Abstract
Chloroplasts produce reactive oxygen species (ROS) during cellular stress. ROS are known to act as regulators of programmed cell death (PCD) in plant and animal cells, so it is possible that chloroplasts have a role in regulating PCD in green tissue. Arabidopsis thaliana cell suspension cultures are model systems in which to test this, as here it is shown that their cells contain well-developed, functional chloroplasts when grown in the light, but not when grown in the dark. Heat treatment at 55 °C induced apoptotic-like (AL)-PCD in the cultures, but light-grown cultures responded with significantly less AL-PCD than dark-grown cultures. Chloroplast-free light-grown cultures were established using norflurazon, spectinomycin, and lincomycin and these cultures responded to heat treatment with increased AL-PCD, demonstrating that chloroplasts affect AL-PCD induction in light-grown cultures. Antioxidant treatment of light-grown cultures also resulted in increased AL-PCD induction, suggesting that chloroplast-produced ROS may be involved in AL-PCD regulation. Cycloheximide treatment of light-grown cultures prolonged cell viability and attenuated AL-PCD induction; however, this effect was less pronounced in dark-grown cultures, and did not occur in antioxidant-treated light-grown cultures. This suggests that a complex interplay between light, chloroplasts, ROS, and nuclear protein synthesis occurs during plant AL-PCD. The results of this study highlight the importance of taking into account the time-point at which cells are observed and whether the cells are light-grown and chloroplast-containing or not, for any study on plant AL-PCD, as it appears that chloroplasts can play a significant role in AL-PCD regulation.
Keywords: Antioxidant, apoptosis, Arabidopsis thaliana, cell suspension culture, chloroplast, light, programmed cell death, reactive oxygen species
Introduction
Programmed cell death (PCD) is a cellular process in plants that is an essential component of several developmental programmes. It can occur in response to environmental stresses and is often a part of the defence mechanism against pathogen attack via the hypersensitive response (reviewed by Jones, 2001; Williams and Dickman, 2008). Apoptotic-like PCD (AL-PCD) is one type of cell death that can be identified by a distinctive corpse morphology where the cytoplasm has condensed away from the cell wall and nuclear DNA has been fragmented. AL-PCD is a controlled process that is initiated by the cells themselves (McCabe et al., 1997; Danon et al., 2000; Reape et al., 2008) and is distinct from necrosis, an unorganized cell death that results from overwhelming stress and severe cell damage and does not result in cytoplasmic condensation. As condensed cell morphology and fragmented DNA are distinguishing features of AL-PCD in plants, they can be used as markers to determine levels of AL-PCD in plant cells (Danon et al., 2000; Reape and McCabe, 2008).
The mitochondrion has been shown to regulate PCD in a number of ways; for example, cytochrome c is released early in the PCD process (Balk et al., 1999). Cytochrome c is a component of the electron transport chain and one effect of its release is the production of reactive oxygen species (ROS), which have been implicated as regulators of PCD in both animal and plant cells (Jabs, 1999; Jones, 2001). Environmental stresses result in excess excitation energy within the chloroplast, which leads to production of ROS, so this organelle may also be involved in the regulation of stress responses including AL-PCD, and may act as a sensor of cellular stress (Mullineaux and Karpinski, 2002). A number of studies have shown that plant AL-PCD, including hypersensitive response progression, is affected by light (Genoud et al., 2002; Danon et al., 2004; Zeier et al., 2004; Dzyubinskaya et al., 2006). It has been found that the hypersensitive response was accelerated by the loss of chloroplast function (Seo et al., 2000) and several recent studies have linked chloroplast-produced ROS with the hypersensitive response (reviewed by Mur et al., 2008). ROS have been shown to influence senescence in tobacco, as Zapata et al. (2005) demonstrated that inactivating the plastid ndhF gene, thereby altering levels of ROS, resulted in delayed senescence, while chloroplast-produced ROS have been shown to be capable of transmitting the spread of wound-induced PCD through maize tissue (Gray et al., 2002). In addition, research by Chen and Dickman (2004) on the transgenic expression of animal anti-apoptotic Bcl-2 family members in tobacco have implicated chloroplast involvement in oxidative stress-induced PCD. Bcl-2 family proteins were found to localize to chloroplasts and to suppress PCD induction by herbicides that generate oxidative stress, suggesting that chloroplasts can mediate plant PCD under oxidative stress conditions.
AL-PCD can be initiated in undifferentiated Arabidopsis thaliana cells in suspension culture and may easily be observed and quantified using the hallmark features of condensed cell morphology and fragmented DNA (McCabe and Leaver, 2000). Cell suspension cultures are often used in plant PCD studies, but because the cells are grown in sucrose medium, they may be grown in the dark and usually do not contain chloroplasts even when grown in the light. Therefore, many of these cell culture-based studies cannot test any possible effects of chloroplasts on the regulation of plant PCD. However, our A. thaliana cultures are green in colour if grown in the light and it is shown here that growth in the light stimulates the biosynthesis of functional chloroplasts. This system is ideally suited to studies on the involvement of chloroplasts in plant AL-PCD because the cells contain chloroplasts if grown in the light, but not if grown in the dark. The present study shows that light-grown and dark-grown culture cells respond differently to a PCD-inducing stimulus, resulting in different levels of cell condensation and DNA fragmentation. Evidence that chloroplasts regulate plant AL-PCD, and that this regulation may involve chloroplast-produced ROS, is presented. Data are also presented suggesting a complex interplay between light, chloroplasts, ROS, and nuclear protein synthesis during AL-PCD. The data suggest the chloroplasts may be an important factor in AL-PCD regulation in green tissue. These results stress the importance, for any study on plant PCD, of taking into account the time-point at which cell death is observed and whether the cells under study are light-grown and chloroplast-containing or not.
