Hutchinson-Gilford progeria syndrome (HGPS) is a rare, sporadic, autosomal dominant disease with phenotypic features of premature aging (1). It is caused by de novo mutations in LMNA that encodes the A-type nuclear lamins, intermediate filament proteins that are components of the nuclear lamina (2, 3). Besides HGPS, a spectrum of diseases sometimes referred to as “laminopathies,” which include cardiomyopathy, muscular dystrophy, partial lipodystrophy, peripheral neuropathy, and variant progeroid syndromes, result from mutations in LMNA (4).
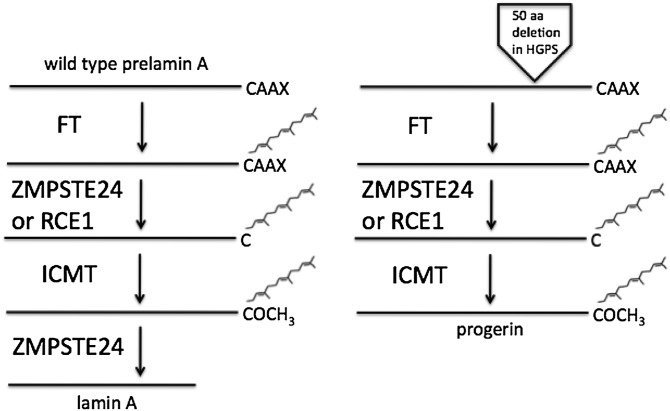
The major A-type lamin isoforms, lamin A and lamin C, are expressed in most differentiated somatic cells and arise by alternative splicing of LMNA premRNA (5). Lamin A is synthesized as a precursor, prelamin A, which undergoes a series of posttranslational chemical reactions (6, 7). A CAAX (cysteine-aliphatic-aliphatic-any amino acid) motif at the carboxyl-terminus of prelamin A triggers three sequential enzymatic reactions leading to farnesylation and carboxymethylation of the cysteine (Fig. 1). The first reaction, catalyzed by protein farnesyltransferase, is the addition of a farnesyl lipid to the cysteine. The next reaction is the endoproteolytic cleavage of the –AAX, which in the case of prelamin A, is catalyzed by RCE1 and ZMPSTE24. The third reaction, catalyzed by isoprenylcysteine carboxyl methyltransferase, is methylation of the farnesylated cysteine. Prelamin A then undergoes a farnesylation-dependent cleavage catalyzed by ZMPSTE24, which leads to removal of a 15-amino acid farnesylated polypeptide from the carboxyl-terminus. As a result, mature lamin A that is incorporated into the nuclear lamina is no longer prenylated.
Fig. 1.
Posttranslational processing of wild-type prelamin A to lamin A (left) and generation of progerin in HGPS (right). See text for details. FT, protein fanesyltransferase; ICMT, isoprenylcysteine carboxyl methyltransferase.
HGPS is caused by mutations in exon 11 of LMNA that optimize an alternative RNA splice donor site resulting in an in-frame deletion of 50 amino acids near the carboxyl-terminus of prelamin A (2, 3). As the CAAX motif is retained in the truncated protein, it undergoes the first three reactions just like wild-type prelamin A (Fig. 1). However, the second ZMPSTE24 cleavage site is lost as a result of the deletion preventing further processing; hence, progerin remains prenylated (Fig. 1).
In 2002, Bergo et al. (8) and Pendás et al. (9) showed that knocking out Zmpste24 in mice resulted in accumulation of unprocessed, farnesylated prelamin A and a progeroid phenotype. In 2004, Fong et al. (10) showed that the progeroid phenotype of these mice was ameliorated by a genetic reduction of unprocessed, farnesylated prelamin A by crossing them to Lmna deficient mice. Progerin is a truncated, permanently farnesylated variant of prelamin A (Fig. 1). This led Stephen Young, Loren Fong and colleagues to hypothesize that, similar to unprocessed prelamin A, progerin is responsible for the progeroid phenotype in HGPS and that blocking its farnesylation would be beneficial. To test this hypothesis, they generated knock in mice with a targeted HGPS mutation, an animal model that recapitulates many of the phenotypic features of the human disease (11, 12). They showed that treatment of fibroblasts from these mice with a protein farnesyltransferase inhibitor (FTI) reversed nuclear shape abnormalities seen in cells from subjects with most laminopathies (11). They (13) and others (14, 15) subsequently showed that FTI treatment reversed the nuclear shape abnormalities in cultured fibroblasts from human subjects with HGPS. Most importantly, the group of Young and Fong showed that systemic treatment with an FTI significantly improved, albeit not completely, the progeroid phenotypes of both HGPS knock in and Zmpste24 knockout mice (12, 16). Two years later, Capell et al. (17) reported that an FTI prevented loss of vascular smooth muscle cells in the media of large arteries in a BAC transgenic mouse that expresses progerin but lacks any pathologic features of HGPS other than the vascular abnormalities.
