Abstract
Salinity stress enhances sugar accumulation in tomato (Solanum lycopersicum) fruits. To elucidate the mechanisms underlying this phenomenon, the transport of carbohydrates into tomato fruits and the regulation of starch synthesis during fruit development in tomato plants cv. ‘Micro-Tom’ exposed to high levels of salinity stress were examined. Growth with 160 mM NaCl doubled starch accumulation in tomato fruits compared to control plants during the early stages of development, and soluble sugars increased as the fruit matured. Tracer analysis with 13C confirmed that elevated carbohydrate accumulation in fruits exposed to salinity stress was confined to the early development stages and did not occur after ripening. Salinity stress also up-regulated sucrose transporter expression in source leaves and increased activity of ADP-glucose pyrophosphorylase (AGPase) in fruits during the early development stages. The results indicate that salinity stress enhanced carbohydrate accumulation as starch during the early development stages and it is responsible for the increase in soluble sugars in ripe fruit. Quantitative RT-PCR analyses of salinity-stressed plants showed that the AGPase-encoding genes, AgpL1 and AgpS1 were up-regulated in developing fruits, and AgpL1 was obviously up-regulated by sugar at the transcriptional level but not by abscisic acid and osmotic stress. These results indicate AgpL1 and AgpS1 are involved in the promotion of starch biosynthesis under the salinity stress in ABA- and osmotic stress-independent manners. These two genes are differentially regulated at the transcriptional level, and AgpL1 is suggested to play a regulatory role in this event.
Keywords: Abscisic acid, ADP-glucose pyrophosphorylase, 13C accumulation, sucrose transport, salinity stress, Solanum lycopersicum, starch biosynthesis, sugar accumulation, tomato
Introduction
Salinity stress improves the fruit quality of tomato (Solanum lycopersicum) by increasing the level of total soluble solids, including sugars, organic acids, and amino acids in fruits (Tal et al., 1979; Ho et al., 1987; Adams, 1991; Balibrea et al., 1996, 1999; Gao et al., 1998; Krauss et al., 2006; Saito et al., 2008). An increase in soluble solids enhances not only the market value of fresh fruit but also its processing efficiency, because it increases flavour and lowers water content (Stark et al., 1996). Sugar content is most important in terms of fruit taste. Many studies have used model plants in an attempt to understand the mechanism of the effect of salinity stress in enhancing the accumulation of metabolites in plant tissues. However, in the tomato plant, most investigations into salinity stress have been agronomic and have tended to explain the phenomenon as a ‘concentration effect’ due to a reduction in the size of the fruit (Ehret and Ho, 1986; Ho et al., 1987; Sakamoto et al., 1999).
Invertase and sucrose synthase have been the enzymes most studied in order to elucidate the control mechanisms of sink strength and sugar level in fruit (Balibrea et al., 1996, 1999; reviewed in Nguyen-Quoc and Foyer, 2001). During the last decade, cell-wall invertase has attracted attention as a key player to determine fruit sugar level (Fridman et al., 2000, 2004; Baxter et al., 2005). In addition, there is positive correlation between cytoplasmic invertase and hexose contents in salinity-stressed fruit (Balibrea et al., 2006). On the other hand, early studies have related the level of soluble solids in ripe tomato fruit to the starch level in the immature and mature green fruit stages (Davies and Cocking, 1965; Dinar and Stevens, 1981; Schaffer and Petreikov, 1997). However, there is much less information regarding the regulation of starch biosynthesis and accumulation by invertase (N'tchobo et al., 1999; Li et al., 2002). ADP-glucose pyrophosphorylase (AGPase, EC 2.7.7.27) has been proposed to regulate starch biosynthesis during the early stages of the developing fruit (Schaffer and Petreikov, 1997; Schaffer et al., 2000). AGPase catalyses the synthesis of ADP-glucose from glucose-1-phosphate and ATP (Preiss, 1988). Evidence from starch-deficient mutants, transgenic plants, and kinetic analyses has confirmed that this reaction is the first regulatory step in starch synthesis in plants (Tsai and Nelson, 1966; Lin et al., 1988; Müller-Röber et al., 1992; Stark et al., 1992). Plant AGPase is a hetero-tetrameric enzyme composed of two small and two large subunits, and all subunits are required for normal enzyme function.
In tomato AGPase, there are two isoforms of the small subunit and three isoforms of the large subunit (Chen and Janes, 1997). One gene encoding the small subunit (AgpS1) and three genes encoding the large subunit (AgpL1, L2, and L3) have so far been isolated as cDNAs (Chen et al., 1998; Park and Chung, 1998). Whereas AgpL3 is a major transcript in leaves, the predominant transcripts in developing fruit are AgpL1 and AgpS1, and the expression of these two genes peaks during the early development stages (Park and Chung, 1998; Petreikov et al., 2006). There are currently three known mechanisms regulating AGPase expression. Post-transcriptionally, AGPase undergoes allosteric regulation by glycerate-3-phosphate (3PGA) and Pi (Sowokinos, 1981; Sowokinos and Preiss, 1982) and by redox modification (Tiessen et al., 2002). At the transcriptional level, AGPase-encoding genes are regulated by phosphate, nitrate, and sugars (Müller-Röber et al., 1990; Scheible et al., 1997; Nielsen et al., 1998; Sokolov et al., 1998). In tomato, elevated expression of AgpL1, L2, and AgpS1 by sucrose in fruit and leaves (Li et al., 2002) and enhanced starch accumulation and AGPase activity in fruit of plants exposed to salinity stress has been observed (Balibrea et al., 1996; Gao et al., 1998). However, because of a lack of detailed information on the molecular background of these processes, the mechanisms underlying salinity stress enhancement of the accumulation of metabolites such as sugars in tomato fruit are not fully understood.
In this study, to address the AGPase in the above processes, we focused on elucidating the expression profiles of the AGPase genes and their regulation in developing fruit of tomato exposed to salinity stress. Our data indicate that salinity stress enhances not only carbohydrate accumulation but also its transport from the source leaves into the fruit during the early fruit development stages of tomato. AgpL1 and AgpS1 expression were involved in the salinity-responsive starch accumulation. This expression was regulated by sugar but not ABA and osmotic stress.
Materials and methods
Plant materials
Seeds of Solanum lycopersicum cv. ‘Micro-Tom’ (Scott and Harbaugh, 1989) were sown on moist paper in a culture room at 25 °C under a light intensity of 110 μmol m−2 s−1 with a 16/8 h light/dark photoperiod with relative humidity 50% in the day and 60% at night. Seedlings were transplanted into rockwool pots (5×5 cm) 1 week after germination and grown for 6 weeks in same culture room. The pots were wrapped with aluminium foil around its base to avoid excessive evaporation and saline accumulation on the surface of pot. These plants were grown by supplying a commercial nutrient solution (Otsuka A; Otsuka Chemical Co. Ltd., Osaka, Japan) adjusted to an electrical conductivity (EC) of 2.0 dS m−1 and maintaining a constant volume of 2.0 l in the plastic trays (534×348×60 mm). When the first truss flowered, 7 weeks after germination, one-half were transferred to a saline nutrient solution that was adjusted to EC 15.0 dS m−1 by adding NaCl (equivalent to 160 mM NaCl), while the remaining plants were kept in nutrient solution at EC 2.0 dS m−1 (0 mM NaCl) as a control. For hardening to the salinity stress, the plants were exposed to EC 9.0 dS m−1 (80 mM NaCl), and then EC 12.0 dS m−1 (120 mM NaCl) for 4 d in each stress intensity before the transfer to the EC 15.0 dS m−1 solution.