Materials and methods
Maintenance of Arabidopsis thaliana cell suspension cultures
Arabidopsis thaliana cell suspension cultures were kindly provided by Professor CJ Leaver, University of Oxford, UK (May and Leaver, 1993). These were maintained on a 7 d culture cycle by adding 10 ml of culture to 100 ml of medium containing 30 g l−1 sucrose, 4.3 g l−1 Murashige and Skoog basal salt mixture, 500 μl l−1 naphthaleneacetic acid, and 50 μl l−1 kinetin in distilled water, pH 5.8. Cultures were maintained at a constant temperature of 23 °C on orbital shaker tables set at 100 rpm. Dark-grown cultures were maintained in constant darkness and light-grown cultures were maintained under a continuous light intensity of 4 μmol photons m−2 s−1.
Heat treatments of cultures
Ten ml samples of 7-d-old cultures were placed on an orbital shaker set at 80 rpm inside a water bath of 55 °C for 10 min. Samples were then returned to the conditions under which their parent cultures were maintained for a set time period (either 5 h or 24 h) before measuring cell death levels.
Chemical treatments of cultures
Chemicals were purchased from Sigma–Aldrich Ireland. Norflurazon, spectinomycin, and lincomycin treatments were applied to cultures at concentrations of 1 μM, 0.2 mM, and 2 mM, respectively, at the time of culturing and the cultures were maintained in light. All other chemical treatments were applied to samples of 7-d-old cultures. Solutions of sodium ascorbate, ascorbate, and reduced glutathione were made directly before the treatment of culture samples. Samples were treated with sodium ascorbate or ascorbate at a concentration of 25 mM, 24 h before heat treatments, or with glutathione at a concentration of 5 mM, directly prior to heat treatments. Culture samples were treated with cycloheximide at a concentration of 25 μg ml−1 (89 μM), 24 h before heat treatments.
Measuring cell death levels
Using fluorescein diacetate as a vital stain, cells were scored as either alive, dead via necrosis, or dead via AL-PCD with the light microscope, using the methods described by McCabe and Leaver (2000) and Reape et al. (2008). Dead cells that displayed the hallmark morphology of condensed cell, retracted away from the cell wall, were scored as dead via AL-PCD (see Fig. 1). Dead cells that were not condensed were scored as necrotic. Viable cells were not condensed. AL-PCD can also be scored by observing the hallmark feature of DNA fragmentation (McCabe et al., 1997; McCabe and Leaver, 2000). The fluorescent FragEL kit (Calbiochem) was used to perform the TUNEL (TdT-mediated deoxyuridine triphosphate nick end labelling) assay to identify DNA fragmentation. Samples were maintained at 4 °C during the procedure and washed with TBS between each step. Cells were fixed in 4% w/v formaldehyde in PBS for 30 min, before being permeabilized in 20 μg ml−1 proteinase K in 10 mM TRIS, pH 8, at room temperature for 5 min. Cells were then transferred to equilibration buffer at room temperature for 20 min and then incubated in a prepared mixture of TdT enzyme and labelling reaction mix at 37 °C in darkness for 1.5 h for fragment end labelling. Cells were then mounted in DAPI and DAPI-stained nuclei and fluorescein-labelled nuclei, containing fragmented DNA, were observed with fluorescence microscopy.
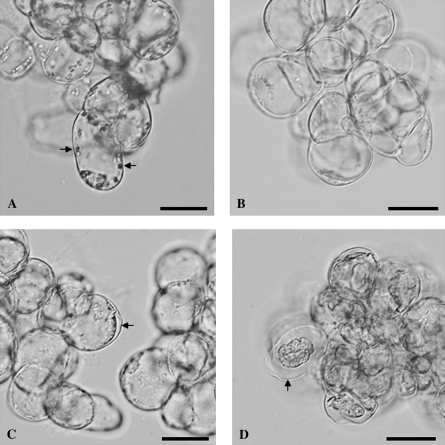
Fig. 1.
Morphology of living and AL-PCD cells from A. thaliana cell suspension cultures. (A, B) Living cells from (A) light-grown culture and (B) dark-grown culture. Note the large organelles in the light-grown cells (A), indicated by the two arrows, which were absent from the dark-grown cells (B). (C, D) Dead cells from (C) light-grown culture and (D) dark-grown culture, 24 h after heat treatment. These cells displayed the AL-PCD hallmark morphology of cell condensation, visible as a gap between the cell wall and cytoplasm, indicated by the arrows in both (C) and (D). Note that the cell condensation was far more extreme in dark-grown (D) than in light-grown (C) cells. Scale bars represent 20 μm.
Transmission electron microscopy
Samples were fixed in a solution of 3% w/v paraformaldehyde and 2.5% w/v glutaraldehyde in 0.08 M pipes buffer for 2 h and then washed twice with 0.08 M pipes buffer, pH 8, before post-fixing in 1% w/v osmium tetroxide for 1 h. Following two washes with distilled water, samples were dehydrated through an ethanol and propylene oxide series as follows: 70% v/v ethanol for 10 min, 90% v/v ethanol for 10 min, 90% v/v ethanol for 10 min, 100% v/v ethanol for 10 min, 100% v/v ethanol for 15 min, 100% v/v propylene oxide for 15 min, 100% v/v propylene oxide overnight, and finally 100% v/v propylene oxide for 1 h. Samples were embedded in low viscosity resin using the hard resin recipe supplied with the resin kit (Agar Scientific). For embedding, samples were firstly transferred to a mixture of 50% v/v resin and 50% v/v propylene oxide for 4 h, then transferred to fresh 100% v/v resin for infiltration overnight, and finally transferred to fresh 100% v/v resin and incubated at 60 °C overnight for polymerization. The resin blocks were trimmed with glass knives before sectioning on a Reichert–Jung microtome with a diamond knife to a thickness of 100 μm. Sections were stained with uranyl acetate for 20 min and then with lead citrate for 10 min before viewing with a Tecnai transmission electron microscope.