These laboratory studies raised the exciting possibility that blocking progerin farnesylation could be a treatment for children with HGPS. However, further work from the group of Young and Fong raised a yellow flag when they created an ingenious knock in model mouse that expressed only nonfarnesylated progerin. These mice, in which the cysteine of the progerin CAAX motif was replaced by a serine, unexpectedly developed the same but milder progeroid phenotypes as knock in mice expressing farnesylated progerin (18). This finding had two important implications. First, it suggested that FTIs might be acting indirectly to improve the phenotypes in HGPS knock in mice by blocking the activities of farnesylated proteins other than progerin. Second, it raised concerns about the overall utility of FTIs as potential treatment for the disease.
In the current issue of the JLR, Fong, Young, and colleagues present new data suggesting that the beneficial effects of an FTI in HGPS model mice likely result from specifically blocking farnesylation of progerin (19). They compared the ability of an FTI to improve the progeroid phenotypes in knock in mice that expressed farnesylated and nonfarnesylated progerin. FTI treatment significantly, although not completely, improved the phenotype and survival of the knock in mice expressing progerin. In contrast, the drug had no significant effect in mice expressing nonfarnesylated progerin. These results suggest that the beneficial effects of an FTI in HGPS model mice are due to a direct effect of drug on progerin. This, of course, does not mean that the FTI is only blocking farnesylation of progerin (the authors even show inhibition of farnesylation of HDJ-2 in both mice). Therefore, FTI administration could still interfere with other farnesylation-dependent protein functions that could have consequences other than improvement of the progeroid phenotype.
Which leads to the second more than academic concern about the overall utility of FTIs as potential treatment for children with HGPS. Based largely on the pioneering research of the Young and Fong group, a clinical trial of an FTI has been initiated in the United States for children with HGPS (20). Based on another study in Zmpste24 knockout mice (21), a combination of a statin and an aminobisphosphonate, which can theoretically inhibit protein prenylation but also have off-target effects, is being used in a similar clinical trial in Europe. But given the results of Young and Fong (12, 18, 19), blocking progerin prenylation will not likely completely reverse or totally halt progression of the disease in children with HGPS. Even a chemical modification of progerin that completely prevents its farnesylation does not cure the disease in mice (18, 19). Furthermore, it is unclear how long FTIs or other drugs that interfere with protein prenylation, which may have other effects as well, can be given safely to children before significant adverse events occur. As with all pharmacological interventions, a risk-benefit assessment based upon the data must be made. Regarding potential benefits, the research published in this issue of the JLR (19) shows that an FTI improves progeroid phenotypes and survival in an animal mode of HGPS and strongly suggests that the mechanism of action is by directly blocking farnesylation of the culprit target protein. However, the same research highlights the potential limitations of blocking progerin farnesylation to treat HGPS using drugs that may have risks. A realistic and cautious approach to such treatment is necessary, especially in discussing the issue with patients, family members, and the lay press.