Fruits were sampled from plants grown under the two growth conditions at 10, 14, 18, 22, 26, 34, and 42 d after flowering (DAF), and frozen at –80 °C until use. The DAF of each salinity-stressed sample corresponds to the exposure period to the EC 15.0 dS m−1 solution. In this experiment, fruit at 10–14, 18–26, 34, and 42 DAF were defined as immature green, mature green, yellow and red stages, respectively.
For transcriptional analysis of LeSUT1, 7-week-old plants were used. Fully-expanded mature leaves were sampled from plants exposed to the control (EC 2.0 dS m−1) and the salinity (EC 15.0 dS m−1) conditions according to the time-course after the onset of the EC 15.0 dS m−1 of salinity stress. In this experiment, plants were transferred directly from the EC 2.0 dS m−1 to the EC 15.0 dS m−1 solution without the hardening procedure. The stress treatment started 2 h after the beginning of the light period. At each time point and condition, leaves were sampled from four plants, combined and frozen at –80 °C until use.
Sugar assay
The content of glucose, fructose, and sucrose was determined using high performance liquid chromatography (HPLC) as described in Zushi and Matsuzoe (2006). On a fresh weight basis, 0.2 g of frozen fruit was ground in liquid nitrogen and ground in 1 ml of Milli Q water. These samples were incubated at 95 °C for 10 min to inactivate sugar degradative enzymes and then centrifuged for 30 min at 15 000 g at 4 °C. In the supernatant, the same amount of acetonitrile was added and centrifuged for 15 min at 15 000 g at 4 °C, and then the supernatant was filtered through filter paper and then through a 0.45 μm filter (Millipore Co., Billerica, MA, USA). Soluble sugars were separated at 40 °C on a Shodex Asahi-pack NH2P-50 4E column (250×4.6 mm, Showa Denko KK, Tokyo, Japan) installed in the LC system 8020 series (Tosoh Co., Tokyo, Japan), and the refractive index (RI) was detected using an RI detector. The mobile phase was acetonitrile/water (75/25, v/v) at a flow rate of 1 ml min−1.
Starch assay
Starch content in fruit was measured using a modified method described by Raskin and Kende (1984). Between 0.1–0.4 g of frozen fruit was extracted in 0.5 ml of 80% (w/v) ethanol, boiled for 1 min at 100 °C, centrifuged at 12 000 g for 10 min, and the resulting pellet was resuspended in 80% (w/v) ethanol. This process was repeated once more to remove soluble sugars. The resulting pellet was extracted in 0.3 ml 200 mM KOH, boiled for 1 min, and centrifuged at 12 000 g for 10 min. The pH of 0.1 ml aliquots of the supernatant was adjusted to 5.0 with 8 μl of 18% (w/v) acetic acid and then 0.1 ml of α-amylase (10 units of α-amylase in 100 mM sodium acetate buffer, pH 5.3) was added and the solution was incubated at 37 °C for 1 h. To this mixture, 0.1 ml of amyloglucosidase was added (1,4-α-D-glucan glucohydrolase from Aspergillus niger (Roche pharmaceuticals, Nutley, NJ, USA); 1 unit of amyloglucosidase in 100 mM sodium acetate buffer, pH 4.6), incubated at 55 °C for 1 h, boiled for 2 min, and centrifuged at 1300 g for 10 min. The resulting glucose in the supernatant was determined with a glucose assay kit (Glucose test CII WAKO, Wako Pure Chemical Industries Ltd., Osaka, Japan).
13C experiments
13CO2 feeding was carried out following the modified method of Mori et al. (2004). For both control and salinity-treated plants, 15 plants setting fruits at 10–42 DAF were assayed. The duration of salinity-stress was about 50 d. Groups of five plants were placed in a 534×348×60 mm plastic tray supplying 2.0 l of nutrient solution adjusted to EC 2.0 dS m−1 or 15.0 dS m−1 (0 mM or 160 mM NaCl), and the trays were covered with a 60 l clear plastic bag (0.03 mm thick). Because ‘Micro-Tom’ is a miniature dwarf cultivar, and it was difficult to supply carbon dioxide to a specific leaf, 13C-labelled carbon dioxide (13CO2) was fed into the plastic tray. The 13CO2 was produced by mixing 1 g Ba13CO3 (99 atom%; Cambridge Isotope Laboratories, Inc., Andover, MA, USA) with 1 N HCl. The trays containing the plants were fed with 2000 ppm of 13CO2 for 4 h under light conditions. Fruits were harvested separately at 24 h and 48 h after the end of the 13CO2 treatment by the following fruit stages, at 10–14 (immature green), 18–26 (mature green), 34 (yellow), and 42 DAF (red). The harvested fruits were completely dried in a circulation drier at 80 °C and crushed into a fine powder in a vibrating sample mill (TI-200; Heiko Seisakusho Co., Ltd.). The content of 13C atom% and total carbon was measured in an infrared 13CO2 analyser (EX-130, Jasco Corp., Tokyo, Japan). 13C atom% excess was calculated from differences between the 13CO2-treated samples and the untreated controls.
AGPase activity
Soluble proteins were extracted by crushing frozen fruits (<0.5 g FW) in 5 ml of precooled extraction buffer (50 mM TRIS-HCl, 5 mM EDTA, pH 7.5, and 5 mM 2-mercaptoethanol) using a Polytron (Kinematica AG, Littau-Lucerne, Switzerland). The extracts were centrifuged at 12 000 g for 5 min at 4 °C, and 4 ml of the supernatant were transferred to new tubes. The crude supernatant was purified and concentrated using an Amicon Ultra-4 10 000 MW CO (Millipore, Billerica, MA, USA), according to the manufacturer's instructions, to a volume of 0.25 ml. This resulting extract was used in the assay for AGPase activity. Protein quantification was carried out according to the method of Bradford (1976). A purified 35 μl extract was mixed with 165 μl of reaction buffer [100 mM HEPES-NaOH pH 8.0, 5 mM MgCl2, 3 mM DTT, 0.5 mM glucose-1-phosphate, 1.5 mM ATP, 5 mM 3-phosphoglyceric acid, 0.4 mg ml−1 BSA, and 100 kcpm (α-14C) glucose-1-phosphate; GE Healthcare Bioscience] and reacted for 30 min at 37 °C. The reaction was stopped by heating the extract to boiling point. Four units of alkaline phosphatase (Funakoshi Co., Ltd., Tokyo, Japan) were then added to the solution and incubated for 40 min at 37 °C to dephosphorylate the unreacted [α-14C] glucose-1-phosphate. One hundred μl of the sample solution was spotted onto a DEAE cellulose membrane (Whatman, Kent, UK) and immediately washed five times in distilled water for 1 min to remove unreacted [α-14C] glucose. After washing, the cellulose membrane was dried and radioactivity was measured in a liquid scintillation counter.
RNA isolation, cDNA synthesis, and quantitative RT-PCR
Total RNA was extracted from frozen samples with the RNeasy plant Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instruction. The extracted RNA was dissolved in RNase free-water, and stored at –80 °C until use. For cDNA synthesis, 1 μg of total RNA were reverse-transcribed with the First Strand cDNA Synthesis kit (Takara Bio Inc., Otsu, Japan) according to the manufacturer's instruction.
The gene-specific primers were designed with Amplify ver.3.1.4 (B Engels, University of Wisconsin, USA; http://engels.genetics.wisc.edu/amplify/) based on published sequences (see Supplementary Table 1 at JXB online). Quantitative RT-PCR (qRT-PCR) reactions were carried out on an Mx 3000P qRT-PCR system (Stratagene, San Diego, CA, USA). For normalizing the qRT-PCR reaction, the endogenous actin gene was used as an internal standard (accession number U60482; Moniz and Drouin, 1996). The reaction cycles were as follows: for AGPase genes, 95 °C for 10 min as an initial denaturation, 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and 1 cycle of 95 °C for 30 s, 55 °C for 30 s, and 95 °C for 30 s; for LeSUT1genes, 95 °C for 10 min for initial denaturation, 40 cycles of 95 °C for 20 s, 50 °C for 30 s, and 72 °C for 20 s, and 1 cycle of 95 °C for 30 s, 55 °C for 30 s, and 95 °C for 30 s. Specific amplifications were confirmed by single transcript amplification in agarose gel, single dissociation peaks, and calibration curves. Gene expression was calculated in relation to the level of actin gene expression according to the instructions provided by Stratagene based on the method reported by Pfaffl (2001).