Pigment absorbance spectra
Pigments were extracted from approximately 0.02 g of leaves from 6-week-old A. thaliana plants and from 2–5 g of 7-d-old A. thaliana culture cells by grinding in 80% v/v acetone for several minutes. Samples were then centrifuged and the supernatants analysed for their absorption spectra (wavelength range of 400–750 nm) using a Varian Cary 300 Bio UV-Visible spectrophotometer. Chlorophyll concentration was calculated using the following formula, developed by Arnon (1949) using the absorption coefficients calculated by Mackinney (1941):
Chlorophyll concentration (g m−3)=(20.2)(absorbance at 645 nm)+(8.02)(absorbance at 663 nm)
Fluorescence yield (Fv/Fm) measurements
One to 2 ml samples of 7-d-old cultures were filtered onto glass microfibre filter papers using a suction device. The resulting thin, uniform layer of cells was then dark-adapted for 25 min. Fluorescence yield (Fv/Fm) was then measured with a portable chlorophyll fluorometer: the Walz mini-PAM (pulse amplitude modulation) photosynthesis yield analyser.
Statistical analyses
Data sets were subjected to the Shapiro–Wilk test for normal distribution and the Levene test for equality of error variances and then either an independent samples t test or univariate or multivariate analysis of variance was performed. The Tukey HSD and Scheffe post hoc tests were used for equal and unequal n, respectively.
Results
Light-grown A. thaliana suspension culture cells contain functional chloroplasts
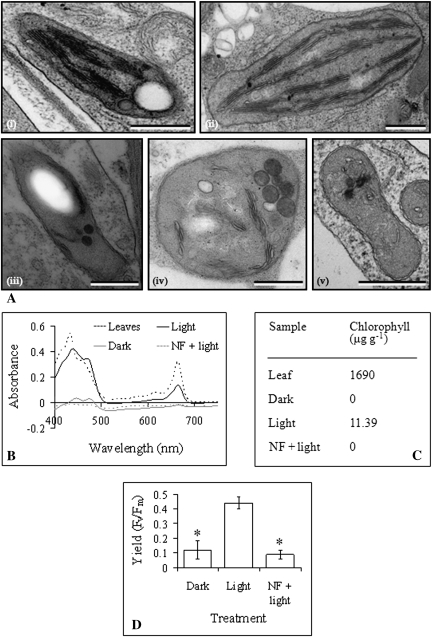
Our A. thaliana suspension cultures are green in colour when light-grown, but are beige in colour when dark-grown, and large organelles were observed with the light microscope in light-grown but not in dark-grown culture cells (Fig. 1). Transmission electron microscopy revealed that these organelles were chloroplasts, containing well-organized grana (Fig. 2A). These were compared to chloroplasts from A. thaliana leaves, and were found to be smaller on average, to contain less numerous grana, and to be less regular in shape than the leaf chloroplasts. Transmission electron microscopy also revealed the presence of proplastids in cells from dark-grown cultures (Fig. 2A), which were smaller than chloroplasts from light-grown cultures and contained only a few thylakoid membranes, which were not well-organized. Pigment extraction and absorbance measurements confirmed that dark-grown cultures did not contain chlorophyll, while light-grown cultures did, however, at a much lower concentration than is found in A. thaliana leaves (Fig. 2B, C). Measurements of fluorescence yield (Fv/Fm) suggested the presence of at least partial photosynthetic pathways in light-grown, but not in dark-grown cultures (Fig. 2D). An average fluorescence yield of 0.44 was measured in light-grown cultures, while an average fluorescence yield of just 0.12 was measured in dark-grown cultures.
Fig. 2.
Evidence for the presence of functional chloroplasts in light-grown A. thaliana culture cells. (A) Transmission electron microscopy images of a typical plastid from A. thaliana (i) light-grown culture, (ii) leaf tissue, (iii) dark-grown culture, (iv) norflurazon-treated light-grown culture, and (v) spectinomycin-treated light-grown culture. Scale bars represent 500 nm. (B) Absorbance spectra of pigments extracted from A. thaliana leaves and cells from dark-grown, light-grown, and norflurazon-treated light-grown cultures. (C) Chlorophyll concentrations (per g fresh weight) in A. thaliana leaves and cells from dark-grown, light-grown, and norflurazon-treated light-grown cultures, as determined using absorbance values taken from the absorbance spectra in (B). (D) Average fluorescence yield (Fv/Fm), representing photosynthetic potential of chloroplast photosystem II, of A. thaliana dark-grown, light-grown, and norflurazon-treated light-grown (NF+light) culture cells. Values are means and error bars represent SD (n ≥3 replicates). Fluorescence yield values marked with an asterisk were significantly different from fluorescence yield in light-grown cultures (P <0.001).