Footnotes
Abbreviations:
- CAAX
- cysteine-aliphatic-aliphatic-any amino acid
- FTI
- farnesyltransferase inhibitor
- HGPS
- Hutchinson-Gilford progeria syndrome
REFERENCES
- 1.Merideth M. A., Gordon L. B., Clauss S., Sachdev V., Smith A. C., Perry M. B., Brewer C. C., Zalewski C., Kim H. J., Solomon B., et al. 2008. Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 358: 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksson M., Brown W. T., Gordon L. B., Glynn M. W., Singer J., Scott L., Erdos M. R., Robbins C. M., Moses T. Y., Berglund P., et al. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 423: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C. L., Munnich A., Le Merrer M., et al. 2003. Lamin a truncation in Hutchinson-Gilford progeria. Science. 300: 2055. [DOI] [PubMed] [Google Scholar]
- 4.Worman H. J., Bonne G., et al. 2007. “Laminopathies”: a wide spectrum of human diseases. Exp. Cell Res. 313: 2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin F., Worman H. J., et al. 1993. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268: 16321–16326. [PubMed] [Google Scholar]
- 6.Young S. G., Fong L. G., Michaelis S., et al. 2005. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria–new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J. Lipid Res. 46: 2531–2558. [DOI] [PubMed] [Google Scholar]
- 7.Rusiñol A. E., Sinensky M. S. 2006. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 119: 3265–3272. [DOI] [PubMed] [Google Scholar]
- 8.Bergo M. O., Gavino B., Ross J., Schmidt W. K., Hong C., Kendall L. V., Mohr A., Meta M., Genant H., Jiang Y., et al. 2002. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. USA. 99: 13049–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pendás A. M., Zhou Z., Cadiñanos J., Freije J. M., Wang J., Hultenby K., Astudillo A., Wernerson A., Rodríguez F., Tryggvason K., et al. 2002. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31: 94–99. [DOI] [PubMed] [Google Scholar]
- 10.Fong L. G., Ng J. K., Meta M., Coté N., Yang S. H., Stewart C. L., Sullivan T., Burghardt A., Majumdar S., Reue K., et al. 2004. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc. Natl. Acad. Sci. USA. 101: 18111–18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S. H., Bergo M. O., Toth J. I., Qiao X., Hu Y., Sandoval S., Meta M., Bendale P., Gelb M. H., Young S. G., et al. 2005. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. USA. 102: 10291–10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S. H., Meta M., Qiao X., Frost D., Bauch J., Coffinier C., Majumdar S., Bergo M. O., Young S. G., Fong L. G. 2006. Treatment with a proteinfarnesyltransferase inhibitor improves disease phenotypes in mice with a targeted Hutchinson- Gilford progeria syndrome mutation. J. Clin. Invest. 116: 2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth J. I., Yang S. H., Qiao X., Beigneux A. P., Gelb M. H., Moulson C. L., Miner J. H., Young S. G., Fong L. G. 2005. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA. 102: 12873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capell B. C., Erdos M. R., Madigan J. P., Fiordalisi J. J., Varga R., Conneely K. N., Gordon L. B., Der C. J., Cox A. D., Collins F. S., et al. 2005. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 102: 12879–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn M. W., Glover T. W. 2005. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 14: 2959–2969. [DOI] [PubMed] [Google Scholar]
- 16.Fong L. G., Frost D., Meta M., Qiao X., Yang S. H., Coffinier C., Young S. G. 2006. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science. 311: 1621–1623. [DOI] [PubMed] [Google Scholar]
- 17.Capell B. C., Olive M., Erdos M. R., Cao K., Faddah D. A., Tavarez U. L., Conneely K. N., Qu X., San H., Ganesh S. K., et al. 2008. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc. Natl. Acad. Sci. USA. 105: 15902–15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S. H., Andres D. A., Spielmann H. P., Young S. G., Fong L. G. 2008. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J. Clin. Invest. 118: 3291–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S. H., Chang S. Y., Andres D. A., Spielmann H. P., Young S. G., Fong L. G. 2010. Assessing the efficacy of protein farnesyltransferase inhibitors in mouse models of progeria. J. Lipid Res. 51: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon L. B., Harling-Berg C. J., Rothman F. G. 2007. Highlights of the 2007 Progeria Research Foundation scientific workshop: progress in translational science. J. Gerontol. A Biol. Sci. Med. Sci. 63: 777–787. [DOI] [PubMed] [Google Scholar]
- 21.Varela I., Pereira S., Ugalde A. P., Navarro C. L., Suárez M. F., Cau P., Cadiñanos J., Osorio F. G., Foray N., Cobo J., et al. 2008. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat. Med. 14: 767–772. [DOI] [PubMed] [Google Scholar]