Treatments with ABA, sucrose, and/or mannitol
Fruits at 10 DAF of the plants grown in control condition were sampled and submitted to the following treatments. The fruits were cut in half with a razor blade along the equatorial plane and placed on the agar plate of half-strength MS medium containing 0.01–100 μM ABA alone, 150 mM sucrose plus 0–100 μM ABA, 150 mM mannitol plus 0–100 μM ABA, half-strength MS medium, and 1% agar medium as the control, and then incubated for 6 h at 25 °C. In those treatments, 0 μM ABA plus sucrose or mannitol indicates a single treatment with each compound. After the incubation, the fruits were stored at –80 °C until use for qRT-PCR analyses.
Quantitative determination of ABA
ABA was quantified by an enzyme-linked immunosorbent assay (ELISA) with a Phytodetek ABA test Kit (Agdia Incorporated, IN, USA). Five grams of flesh fruits were frozen in liquid nitrogen, lyophilized for 3 d and stored at –80 °C until use. For ABA, extractions were performed using 0.2 g of lyophilized fruit ground in a mortar, homogenized in 80% methanol (v/v), and extracted for 12 h on a shaker at 4 °C in dark conditions. The extracts were centrifuged at 10 000 g for 10 min and the supernatants were diluted 2000-fold with the detection buffer (25 mM trizma base, 100 mM NaCl, and 1 mM MgCl2.6H2O). The ELISA procedures were performed according to the manufacturer's instructions.
Statistical analyses were performed with StatView® ver. 4.5 (SAS Institute Inc., NC, USA) and SPSS ver. 17.0 (SPSS Inc., IL, USA).
Results
Fruit weight and carbohydrate accumulation in developing fruit
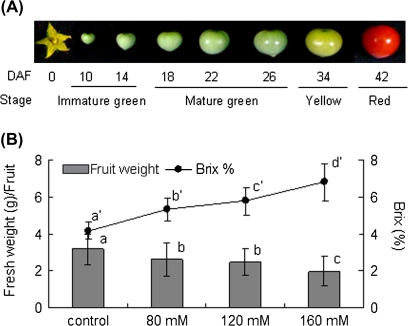
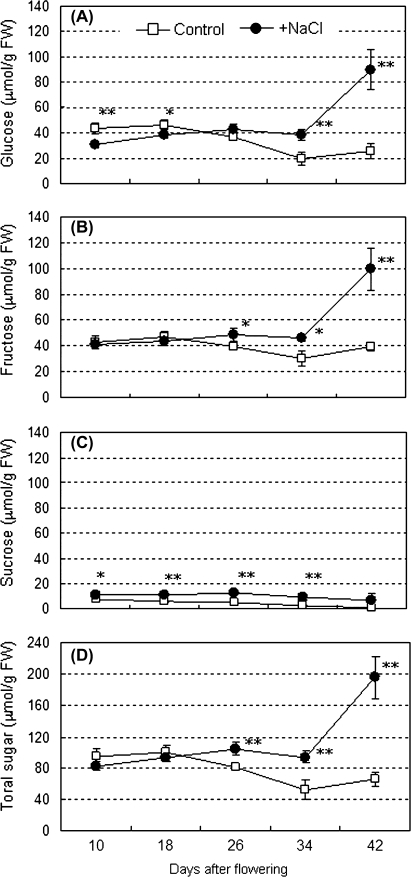
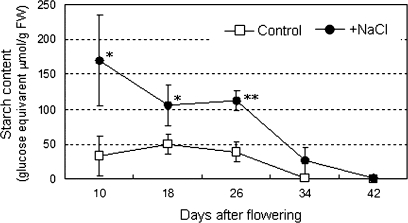
Fruits were sampled from plants grown under the two growth conditions at 10, 14, 18, 22, 26, 34, and 42 DAF as shown in Fig. 1A. At the start of this experiment, the effect of salinity intensity on fruit brix (%) and fresh weight of red ripe fruit in cv. ‘Micro-Tom’ was examined (Fig. 1B). Fruit brix (%) increased in response to the elevation of the stress level of the salinity and finally reached 1.6-times that of control fruit in the 160 mM NaCl condition. By contrast, fresh fruit weight decreased to 82–62% of the control in the given salinity conditions exhibiting an inverse correlation to the brix. Although size of the plants was concomitantly decreased under the salinity stress, the ratio of fruits to foliage was kept almost the same in both of the conditions on a fresh and a dry weight basis (see Supplementary Fig. S2 at JXB online). In order to dissect the dynamic alteration of carbohydrate in salinity-stressed fruit, plants treated with 160 mM NaCl were investigated using the following analyses. To avoid an excessive concentration effect due to the salinity stress, similar sized fruits were selectively used between both conditions in each developmental stage through the present work (see Supplementary Fig. S1 at JXB online). Soluble sugar contents (glucose, fructose, and sucrose) of developing fruits were determined in fresh fruit grown under saline and normal conditions (Fig. 2). In the control fruits, sugar levels gradually decreased (glucose, sucrose, and total sugar) or were unchanged (fructose) during fruit development (10–34 DAF) and, apart from sucrose, increased slightly by the end of the maturation stage (42 DAF). Sugar levels of salinity-stressed fruit were unchanged at 34 DAF but had increased substantially by 42 DAF; however, only sucrose had been kept at a similar level during this period even under saline conditions. Salinity stress enhanced the accumulation of glucose by 2.43 times, fructose by 2.05 times, sucrose by 7.87 times, and total sugars by 2.27 times at 42 DAF compared with those of the control. The promotion effect of the salinity stress on the accumulation of soluble sugars in red-ripe fruit was also confirmed on a dry weight basis (see Supplementary Fig. S3 at JXB online). Water contents were 90.7 (10 DAF), 91.8 (18 DAF), 89.9 (26 DAF), and 93.2% (42 DAF) in control fruits and 85.9 (10 DAF), 85.8 (18 DAF), 86.7 (26 DAF), and 90.9% (42 DAF) in the salinity-stressed fruits (data not shown). In contrast to the sugar contents, salinity stress enhanced starch accumulation during the early fruit development stages by 5.04 (10 DAF), 2.13 (18 DAF), and 2.91 (26 DAF) times compared to those of the control, respectively. Accumulation of starch granules in the fruits at 10 DAF was visualized by Periodic acid–Schiff (PAS) staining (see Supplementary Fig. S4 at JXB online). Figure 3 shows that enhanced starch accumulation was observed in the fruit of plants exposed to the salinity stress in developmental fruits. However, in the pericarp, starch only accumulated in the inner pericarp but not in the outer pericarp and exocarp (see Supplementary Fig. S4A, D at JXB online). By 26 DAF, starch had started to disappear from control fruit but was still accumulating in the salinity-stressed fruit. In those fruit, starch disappearance was delayed compared to the control (34 DAF).
Fig. 1.