Light-grown and dark-grown culture cells react differently to an AL-PCD-inducing heat treatment
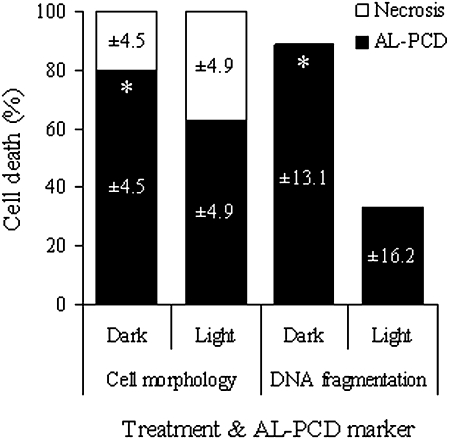
A heat treatment of 10 min duration at 55 °C will induce AL-PCD in the majority of cells in plant suspension cultures (McCabe et al., 1997), including A. thaliana cultures. However, the level of AL-PCD induced by this treatment was significantly higher for dark-grown than for light-grown cultures and this was shown using both the cell morphology and DNA fragmentation markers of AL-PCD (Fig. 3). AL-PCD was measured 24 h after the heat treatment because it was found that maximal cell death and cell condensation levels were observable at this time (data not shown). According to the cell morphology marker, the fraction of AL-PCD induced by the heat treatment was 80% in dark-grown and 63% in light-grown cultures, with all remaining cells necrotic. Using the DNA fragmentation marker (TUNEL assay), the fraction of cells with nuclei positive for DNA fragmentation was 89% in dark-grown and 33% in light-grown cultures.
Fig. 3.
Light-grown and dark-grown culture cells react differently to an AL-PCD-inducing heat treatment. Percentage of AL-PCD, necrosis, and living cells, scored using both cell morphology and DNA fragmentation markers of AL-PCD, in A. thaliana dark-grown and light-grown cultures, 24 h after heat treatment. Values are means ±SD (n ≥3 replicates). PCD values marked with an asterisk were significantly different from PCD in light-grown cultures for the same PCD marker (P=0.006 for cell morphology marker and P=0.001 for DNA fragmentation marker).
Suppressing chloroplast function in light-grown cultures results in dark-grown levels of AL-PCD
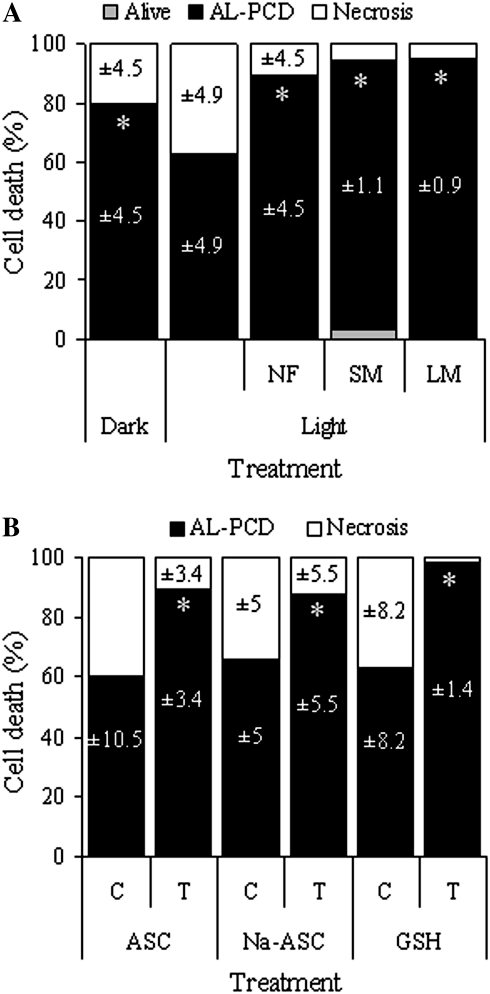
To determine whether the reduction in AL-PCD induction in light-grown cultures was specifically related to the presence of chloroplasts, light-grown cultures were developed in which chloroplast function was suppressed by the herbicide norflurazon (Strand et al., 2003) and in which chloroplast protein synthesis was suppressed by the antibiotics spectinomycin and lincomycin (Thomson and Ellis, 1972; Mulo et al., 2003). These light-grown cultures changed in colour to beige, as seen after several days of growth. Transmission electron microscopy revealed the presence of plastids in norflurazon culture cells, which were simpler in structure and smaller on average than chloroplasts from light-grown culture cells, yet more complex in structure than proplastids from dark-grown culture cells (Fig. 2A). These plastids usually contained many plastoglobuli and were often irregular in shape. Transmission electron microscopy revealed the presence of a very low number of small and simple proplastids in spectinomycin culture cells, which contained very few thylakoid membranes (Fig. 2A). Similar proplastids were also present in lincomycin culture cells (data not shown). Pigment extraction and absorbance confirmed that the norflurazon culture cells did not contain chlorophyll (Fig. 2B, C) and the fluorescence yield of the norflurazon culture cells averaged just 0.09 (Fig. 2D). AL-PCD induction in norflurazon, spectinomycin, and lincomycin cultures was increased to 90%, 91%, and 95% of cells, respectively, in comparison with untreated light-grown cultures (63% of cells; Fig. 4A). In spectinomycin cultures, 3.7% of cells remained alive. All remaining cells were necrotic.
Fig. 4.
Evidence suggesting chloroplast and ROS involvement in AL-PCD. Percentage of AL-PCD, necrosis, and living cells in cultures, scored using the cell morphology marker of AL-PCD, 24 h after heat treatment. Values are means ±SD (n ≥3 replicates). (A) Dark-grown cultures and light-grown cultures, untreated and treated with the chloroplast inhibitors norflurazon (NF), spectinomycin (SM), and lincomycin (LM). PCD values marked with an asterisk were significantly different from PCD in untreated light-grown cultures (P=0.026 for dark-grown cultures and P <0.001 for NF, SM, and LM-treated cultures). (B) Light-grown cultures, control (C; untreated) and treated (T) with the antioxidants ascorbate (ASC), sodium ascorbate (Na-ASC), and reduced glutathione (GSH). PCD values marked with an asterisk were significantly different from PCD in the untreated control for that treatment (P=0.001 for ASC and Na-ASC and P <0.001 for GSH).
