1994;6:1227–1239. doi: 10.1105/tpc.6.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker ML, Burke A, Murphy CA, Thai VK, Ehrenfried ML. Gene expression profiles for cell wall-modifying proteins associated with soybean cyst nematode infection, petiole abscission, root tips, flowers, apical buds, and leaves. Journal of Experimental Botany. 2007;58:3395–3406. doi: 10.1093/jxb/erm188. [DOI] [PubMed] [Google Scholar]
- Tucker ML, Xue P, Raina A, Ehrenfried ML, Asif M, Thai VK. Characterization of several Heterodera glycines mRNA that encode small proteins with putative signal peptides. Journal of Nematology. 2005;37:422–428. [PMC free article] [PubMed] [Google Scholar]
- Wieczorek K, Golecki B, Gerdes L, et al. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. The Plant Journal. 2006;48:98–112. doi: 10.1111/j.1365-313X.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- Williamson VM, Gleason CA. Plant–nematode interactions. Current Opinion in Plant Biology. 2003;6:327–333. doi: 10.1016/s1369-5266(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Wrather JA, Koenning SR. Estimates of disease effects on soybean yields in the United States 2003 to 2005. Journal of Nematology. 2006;38:173–180. [PMC free article] [PubMed] [Google Scholar]
- Wubben MJE, Rodermel SR, Baum TJ. Mutation of a UDP-glucose-4-epimerase alters nematode susceptibility and ethylene responses in Arabidopsis roots. The Plant Journal. 2004;40:712–724. doi: 10.1111/j.1365-313X.2004.02257.x. [DOI] [PubMed] [Google Scholar]
- Wubben MJE, Su H, Rodermel SR, Baum TJ. Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Molecular Plant-Microbe Interactions. 2001;14:1206–1212. doi: 10.1094/MPMI.2001.14.10.1206. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. Journal of Biological Chemistry. 2003;278:49102–49112. doi: 10.1074/jbc.M308297200. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nagata M, Saito K, Wang KL, Ecker JR. Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biology. 2005;5:14. doi: 10.1186/1471-2229-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.