Fruit development of cv. ‘Micro-Tom’. (A) Flowering (0) and fruit at 10, 14, 18, 22, 26, 34, and 42 d after flowering (DAF) grown under control conditions. In this work, fruit at 10–14, 18–26, 34, and 42 DAF were defined as immature green, mature green, yellow, and red stages, respectively. (B) Fresh weight (shaded bars) and brix (%) (black circles) of red-ripe fruit grown under control (0 mM NaCl) and various salinity conditions (80–160 mM NaCl). Right and left vertical axes correspond to brix (%) and fresh weight, respectively. Values are means ±SD (n=50). Different letters indicate statistical significance of means estimated using Fisher's PLSD test (P <0.05).
Fig. 2.
Change in soluble sugar content in developing fruits grown under control and saline conditions. (A) Glucose; (B) fructose; (C) sucrose; (D) total sugars. White squares and black circles indicate control (0 mM NaCl) and salinity treatments (160 mM NaCl), respectively. The horizontal axis indicates fruit developing stages (DAF). Values are means ±SD (n=3). The asterisks indicate statistical significance of the means in the same developing stage estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
Fig. 3.
Change in starch content in developing fruits grown under control (0 mM NaCl) and saline conditions (160 mM NaCl). White squares and black circles indicate control and salinity treatments, respectively. The horizontal axis indicates fruit developing stages (DAF). Values are means ±SD (n=3). The asterisks indicate statistical significance of the means in the same developing stage estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
Carbohydrate accumulation in developing fruit and expression of the sucrose transporter in source leaves
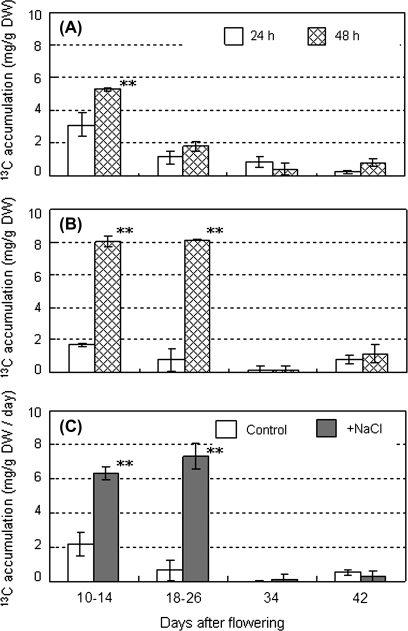
To determine carbohydrate accumulation in the fruit during each development stage, tracer analysis was carried out with 13C-labelled carbon dioxide (Fig. 4). 13C taken up by control fruits increased from 3.12 mg g−1 DW at 24 h to 5.31 mg g−1 DW at 48 h after 13CO2 feeding in the fruits at 10–14 DAF (immature green), and from 1.13 mg g−1 DW at 24 h to 1.79 mg g−1 DW at 48 h in the fruits at 18–26 DAF (mature green) (Fig. 4A). On the other hand, 13CO2 taken up by salinity-stressed fruits increased from 1.69 mg g−1 DW at 24 h to 8.04 mg g−1 DW at 48 h in the fruits at 10–14 DAF, and from 0.79 mg g−1 DW at 24 h to 8.14 mg g−1 DW at 48 h in the fruits at 18–26 DAF (Fig. 4B). The increases in rate of 13C accumulation in fruit of the immature green and the mature green stages were 2.18 mg g−1 DW d−1 and 0.67 mg g−1 DW d−1 in the control fruit, 6.24 mg g−1 DW d−1, and 7.44 mg g−1 DW d−1 in salinity-stressed fruit. Carbon accumulation in the salinity-stressed fruit was 2.86 times higher in immature green and 11.1 times in mature green fruit than those into control fruit. In contrast to the early development stages of fruit, no active accumulation was observed after 34 DAF (yellow) in both conditions (Fig. 4C).
Fig. 4.
Change in 13C accumulation in developing fruits of plants grown under control and saline conditions. (A) Control (0 mM NaCl), (B) saline conditions (160 mM NaCl). Open and shaded columns indicate the time for sampling at 24 h and 48 h after feeding 13C-labelled carbon dioxide, respectively. (C) 13C accumulation in fruit per day calculated based on the mean values from (A) and (B). Open and shaded columns indicate control and salinity treatments. The horizontal axis indicates fruit developing stages. 10–14 DAF and 18–26 DAF correspond to the immature green and mature green stages, respectively. Values are means ±SD (n=3). The asterisks in (A) and (B) indicate statistical significance of the means in the same developing stage estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
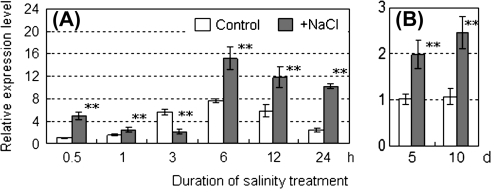
To elucidate the sugar loading activity in source leaves under salinity stress, transcriptional levels of LeSUT1 were investigated (Fig. 5). This gene encodes the major isoform of a sucrose transporter in photosynthetic source leaves that functions in phloem loading of sucrose in tomato plants (Kühn et al., 1997). At the transcriptional level, LeSUT1 exhibited diurnal cycles under control and the salinity conditions, and the setting time-course shown in Fig. 5. In plants exposed to salt stress for 6–24 h, although LeSUT1 expression decreased in the first 3 h, it was up-regulated 1.98–4.19 times compared to the control at the same time point (Fig. 5A). The LeSUT1 transcription was enhanced for at least 10 d after the onset of salinity stress (Fig. 5B), when the prominent starch accumulation was observed in the fruit (Figs 3, 4).
Fig. 5.
Relative expression levels of LeSUT1 genes in leaves of plants grown under control and saline conditions for short (A) and long (B) periods. (A) Leaves were sampled at 0.5, 1, 3, 6, 12, and 24 h after the start of exposure to salinity stress. (B) Leaves were sampled 5 d and 10 d after the start of exposure to salinity stress. Open and shaded columns indicate control (0 mM NaCl) and salinity treatments (160 mM NaCl), respectively. Values are means ±SE (n=4). The asterisks indicate statistical significance of the means estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
AGPase activity and differential expression of Agp genes during fruit development
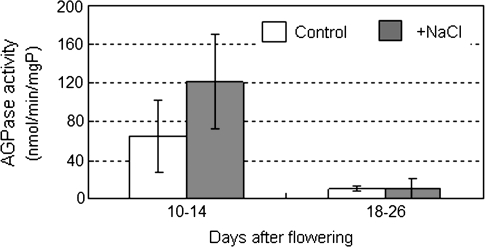
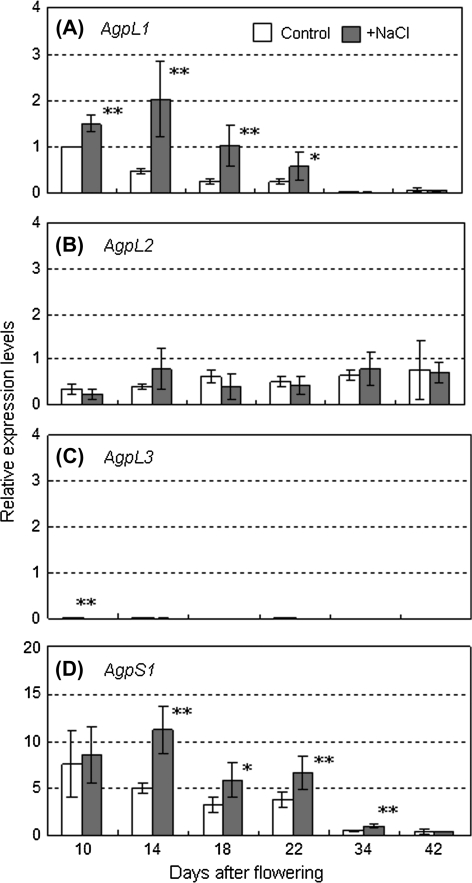
Before performing a transcriptional analysis of AGPase, the effect of salinity stress on AGPase activity in ‘Micro-Tom’ fruit was confirmed. As shown in Fig. 6, fruit at the immature green stage (10–14 DAF) had high AGPase activity, but this activity declined at the mature green stage (18–26 DAF). Although it was not significant, salinity stress tended to enhance AGPase activity by almost 1.87 times that of the control at the immature green fruit stage, but activity was not affected in mature green fruit. To clarify how AGPase genes are regulated under the salinity stress at different development stages, the expression patterns of AgpL1, L2, L3, and S1 genes in developing fruit were investigated by qRT-PCR (Fig. 7). Transcriptional levels of the genes are presented relative to that of AgpL1 in control fruit at 10 DAF. In ‘Micro-Tom’ fruit, the major Agp isoforms were AgpS1, L1, and L2. The AgpL3 transcript was also detectable; however, it was only 1.8% of the AgpL1 transcription level in control fruit at 10 DAF (Fig. 7C). Of all the AGPase genes, AgpS1 showed the highest expression throughout fruit development, which corresponded to 7.6–11.1 times that of AgpL1 during the early developing stages (Fig. 7D). Of the large subunit-encoding genes, AgpL1 was the most highly expressed (Fig. 7A). During the early fruit development stages (10–22 DAF), AgpL1 and AgpS1 were strongly expressed and both were clearly up-regulated by salinity stress. The expression of both of these genes peaked at 10 DAF in the control and at 14 DAF in the salinity-stressed fruit. These expression levels declined rapidly after the peak. At 34 DAF, they decreased to 1.5% and 0.8% of each peak level in AgpL1, and 6.9% and 11.8% in AgpS1 in the control and salinity-stressed fruits, respectively. AgpL2 expression was less than half that of AgpL1 at most developmental stages and was relatively lower than the other two genes, changing little throughout fruit development (Fig. 7B).