Treating light-grown cultures with antioxidants results in dark-grown levels of AL-PCD
As chloroplasts are important producers of ROS in plant cells (Mittler et al., 2004) and ROS are thought to be important cell signals during plant PCD (Jabs, 1999; Mittler, 2002; Overmyer et al., 2003), it was of interest to determine whether the effects of light and chloroplast suppression on AL-PCD induction could be associated with ROS. Light-grown cultures were treated with ascorbate and glutathione, two acidic, powerful antioxidants in plant cells (Noctor and Foyer, 1998), and with sodium ascorbate, a pH-neutral antioxidant. AL-PCD induction in antioxidant cultures was increased to 90%, 88%, and 98% of cells for ascorbate, sodium ascorbate, and glutathione, respectively, in comparison to untreated light-grown cultures (60–65% of cells; Fig. 4B). All remaining cells were necrotic.
Nuclear protein synthesis may interact with chloroplasts and ROS during AL-PCD regulation
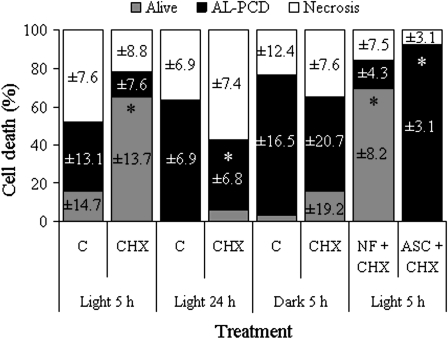
To investigate whether de novo protein synthesis is important in AL-PCD in A. thaliana cell-suspension cultures, light-grown cultures were treated with the translational inhibitor cycloheximide and effects on AL-PCD induction were apparent 5 h after heat treatment (Fig. 5A). At this early time point after heat treatment, maximal cell death levels had not yet occurred in controls and cycloheximide was shown to promote cell viability and attenuate cell death at this time. Cell viability increased to 65% and AL-PCD decreased to 13% of cells, compared to 16% and 37% of cells, respectively, in untreated light-grown cultures. The increase in cell viability was highly significant (P=0.004). However, when cells were observed 24 h after heat treatment, the difference in viability levels between the control and cycloheximide cultures was much reduced, although there was still suppression of AL-PCD from 63% in the control to 37% of cells in cycloheximide cultures (Fig. 5A). The effects of cycloheximide observed 5 h after heat treatment were less pronounced in dark-grown cultures: although cycloheximide did increase cell viability and decrease AL-PCD somewhat, the differences compared to the control were not statistically significant (Fig. 5B). To determine whether this could be due to the absence of chloroplasts in dark-grown cultures, the effects of cycloheximide on AL-PCD induction in norflurazon cultures were tested (Fig. 5B), but little differences in cell viability and cell death were found between these cultures and cycloheximide-treated light-grown cultures 5 h after heat treatment. Interestingly, combining cycloheximide treatment of light-grown cultures with antioxidant treatment seemed to eliminate the effects of cycloheximide: ascorbate treatment resulted in no living cells and high levels of AL-PCD 5 h after the heat treatment, with all remaining cells necrotic (Fig. 5B).
Fig. 5.
Nuclear protein synthesis, light, chloroplasts, and ROS may interact during AL-PCD regulation. Percentage of AL-PCD, necrosis, and living cells in control (C; untreated) or cycloheximide (CHX)-treated light-grown or dark-grown cultures, some further treated with norflurazon (NF) or ascorbate (ASC), scored using the cell morphology marker of AL-PCD, either 5 h or 24 h after heat treatment. Values are means ±SD (n=4 replicates). In light-grown cultures at 5 h, alive values marked with an asterisk were significantly different from alive values in the control (P=0.003 for CHX-treated cultures and P <0.001 for NF+CHX-treated cultures) and PCD value in ASC-treated cultures (marked with an asterisk) was significantly different from PCD value in the control (P < 0.001). In light-grown cultures at 24 h, the PCD value in CHX-treated cultures (marked with an asterisk) was significantly different from PCD value in the control (P=0.002). In dark-grown cultures, alive values were not significantly different (α=0.05) and PCD values were not significantly different (α=0.05).
Discussion
Many studies have shown that mitochondria are important regulators of animal apoptosis (reviewed by Desagher and Martinou, 2000) and plant PCD (reviewed by Diamond and McCabe, 2007). Mitochondria are important sources of ROS, which are thought to act as signals during plant PCD regulation (Diamond and McCabe, 2007). As chloroplasts are also important sources of ROS in plant cells (Foyer and Noctor, 2003), chloroplasts may also regulate PCD in plants. This would be difficult to test in planta due to the complexity of plant tissues; however, A. thaliana suspension cultures provide an ideal system, due to their easily accessible, undifferentiated cells. Transmission electron microscopy, culture pigment analysis, and PAM fluorometry all provided evidence that functional chloroplasts were present in light-grown cultures but absent from dark-grown cultures (Fig. 2). PAM fluorometry is used to measure the photosynthetic potential of photosystem II and the fluorescence yield of healthy leaves should measure around 0.8 (Maxwell and Johnson, 2000). The fluorescence yield of light-grown culture cells measured around 0.4, which, although much lower than that of healthy leaves, was significantly higher than that of dark-grown culture cells, which measured just 0.1 (Fig. 2D). Black and Osborne (2004) used PAM fluorometry to measure the fluorescence yield of photosynthetic cyanobacterial (Nostoc sp.) cells from suspension cultures. The fluorescence yield of free-living cyanobacteria measured around 0.4, which was reduced to around 0.1 when the cyanobacteria were in symbiosis with their plant partner (Gunnera tinctoria) and no longer needed to photosynthesize. Although the fluorescence yield measurements were much lower than those measured in healthy leaves, the free-living cyanobacteria were grown in the absence of a carbon source and therefore must have been photosynthetically active, despite a fluorescence yield of just 0.4. In the present study, the fluorescence yield results suggest that although photosynthesis is not necessary for their survival, A. thaliana culture cells produce functional chloroplasts when grown in the light.