Fig. 6.
Change of AGPase activity in developing fruits grown under control and saline conditions. Open and shaded columns indicate control (0 mM NaCl) and salinity (160 mM NaCl) treatments, respectively. The horizontal axis indicates fruit developing stages. 10–14 DAF, immature green stage, 18–26 DAF, mature green stage. Values are means ±SE (n=3).
Fig. 7.
Relative expression levels of AGPase genes in developing tomato fruit grown under control and saline conditions. (A) AgpL1, (B) AgpL2, (C) AgpL3, (D) AgpS1. Open and shaded columns indicate control (0 mM NaCl) and salinity (160 mM NaCl) treatments, respectively. The horizontal axis indicates fruit developing stages (DAF). Values are means ±SE (n=5). The asterisks indicate statistical significance of means in the same developing stage estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
Effect of ABA, sucrose, and osmotic stress on AGPase gene expression
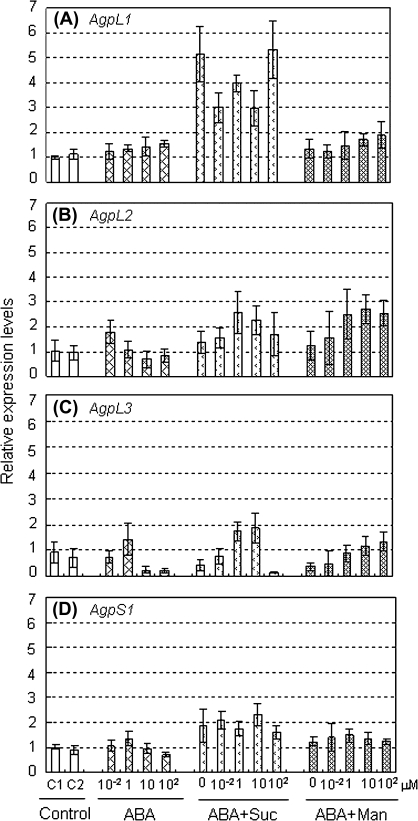
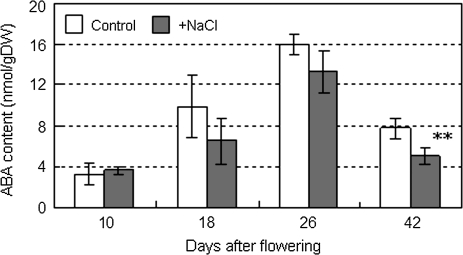
To elucidate the response of Agp genes to possible physiological effectors in early developing fruit, the fruits at 10 DAF were exposed to exogenous ABA, sucrose, and the same level of osmotic stress given by mannitol. The transcription level of the Agp genes was analysed in fruits at 10 DAF which were cultured for 6 h on agar plates of half-strength MS medium containing 0.01–100 μM ABA, 150 mM sucrose, and 150 mM mannitol as single or multiple effectors. AgpL1 and AgpS1 were significantly up-regulated by sucrose and sucrose+ABA but not by ABA, mannitol, and mannitol+ABA (Fig. 8A, D; see Supplementary Table S2 at JXB online). Results of the two-way repeated measures ANOVA showed the sucrose-response of AgpL1 was affected by ABA; however, there was no interaction effect on AgpS1 expression (see Supplementary Table S2 at JXB online). On the other hand, AgpL2 transcription was slightly up-regulated by mannitol, and AgpL3 was affected by the ABA level with sucrose and mannitol, while these were not effective as single effectors (Fig. 8B, C; see Supplementary Table S2 at JXB online). To ensure the validity of this analysis, the endogenous ABA content in developing fruit was assayed (Fig. 9). Fruit ABA contents ranged from 3 nmol g−1 DW to 16 nmol g−1 DW and peaked at 26 DAF in both growth conditions. However, ABA tended to be lower in the salinity-stressed fruits than control fruits at 10 DAF. Based on the water content ratio in the fruits, 91.4% and 87.6% on average in the control and salinity-stressed fruits, respectively, endogenous ABA concentration in fresh fruit was roughly estimated to be 0.3 μM to 2 μM, which fell within the dose range administered in Fig. 8.
Fig. 8.
Relative expression levels of AGPase genes in fruit treated with ABA, sucrose, and mannitol as single or multiple effector(s). (A) AgpL1, (B) AgpL2, (C) AgpL3, (D) AgpS1. Fruits at 10 DAF of plants grown in control condition were cut in half and incubated for 6 h on half-strength MS medium plate containing the indicated effector(s). Suc, 150 mM sucrose; Man, 150 mM mannitol; C1 (control 1), 1% agar; C2 (control 2), the half-strength MS plate without effectors. The ABA concentrations were indicated under the each column. Values are means ±SE (n=4).
Fig. 9.
Changes in endogenous ABA content in the developing fruits grown under control and saline conditions. Open and shaded columns indicate control (0 mM NaCl) and salinity (160 mM NaCl) treatments, respectively. The horizontal axis indicates fruit developing stages (DAF). Values are means ±SD (n=5). The asterisks indicate statistical significance of means in the same developing stage estimated using Fisher's PLSD test (*P <0.05, **P <0.01).
Discussion
In this work, the role and regulation mechanisms of starch accumulation and AGPase genes underlying sugar accumulation in tomato fruits exposed to salinity stress were investigated. This cultivar ‘Micro-tom’ exhibited increasing brix (%) and a decreasing size of fruits, keeping almost the same level of the source–sink balance with exposure to high salinity stress (Fig. 1B; see Supplementary Fig. S2 at JXB online) without serious blossom-end rot, indicating this laboratory-grown cultivar is available for a study of carbohydrate metabolic shift under salinity stress in tomato plants.
As shown in Fig. 2, salinity stress strikingly enhanced soluble sugar accumulation after the start of ripening. The effect of the stress on the accumulation of sugars was apparently higher than those on the suppression of fruit weight (see Supplementary Fig. S1 at JXB online). Considering that the sugar contents on a dry weight basis also increased under the stress (see Supplementary Fig. S3 at JXB online), activation of sugar metabolism should be involved in this event together with a concentration effect due to the reduction of fruit size. Under the control conditions in our experiment, hexose contents in the ‘Micro-Tom’ fruit decreased by 34 DAF (Fig. 2) unlike other Solanum lycopersicum cultivars (Robinson et al., 1988; Damon et al., 1988; Yelle et al., 1988). The sugar contents were also measured using the hexokinase-coupled assay and H-NMR with ‘Micro-Tom’ fruit from another replicated cultivation, and a similar hexose profile to the present result was obtained (data not shown). In addition, Hazem et al. (2004) reported the continuous decrease of glucose and unchanged fructose contents until the start of ripening in ‘Micro-Tom’ fruit, suggesting such a hexose profile is an attribute of this cultivar.
Our data also indicate that salinity stress promotes and extends the accumulation of starch during early fruit development (Fig. 3). Such effects of salinity stress on starch accumulation were also reported by Balibrea et al. (1996, 1999). Because the transiently produced starch in immature tomato fruits is completely degraded to soluble sugars in ripe fruits (Dinar and Stevens, 1981), it is probable that enhanced starch accumulation results in a higher sugar content in ripe red fruits under salinity stress (Fig. 2). The higher level and the extended period of starch accumulation in the early stages of tomato fruit development were also reported in tomato introgression lines having a high soluble solids trait that have alleles derived from the wild species S. pennellii (Baxter et al., 2005) and S. habrochaites (Petreikov et al., 2006). Our results and those from the introgression lines suggest a regulation mechanism of soluble solids level in the ripe red stage via transient starch accumulation in the early developmental stages in tomato fruit and its variability is influenced by both environmental conditions and genotype. In addition, Balibrea et al. (2006) reported that sucrose import during ripening played an important role for the increase in sugar content under salinity stress in wild species, suggesting there are different mechanisms to determine the sugar level within the germplasm.
The effect of salinity stress on promoting starch accumulation and AGPase activity in developing tomato fruit was also reported in a normal cultivar (Gao et al., 1998). A similar increase in AGPase activity was found in salinity-stressed fruit of ‘Micro-Tom’ (Fig. 6) and therefore the response of AGPase genes to salinity stress was examined. In control fruit grown without salt, these genes exhibited similar expression patterns to those previously reported: AgpS1 is most strongly expressed, followed by AgpL1 and AgpL2, then AgpL3, in which expression is low or absent (Park and Chung, 1998; Li et al., 2002; Petreikov et al., 2006) (Fig. 7). When grown under saline conditions, AgpS1 and AgpL1 were up-regulated at the transcriptional level in fruits from 10–22 DAF, while AgpL2 and AgpL3 showed little response to salinity (Fig. 7). Since the AGPase activity declined earlier than AgpS1 and AgpL1 transcription, post-transcriptional regulation could be involved in those events. In plants, the large subunit of AGPase functions as an allosteric modulator, whereas the small subunit functions as a catalytic molecule (Okita et al., 1990). Functional AGPase requires a heterotetramer consisting of two large and two small subunit genes (Chen and Janes, 1997). In the early development of tomato fruits, transcriptional levels of the large subunit genes (AgpL1–L3) are much lower than that of the small subunit gene (AgpS1), indicating that expression of the large subunit genes limits AGPase expression at the transcriptional level in young tomato fruit. Because expression of AgpL1 was highest among the large subunit genes during early fruit development, and is expressed in a co-ordinated manner to the enzymatic activity and starch accumulation pattern during fruit development, AgpL1 is likely to be responsible for the enhanced starch biosynthesis under salinity stress. The starch accumulation pattern observed in Supplementary Fig. S4 at JXB online also strongly supported the regulatory role of AgpL1 in fruit because it entirely corresponded to the GUS expression pattern driven by the AgpL1 promoter in an immature-green fruit (Xing et al., 2005).
13C analysis showed that salinity stress strongly promoted 13C accumulation in immature green fruits (Fig. 4). This could be interpreted as the result of a suppressed respiration activity and subsequent lower utilization of carbohydrate for energy production in the salinity-stressed fruits. It was supported by the hexose profiles during 10–34 DAF, in which the hexose contents slightly increased under the salinity stress whereas they gradually decreased in control condition (Fig. 2A, B). On the other hand, it was also likely to be caused by an increased influx of carbohydrate in the salinity-stressed fruit. If the higher 13C accumulation is due to the lower carbohydrate utilization, sink strength should be higher in the control than in the salinity-stressed fruit. However, expression of AgpL1 and AgpS1 in the sink organs (fruit) were actually higher in salinity than control condition (Figs 5, 6, 7). An increased allocation of photosynthetic 14C/13C to fruits (Gao et al., 1998; Saito et al., 2009) and the higher expression of LeSUT1 in source leaves of tomato plants exposed to salinity stress (Fig. 5) supported this possibility. To examine these hypotheses, a direct measurement of carbon transport using a positron-emitting tracer imaging system (PETIS; Suwa et al., 2008) will be needed. Although a small green fruit could have some photosynthetic activity, the possibility that the salinity stress promotes phyto-assimilation on the fruit surface was ruled out, because the accumulation of starch granules only appeared in the inner pericarp, the columella, and placenta tissue but not in the outer pericarp and exocarp where photosynthetic chloroplasts are localized (see Supplementary Fig. S4 at JXB online). In addition, the 13C value just after the 13CO2 feeding was subtracted from all of the measured figures of the samples taken after 24 h and 48 h. Therefore, the data presented in Fig. 4 reflected the net influx and consumption in the fruit after the 13CO2 feeding. The 13C analyses also revealed that carbohydrate accumulation in the fruit was almost completed by the mature green stage and did not occur after the start of ripening (Fig. 4). It indicated that the higher sugar accumulation in salinity-stressed ripe fruit is due to active starch accumulation during the early stages of fruit development. Furthermore, the starch completely disappeared from the fruit by the red stage under both the control and salt-stressed conditions (Fig. 3), indicating that there is sufficient enzymatic activity for starch breakdown in ripening fruit. This leads us to conclude that the increase in carbohydrate accumulation in the fruit, probably caused by a promoted sugar transport at the early development stages, plays an important role in ensuring the soluble sugar content in the mature red fruit under these salinity conditions.
Because our analyses indicated that the expression of LeSUT1 in source leaves was up-regulated at the transcriptional level (Fig. 5) under the salinity stress, a systemically co-ordinated mechanism is likely to be involved in this event. It will be interesting to elucidate which factor functions as the driving force for the amplification of carbohydrate flux; increased sink capacity of the fruit or intensification of the loading ability in source leaves. Overexpression of the bacterial small subunit gene driven by the potato spADPGPP promoter in the tomato plant resulted in a 20–30% increase in total soluble solids in ripe fruit (Stark et al., 1996), indicating that fruit sink capacity can be controlled by a modification of AGPase gene expression. To clarify how AGPase genes contribute to the determination of sink strength during fruit development of tomato, functional analyses using overexpression or knockdown strategies in an organ- and stage-specific manner will be indispensable. Those transgenic plants would be available to analyse the balance of contribution of AGPase and other sucrolytic enzymes, such as invertase and sucrose synthase, in fruit sugar levels and sink strength.