AL-PCD was significantly higher in dark-grown than in light-grown cultures and this result was confirmed using two different methods for the identification of AL-PCD: cell morphology observation and the TUNEL assay (Fig. 3). As both cell condensation and DNA fragmentation occurred in the cells, it can be confirmed that the heat treatment activated AL-PCD, specifically. The number of TUNEL-positive cells correlated very well with cell condensation in dark-grown cultures, however, in light-grown cultures the number of TUNEL-positive cells was significantly lower than the number of condensed cells. As cell condensation was much more extreme in dark-grown than in light-grown heat-treated cells (Fig. 1C, D), it is conceivable that DNA fragmentation may also be more extensive in dark-grown than in light-grown heat-treated cells, which may explain the different level of AL-PCD measured by the cell morphology and TUNEL methods in light-grown cultures (Fig. 3). TUNEL may therefore be an accurate marker of AL-PCD in dark-grown or chloroplast-free cells but may significantly underestimate levels of AL-PCD in light-grown or chloroplast-containing cells. Consequently, further experiments used cell condensation as a more accurate marker for levels of AL-PCD in both light-grown and dark-grown cultures.
The presence of functional chloroplasts in light-grown but not dark-grown cultures and the difference in AL-PCD levels between the two culture types suggested that chloroplasts may affect AL-PCD progression. To test this, light-grown cultures lacking functional chloroplasts were established. Norflurazon is a herbicide that inhibits phytoene desaturase, an early enzyme in the carotenoid biosynthesis pathway (Sandmann, 1994). As carotenoids protect thylakoid membranes from photo-oxidative damage, norflurazon treatment results in early arrest of plastid development in the light. Norflurazon treatment may also indirectly result in the down-regulation of nuclear genes encoding chloroplast proteins (Susek and Chory, 1992; Gray et al., 2003), over 90% of which are encoded by the nucleus rather than the chloroplast itself. Although plastids were observed in norflurazon culture cells using transmission electron microscopy (Fig. 2A), the lack of chlorophyll in the cells (Fig. 2B, C) and the low fluorescence yield measurements (Fig. 2D) indicated that these plastids did not possess photosynthetic function. Under low light conditions, photo-oxidative stress is reduced and chloroplasts may form in the presence of norflurazon (Sagar et al., 1988). Although carotenoid formation is inhibited by norflurazon, carotenoids are not always strictly necessary for chloroplast biosynthesis, but are needed for the assembly of photosystem II (Susek and Chory, 1992). It appears likely therefore that chloroplast biosynthesis took place but that the thylakoid membranes were photosynthetically impaired. Norflurazon cultures responded to heat treatment with significantly higher levels of AL-PCD than untreated light-grown cultures (Fig. 4A), as well as more extreme cell condensation in the cells (data not shown), suggesting that suppression of chloroplast function affects AL-PCD progression.
Spectinomycin and lincomycin are antibiotics that inhibit prokaryotic and organellar protein synthesis but do not affect cytosolic translation (Thomson and Ellis, 1972); however, they may indirectly result in a decrease in the expression of nuclear genes that regulate chloroplast biosynthesis and function (Gray et al., 2003; Mulo et al., 2003). Antibiotic treatment did not affect culture growth or cell viability and the culture cells contained many mitochondria (observed with transmission electron microscopy; data not shown); therefore, the antibiotic concentrations used did not appear to affect mitochondrial biogenesis. Spectinomycin and lincomycin block plastid protein synthesis, which is necessary for correct thylakoid membrane structure (Thomson and Ellis, 1972) and transmission electron microscopy revealed that the cells of these cultures only contained very simple proplastids (Fig. 2A). These cultures responded to heat treatment with significant increases in levels of AL-PCD compared with untreated light-grown cultures (Fig. 4A) and an increase in the extent of cell condensation (data not shown), again suggesting that in light-grown cultures, the presence of functional chloroplasts attenuates AL-PCD progression.
Ascorbate is a highly abundant, key antioxidant in plants and can directly eliminate singlet oxygen, hydroxyl radicals, and superoxide and indirectly eliminate hydrogen peroxide through the activity of ascorbate peroxidase (Noctor and Foyer, 1998). Sodium ascorbate is a pH-neutral form of ascorbate, which is acidic (data not shown). Reduced glutathione (also acidic) is a key redox buffer in plants, forming a barrier between protein Cys groups and ROS (Foyer and Noctor, 2005). These antioxidants had no effect on light-grown cultures in the absence of heat treatment (data not shown) but heat-treating these cultures resulted in the induction of high levels of AL-PCD (Fig. 4B), with extreme cell condensation (data not shown), similar to AL-PCD induction in dark-grown cultures. The results achieved by sodium ascorbate treatment suggest that the effects of ascorbate and glutathione on AL-PCD induction were not due to their acidity. Antioxidant treatment not only increased AL-PCD induction but also decreased necrosis. The more severe the stress level cells are exposed to, the more likely that necrosis will result, rather than PCD (Reape et al., 2008). Considering that light stimulates ROS production by chloroplasts and peroxisomes (Foyer and Noctor, 2003) and ROS can be extremely destructive (Mittler, 2002; Mittler et al., 2004), ROS levels are likely to be high enough in light-grown cultures to, combined with the stress of a heat treatment, result in cell necrosis. Antioxidant treatment may have reduced ROS to levels similar to that in dark-grown cultures, thus reducing the stress levels and allowing the cells to initiate AL-PCD during the heat treatment. These results suggest that chloroplast-produced ROS may lower AL-PCD levels by switching death to necrosis in light-grown cultures.