Transcriptional regulation of the AGPase gene by sugar has been reported in several plants (Salanoubat and Belliard, 1989; Müller-Röber et al., 1990; Sokolov et al., 1998; Akihiro et al., 2005). In tomato, sucrose regulation of AgpL1 expression in fruit was also reported by Li et al. (2002). In the present study, it is demonstrated that both AgpL1 and AgpS1 are specifically up-regulated by sucrose but not by ABA and osmotic stress (Fig. 8A, D). The ANOVA results indicate that the sucrose response of AgpL1 transcription was affected by ABA. However, we think that ABA does not have an effect on sucrose-regulation because the sucrose treatment strikingly induced AgpL1 transcription even in the absence of ABA, and it did not exhibit a dose-response to ABA (Fig. 8A). Synergistic effects of ABA and sugar on AGPase gene expression were reported in Apl3 in Arabidopsis leaves (Rook et al., 2001) and OsAPL3 in rice cultured cells (Akihiro et al., 2005). In contrast to those tissues and species, our results indicate that starch biosynthesis in tomato fruit is mainly regulated by sugar in an ABA- and osmotic stress-independent manner. It was also supported by the lower level of endogenous ABA contents in immature-green fruit and the poor response to the salinity-stress (Fig. 9). Considering the prominent activation of 13C accumulation under salinity conditions (Fig. 4B), the increase of carbohydrate availability in the developing fruits plays an important role in the up-regulation of AGPase genes and consequent starch accumulation, resulting in later starch breakdown and more sugar accumulation in the salinity-stressed ripe fruit. In contrast to AgpL1, AgpS1 exhibited a much lower response to sucrose, even though it was strongly up-regulated by salinity stress (Figs 8D, 9D). These results indicate differential regulation pathways of those genes under the salinity stress in tomato fruit. On the other hand, the possibility was ruled out that AgpL2 and AgpL3 participate in starch accumulation in fruit because of the lower expression levels and poor responsiveness to salinity stress (Fig. 7B, C). In addition, our preliminary analysis suggested that AgpL2 is mainly expressed in seeds (data not shown). AgpL3 was the only isoform in which the synergistic effects of ABA/osmotic stress and sucrose were observed (Fig. 8C; see Supplementary Table S2 at JXB online). Since AgpL3 is specifically expressed in source tissue including photosynthetic leaves (Xing et al., 2005), such a regulation manner would be general for source-type AGPase genes in plant. Tiessen et al. (2003) reported that sucrose and glucose lead to transcriptional and post-translational up-regulation of AGPase by affecting its redox state and SNF1-related kinase- and hexokinase-mediated signalling pathways in potato tuber. In tomato fruit, it is likely that the elevated sugar transport under the salinity stress stimulates AGPase activity in a similar manner.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table 1. Primer sequences and accession numbers of target genes for quantitative RT-PCR analyses.
Supplementary Table 2. Statistical analyses of the effects of ABA, sucrose, mannitol, and those in combination on the expression of tomato AGPase genes.
Supplementary Fig. 1. Fresh weight of developing fruits used in the present experiments.
Supplementary Fig. 2. Ratio of fruits to foliage on a dry weight basis of the 14-week-old plants grown under control and 160 mM of salinity condition for 7 weeks after flowering.
Supplementary Fig. 3. Soluble sugar contents on a dry weight basis in ripe fruits (42 DAF) of plants grown under control and salinity conditions.
Supplementary Fig. 4. Accumulation pattern of starch granules in tomato fruit at 10 DAF.
Supplementary Material
Acknowledgments
We thank Dr Takashi Akihiro, Shimane University, for technical advice on the AGPase enzyme assay. We also appreciate Ms Atsuko Ushitani, Junko Yamasaki, Yasuko Shirai and Izumi Ohshima for their technical support. This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (No. 16380020 and No. 21580075) and Research and Development projects for application in promoting new policy of Agriculture, Forestry, and Fisheries (No. 2050).
Glossary
Abbreviations
- ABA
abscisic acid
- AGPase
ADP-glucose pyrophosphorylase
- DAF
days after flowering
- EC
electrical conductivity
- qRT-PCR
quantitative RT-PCR
- SUT
sucrose transporter
References
- Adams P. Effects of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomatoes grown in rockwool. Journal of Horticultural Science. 1991;66:201–207. [Google Scholar]
- Akihiro T, Mizuno K, Fujimura T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant and Cell Physiology. 2005;46:937–946. doi: 10.1093/pcp/pci101. [DOI] [PubMed] [Google Scholar]
- Balibrea M, Martinez-Andújar C, Cuartero J, Bolarín M, Pérez-Alfocea F. The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Functional Plant Biology. 2006;33:279–288. doi: 10.1071/FP05134. [DOI] [PubMed] [Google Scholar]
- Balibrea M, Parra M, Bolarín M, Pérez-Alfocea F. Cytoplasmic sucrolytic activity controls tomato fruit growth under salinity. Australian Journal of Plant Physiology. 1999;26:561–568. [Google Scholar]
- Balibrea M, Santa Cruz A, Bolarín M, Pérez-Alfocea F. Sucrolytic activities in relation to sink strength and carbohydrate composition in tomato fruit growing under salinity. Plant Science. 1996;118:47–55. [Google Scholar]
- Baxter CJ, Carrari F, Bauke A, Overy S, Hill SA, Quick PW, Fernie AR, Sweetlove LJ. Fruit carbohydrate metabolism in an introgression line of tomato with increased fruit soluble solids. Plant and Cell Physiology. 2005;46:425–437. doi: 10.1093/pcp/pci040. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen B-Y, Janes HW. Multiple forms of ADP-glucose pyrophosphorylase from tomato fruit. Plant Physiology. 1997;113:235–241. doi: 10.1104/pp.113.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B-Y, Janes HW, Gianfagna T. PCR cloning and characterization of multiple ADP-glucose pyrophosphorylase cDNA from tomato. Plant Science. 1998;6:59–67. doi: 10.1016/s0168-9452(98)00095-8. [DOI] [PubMed] [Google Scholar]
- Damon S, Hewitt JD, Nieder M, Bennett AB. Sink metabolism in tomato fruit. II. Phloem unloading and sugar uptake. Plant Physiology. 1988;87:731–736. doi: 10.1104/pp.87.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JN, Cocking EC. Changes in carbohydrates, proteins and nucleic acids during cellular development in tomato fruit locule tissue. Planta. 1965;67:242–253. [Google Scholar]
- Dinar M, Stevens MA. The relationship between starch accumulation and soluble solids content of tomato fruits. Journal of the American Society for Horticultural Science. 1981;106:415–418. [Google Scholar]
- Ehret DL, Ho LC. The effect of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. Journal of Horticultural Science. 1986;61:361–367. [Google Scholar]
- Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science. 2004;305:1786–1789. doi: 10.1126/science.1101666. [DOI] [PubMed] [Google Scholar]
- Fridman E, Pleban T, Zamir D. A recombination hotspot delimits a wild-species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proceedings of the National Academy of Sciences, USA. 2000;97:4718–4723. doi: 10.1073/pnas.97.9.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Sagi M, Lips SH. Carbohydrate metabolism in leaves and assimilate partitioning in fruits of tomato (Lycopersicon esculentum L.) as affected by salinity. Plant Science. 1998;135:149–159. [Google Scholar]
- Hazem OA, Fernie AR, Kossmann J, Lloyd JR. Developmental analysis of carbohydrate metabolism in tomato (Lycopersicon esculentum cv. Micro-Tom) fruits. Physiologia Plantarum. 2004;120:196–204. doi: 10.1111/j.0031-9317.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- Ho L-C, Grange RI, Picken AJ. An analysis of the accumulation of water and dry matter in tomato fruit. Plant. Cell and Environment. 1987;10:157–162. [Google Scholar]