Cycloheximide blocks nuclear protein synthesis by inhibiting cytoplasmic translation only (Mulo et al., 2003). Cycloheximide treatment of light-grown cultures resulted in a temporal promotion of cell viability and repression of AL-PCD compared to untreated controls (Fig. 5A), but these effects were less pronounced in dark-grown cultures and did not occur in antioxidant-treated light-grown cultures, although they did occur in norflurazon cultures (Fig. 5B). Studies on the effects of cycloheximide on plant PCD have produced conflicting results, including PCD suppression (Desikan et al., 1998; Solomon et al., 1999; Vacca et al., 2004), no effect on PCD (Mazel and Levine, 2001; Elbaz et al., 2002) and PCD promotion (Dzyubinskaya et al., 2006). Reape and McCabe (2008) suggested that variations in experimental protocols may partially explain these conflicting conclusions and the results presented here indicate that these variations could include the time-point at which the cells were observed, whether the cells were light-grown or dark-grown, and whether the cells were chloroplast-containing or not. It appears that plant AL-PCD can proceed in the absence of de novo nuclear protein synthesis, but that a complicated balance between protein synthesis, light, chloroplast activity, and ROS exists, which could somehow explain a cycloheximide-induced attenuation of AL-PCD, under certain conditions only.
Light and the presence of functional chloroplasts have been shown to have significant effects on many forms of plant PCD, including UV-induced (Danon et al., 2004), mycotoxin-induced (Asai et al., 2000) and wounding-induced PCD (Gray et al., 2002), leaf senescence (Zapata et al., 2005), and the hypersensitive response (Genoud et al., 2002; Zeier et al., 2004; Chandra-Shekara et al., 2006). Even in low light conditions environmental stresses result in excess excitation energy within the chloroplast, making the chloroplast a likely candidate for a sensor of stress and an initiator of cellular signalling that leads to stress responses (reviewed by Mullineaux and Karpinski, 2002). Chloroplastic generation of ROS increases during times of stress and may regulate PCD pathways in plant cells (Danon et al., 2006).
Studies that test chloroplast involvement in plant PCD vary widely in both experimental protocol and the ultimate conclusions as to the nature of that involvement. Mittler et al. (1997) revealed that changes in chloroplast ultrastructure occurred after hypersensitive response progression, Coffeen and Wolpert (2004) suggested that chloroplast-localized proteases could degrade Rubisco as part of the PCD pathway, and Yao and Greenberg (2006) suggested that chlorophyll breakdown products could induce PCD, possibly by contributing to ROS production. Chen and Dickman (2004) showed that transgenically expressed animal anti-apoptotic proteins in plant cells localized to chloroplasts and suppressed AL-PCD induced by oxidative stress within chloroplasts, suggesting chloroplast involvement in AL-PCD. Seo et al. (2000) found that the hypersensitive response was accelerated by the loss of chloroplast function and although Dzyubinskaya et al. (2006) found that light accelerated cyanide-induced PCD in chloroplast-containing guard cells, PCD was highly inducible by cyanide in epidermal cells, despite their lack of chloroplasts. The data in this paper show that while plant PCD does occur independently of chloroplasts, when functional chloroplasts are present, they tend to play a major role in determining the severity of and the number of cells undergoing AL-PCD and this effect seems to be driven by chloroplast ROS production.
Acknowledgments
This research was funded by The Embark Initiative, a Government of Ireland scholarship, operated by The Irish Research Council for Science, Engineering and Technology and a seed funding grant from University College Dublin.
Glossary
Abbreviations
- AL
apoptotic-like
- DAPI
4′,6-diamidino-2-phenylindole
- PAM
pulse amplitude modulation
- PBS
phosphate buffered saline
- PCD
programmed cell death
- rpm
revolutions per minute
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase
- TBS
TRIS-buffered saline
- TdT
terminal deoxynucleotidyl transferase
- TUNEL
TdT-mediated deoxyuridine triphosphate nick end labelling
References
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. The Plant Cell. 2000;12:1823–1835. doi: 10.1105/tpc.12.10.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Leaver CJ, McCabe PF. Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Letters. 1999;463:151–154. doi: 10.1016/s0014-5793(99)01611-7. [DOI] [PubMed] [Google Scholar]
- Black K, Osborne B. An assessment of photosynthetic downregulation in cyanobacteria from the Gunnera–Nostoc symbiosis. New Phytologist. 2004;162:125–132. [Google Scholar]
- Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. The Plant Journal. 2006;45:320–334. doi: 10.1111/j.1365-313X.2005.02618.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Dickman MB. Bcl-2 family members localize to tobacco chloroplasts and inhibit programmed cell death induced by chloroplast-targeted herbicides. Journal of Experimental Botany. 2004;55:2617–2623. doi: 10.1093/jxb/erh275. [DOI] [PubMed] [Google Scholar]
- Coffeen WC, Wolpert TJ. Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. The Plant Cell. 2004;16:857–873. doi: 10.1105/tpc.017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Delorme V, Mailhac N, Gallois P. Plant programmed cell death: a common way to die. Plant Physiology and Biochemistry. 2000;38:647–655. [Google Scholar]
- Danon A, Rotari VI, Gordon A, Mailhac N, Gallois P. Ultraviolet-c overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. Journal of Biological Chemistry. 2004;279:779–787. doi: 10.1074/jbc.M304468200. [DOI] [PubMed] [Google Scholar]
- Danon A, Sánchez Coll N, Apel K. Cytochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2006;103:17036–17041. doi: 10.1073/pnas.0608139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Martinou J-C. Mitochondria as the central control point of apoptosis. Trends in Cell Biology. 2000;10:369–376. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochemistry Journal. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M, McCabe PF. The mitochondrion and plant programmed cell death. In: Logan DC, editor. Annual plant reviews; plant mitochondria. Oxford: Blackwell Publishing; 2007. pp. 308–329. [Google Scholar]