- Krauss SW, Schnitzler H, Grassmann J, Woitke M. The influence of different electrical conductivity values in a simplified recalculating soilless system on inner and outer fruit quality characteristics of tomato. Journal of Agricultural and Food Chemistry. 2006;54:441–448. doi: 10.1021/jf051930a. [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Li X-Y, Xing JP, Thomas JG, Harry JW. Sucrose regulation of ADP-glucose pyrophosphorylase subunit genes transcript levels in leaves and fruits. Plant Science. 2002;162:239–224. doi: 10.1016/s0168-9452(01)00565-9. [DOI] [PubMed] [Google Scholar]
- Lin T-P, Caspar T, Somerville C, Preiss J. A starch-deficient mutant of Arabidopsis thaliana with low ADP-glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiology. 1988;88:1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz SM, Drouin G. Phylogeny and substitution rates of angiosperm actin genes. Molecular Biology and Evolution. 1996;13:1198–1212. doi: 10.1093/oxfordjournals.molbev.a025685. [DOI] [PubMed] [Google Scholar]
- Mori K, Sugaya S, Gemma H. Regulatory mechanism of anthocyanin biosynthesis in ‘Kyoho’ grape berries grown under different temperature conditions. Environmental Control in Biology. 2004;42:21–30. [Google Scholar]
- Müller-Röber B, Kossamann J, Hannah LC, Willmitzer L, Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Molecular Genetics and Genomics. 1990;224:136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Müller-Röber B, Sonnewald U, Willmitzer L. Inhibition of ADP-glucose pyrophosphorylase leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO Journal. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Quoc B, Foyer CH. A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. Journal of Experimental Botany. 2001;52:881–889. doi: 10.1093/jexbot/52.358.881. [DOI] [PubMed] [Google Scholar]
- Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M. The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP-glucose pyrophosphorylase is modified by nitrogen and phosphate. Plant, Cell and Environment. 1998;21:443–455. [Google Scholar]
- N'tchobo H, Dali N, Nguyen-Quoc B, Foyer CH, Yelle S. Starch synthesis in tomato remains constant throughout fruit development and is dependent on sucrose supply and sucrose synthase activity. Journal of Experimental Botany. 1999;50:1457–1463. [Google Scholar]
- Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J. The subunit structure of potato tuber ADP-glucose pyrophosphorylase. Plant Physiology. 1990;93:785–790. doi: 10.1104/pp.93.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-W, Chung W-I. Molecular cloning and organ-specific expression of three isoforms of tomato ADP-glucose pyrophosphorylase gene. Gene. 1998;206:215–221. doi: 10.1016/s0378-1119(97)00588-x. [DOI] [PubMed] [Google Scholar]
- Petreikov M, Shen S, Yeselson Y, Levin I, Bar M, Schaffer AA. Temporally extended gene expression of the ADP-Glc pyrophosphorylase large subunit (AgpL1) leads to increased enzyme activity in developing tomato fruit. Planta. 2006;224:1465–1479. doi: 10.1007/s00425-006-0316-y. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Biosynthesis of starch and its regulation. In: Preiss J, editor. The biochemistry of plants. Vol. 14. San Diego, CA: Academic Press; 1988. pp. 181–254. [Google Scholar]
- Raskin I, Kende H. Effect of submergence on translocation, starch content and amylolytic activity in deep-water rice. Planta. 1984;162:556–559. doi: 10.1007/BF00399922. [DOI] [PubMed] [Google Scholar]
- Robinson NL, Hewitt JD, Bennett AB. Sink metabolism in tomato fruit. I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiology. 1988;87:727–730. doi: 10.1104/pp.87.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. Impaired sucrose-induction mutants reveal the modification of sugar-induced starch biosynthetic gene expression by abscisic acid signaling. The Plant Journal. 2001;26:421–433. doi: 10.1046/j.1365-313x.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Fukuda N, Matsukura C, Nishimura S. Effect of salinity on distribution of photosynthates and carbohydrate metabolism in tomato grown using nutrient film technique. Journal of the Japanese Society for Horticultural Science. 2009;78:90–96. [Google Scholar]
- Saito T, Matsukura C, Ban Y, Shoji K, Sugiyama M, Fukuda N, Nishimura S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.) Journal of the Japanese Society for Horticultural Science. 2008;77:61–68. [Google Scholar]
- Sakamoto Y, Watanabe S, Nakashima T, Okano K. Effects of salinity at two ripening stages on the fruit quality of single-truss tomato grown in hydroponics. Journal of Horticultural Science and Biotechnology. 1999;74:690–693. [Google Scholar]
- Salanoubat M, Belliard G. The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and sucrose. Gene. 1989;84:181–185. doi: 10.1016/0378-1119(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Schaffer AA, Levin I, Ogus I, Petreikov M, Cincarevsky F, Yeselson E, Shen S, Gilboa N, Bar M. ADP-glucose pyrophosphorylase activity and starch accumulation in immature tomato fruit: the effect of a Lycopersicon hirsutum-derived introgression encoding for the large subunit. Plant Science. 2000;152:135–144. [Google Scholar]
- Schaffer AA, Petreikov M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiology. 1997;113:739–746. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Gonzàlez-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. The Plant Cell. 1997;9:783–798. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Harbaugh BK. MICRO-TOM—a miniature dwarf tomato. Agricultural Experiment Station, Institute of Food and Agricultural Sciences, University of Florida Circular. 1989;S-370:1–6. [Google Scholar]
- Sokolov LN, Dejardin A, Kleczkowski LA. Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress) Biochemical Journal. 1998;336:681–687. doi: 10.1042/bj3360681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR. Pyrophosphorylases in Solanum tuberosum. II. Catalytic properties and regulation of ADP-glucose and UDP-glucose pyrophosphorylase activities in potatoes. Plant Physiology. 1981;68:924–929. doi: 10.1104/pp.68.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR, Preiss J. Phosphorylases in Solanum tuberosum. III. Purification, physical and catalytic properties of ADP-glucose pyrophosphorylase in potatoes. Plant Physiology. 1982;69:1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stark DM, Barry GF, Kishore GM. Improvement of fruit quality traits through enhancement of starch biosynthesis. Annals of the New York Academy of Sciences. 1996;792:26–36. [Google Scholar]
- Suwa R, Fujimaki S, Suzui N, et al. Use of positron-emitting tracer imaging system for measuring the effect of salinity on temporal and spatial distribution of 11C tracer and coupling between source and sink organs. Plant Science. 2008;175:210–216. [Google Scholar]
- Tal M, Katz A, Heikin H, Dehan K. Salt tolerance in the wild relatives of the cultivated tomato: proline accumulation in Lycopersicon esculentum Mill., L. peruvianum Mill. and Solanum pennellii Cor. treated with NaCl and polyethyleneglycol. New Phytologist. 1979;82:349–355. [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P. Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: A noble regulatory mechanism linking starch synthesis to the sucrose supply. The Plant Cell. 2002;14:2191–2213. doi: 10.1105/tpc.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signaling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. The Plant Journal. 2003;35:490–500. doi: 10.1046/j.1365-313x.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Tsai C-Y, Neleson OE. Starch-deficient maize mutant lacking adenosine diphosphate glucose pyrophosphorylase activity. Science. 1966;151:341–343. doi: 10.1126/science.151.3708.341. [DOI] [PubMed] [Google Scholar]
- Xing J-P, Li X-Y, Luo Y-Y, Gianfagna TJ, Janes HW. Isolation and expression analysis of two tomato ADP-glucose pyrophosphorylase S (large) subunit gene promoters. Plant Science. 2005;169:882–893. [Google Scholar]
- Yelle S, Hewitt JD, Nieder M, Robinson NL, Damon S, Bennett AB. Sink metabolism in tomato fruit. III. Analysis of carbohydrate assimilation in a wild species. Plant Physiology. 1988;87:731–736. doi: 10.1104/pp.87.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushi K, Matsuzoe N. Postharvest changes in sugar, organic acid, glutamic acid and antioxidant contents in tomato fruit grown under salinity stress. Environment Control in Biology. 2006;44:111–117. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.