- Dzyubinskaya EV, Kiselevsky DB, Bakeeva LE, Samuilov VD. Programmed cell death in plants: effect of protein synthesis inhibitors and structural changes in pea guard cells. Biochemistry. 2006;71:395–405. doi: 10.1134/s0006297906040079. [DOI] [PubMed] [Google Scholar]
- Elbaz M, Avni A, Weil M. Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death and Differentiation. 2002;9:726–733. doi: 10.1038/sj.cdd.4401030. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum. 2003;119:355–364. [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment. 2005;28:1056–1071. [Google Scholar]
- Genoud T, Buchala AJ, Chua N-H, Métraux J-P. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. The Plant Journal. 2002;31:87–95. doi: 10.1046/j.1365-313x.2002.01338.x. [DOI] [PubMed] [Google Scholar]
- Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS. Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiology. 2002;130:1894–1907. doi: 10.1104/pp.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Sullivan JA, Wang J-H, Jerome CA, MacLean D. Coordination of plastid and nuclear gene expression. Philosophical Transactions of the Royal Society of London B. 2003;358:135–145. doi: 10.1098/rstb.2002.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochemical Pharmacology. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Jones AM. Programmed cell death in development and defense. Plant Physiology. 2001;125:94–97. doi: 10.1104/pp.125.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinney G. Absorption of light by chlorophyll solutions. Journal of Biological Chemistry. 1941;140:315–322. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- May MJ, Leaver CJ. Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiology. 1993;103:621–627. doi: 10.1104/pp.103.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel A, Levine A. Induction of cell death in Arabidopsis by superoxide in combination with salicylic acid or with protein synthesis inhibitors. Free Radical Biology and Medicine. 2001;30:98–106. doi: 10.1016/s0891-5849(00)00452-4. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Leaver CJ. Programmed cell death in cell cultures. Plant Molecular Biology. 2000;44:359–368. doi: 10.1023/a:1026500810877. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Levine A, Meijer P-J, Tapon NA, Pennell RI. A programmed cell death pathway activated in carrot cells cultured at low cell density. The Plant Journal. 1997;12:267–280. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–510. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death in tobacco. Journal of Cell Science. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Current Opinion in Plant Biology. 2002;5:43–48. doi: 10.1016/s1369-5266(01)00226-6. [DOI] [PubMed] [Google Scholar]
- Mulo P, Pursiheimo S, Hou C-X, Tyystjärvi T, Aro E-M. Multiple effects of antibiotics on chloroplast and nuclear gene expression. Functional Plant Biology. 2003;30:1097–1103. doi: 10.1071/FP03149. [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know? Journal of Experimental Botany. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends in Plant Science. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Reape TJ, McCabe PF. Apoptotic-like programmed cell death in plants. New Phytologist. 2008;180:13–26. doi: 10.1111/j.1469-8137.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- Reape TJ, Molony EM, McCabe PF. Programmed cell death in plants: distinguishing between different modes. Journal of Experimental Botany. 2008;59:435–444. doi: 10.1093/jxb/erm258. [DOI] [PubMed] [Google Scholar]
- Sagar AD, Horwitz BA, Elliott RC, Thompson WF, Briggs WR. Light effects on several chloroplast components in norflurazon-treated pea seedlings. Plant Physiology. 1988;88:340–347. doi: 10.1104/pp.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G. Carotenoid biosynthesis in microorganisms and plants. European Journal of Biochemistry. 1994;223:7–24. doi: 10.1111/j.1432-1033.1994.tb18961.x. [DOI] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Iwai T, Iwano M, Fukui K, Isogai A, Nakajima N, Ohashi Y. Reduced levels of chloroplast FtsH protein in tobacco mosaic virus-infected tobacco leaves accelerate the hypersensitive reaction. The Plant Cell. 2000;12:917–932. doi: 10.1105/tpc.12.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. The Plant Cell. 1999;11:431–443. doi: 10.1105/tpc.11.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand Å, Asami T, Alonso J, Ecker JR, Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature. 2003;421:79–82. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- Susek RE, Chory J. A tale of two genomes: role of a chloroplast signal in coordinating nuclear and plastid genome expression. Australian Journal of Plant Physiology. 1992;19:387–399. [Google Scholar]
- Thomson WW, Ellis RJ. Inhibition of grana formation by lincomycin. Planta. 1972;108:89–92. doi: 10.1007/BF00386509. [DOI] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco bright-yellow 2 cells. Plant Physiology. 2004;134:1100–1112. doi: 10.1104/pp.103.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Dickman M. Plant programmed cell death: can't live with it; can't live without it. Molecular Plant Pathology. 2008;9:531–544. doi: 10.1111/j.1364-3703.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Greenberg JT. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. The Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata JM, Guéra A, Esteban-Carrasco A, Martín M, Sabater B. Chloroplasts regulate leaf senescence: delayed senescence in transgenic ndhF-defective tobacco. Cell Death and Differentiation. 2005;12:1277–1284. doi: 10.1038/sj.cdd.4401657. [DOI] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S. Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling sytemic resistance from salicylic acid and PR-1 accumulation. Planta. 2004;219:673–683. doi: 10.1007/s00425-004-1272-z. [DOI] [PubMed] [Google Scholar]